Efficacy of Contractility Modulation Therapy in Patients with Transthyretin Amyloid Cardiomyopathy, Mildly Reduced to Reduced EF and NYHA III and IV: A Multicentric, Prospective Pilot Study for AMY-CCM Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Primary Outcome
3.3. Secondary Outcome
3.4. Tafamidis Eligibility After CCM
4. Discussion
4.1. Clinical Implications of CCM in ATTR-CM
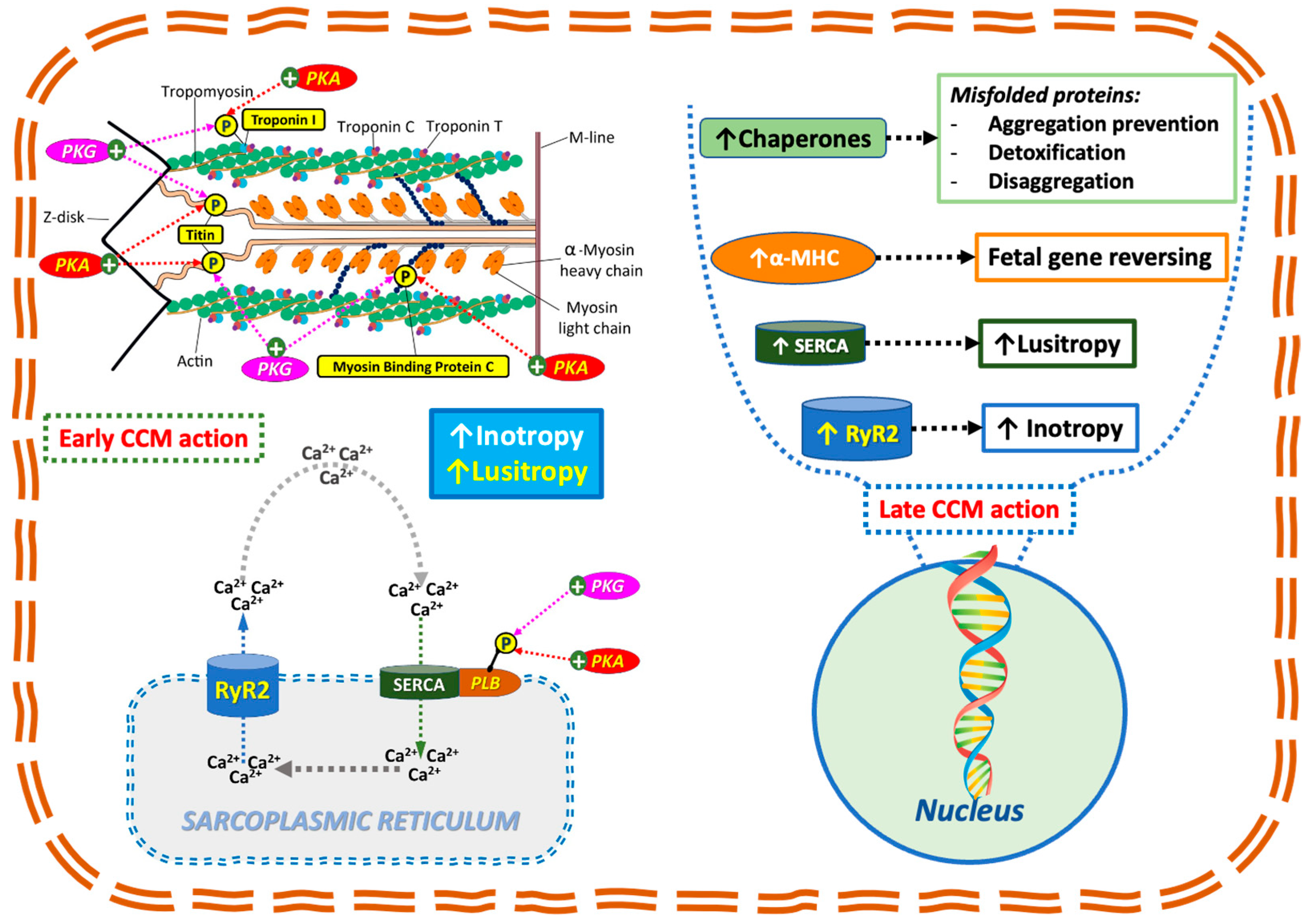
4.2. Mechanistic Rationale for CCM in Amyloidosis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE-I | Angiotensin-Converting Enzyme Inhibitor |
| AL | Light-Chain Amyloidosis |
| AMY-CCM | Amyloidosis–Cardiac Contractility Modulation Registry |
| ARB | Angiotensin Receptor Blocker |
| ARNI | Angiotensin Receptor–Neprilysin Inhibitor |
| ATTR | Transthyretin |
| ATTR-CM | Transthyretin Cardiac Amyloidosis |
| BB | Beta-Blocker |
| BMI | Body Mass Index |
| CABG | Coronary Artery Bypass Grafting |
| CaMKII | Calcium/Calmodulin-Dependent Protein Kinase II |
| CCB | Calcium Channel Blocker |
| CCM | Cardiac Contractility Modulation |
| CMR | Cardiac Magnetic Resonance |
| CRT | Cardiac Resynchronization Therapy |
| ECG | Electrocardiogram |
| EDV | End-Diastolic Volume |
| EF | Ejection Fraction |
| eGFR | Estimated Glomerular Filtration Rate |
| ESV | End-Systolic Volume |
| H | Hereditary |
| HF | Heart Failure |
| HSP70 | Heat Shock Protein 70 |
| ICD | Implantable Cardioverter-Defibrillator |
| IQR | Interquartile Range |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| MAPK | Mitogen-Activated Protein Kinase |
| MRA | Mineralocorticoid Receptor Antagonist |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association (Functional Class) |
| OMT | Optimal Medical Therapy |
| PCI | Percutaneous Coronary Intervention |
| PKA | Protein Kinase A |
| PKB | Protein Kinase B |
| PLB | Phospholamban |
| ROS | Reactive Oxygen Species |
| RyR2 | Ryanodine Receptor 2 |
| SERCA | Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase |
| SGLT2-i | Sodium-Glucose Cotransporter-2 Inhibitor |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TTR | Transthyretin Protein |
| WHF | Worsening Heart Failure |
| WT | Wild-Type |
| α-MHC | Alpha-Myosin Heavy Chain |
References
- Liao, R.; Ward, J.E. Amyloid Cardiomyopathy: Disease on the Rise. Circ. Res. 2017, 120, 1865–1867. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2021, 23, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Garcia-Pavia, P.; Hanna, M.; Judge, D.P.; Merlini, G.; Gundapaneni, B.; Patterson, T.A.; Riley, S.; Schwartz, J.H.; Sultan, M.B.; et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur. J. Heart Fail. 2021, 23, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Marchese, P.; Gennaro, F.; Mazzotta, G.; Acciarri, C.; Amabili, S.; Bonanni, C.; D’antonio, A.; Delfino, D.; Di Vito, L.; Partemi, M.; et al. Cardiac Contractility Modulation Therapy in Patients with Amyloid Cardiomyopathy and Heart Failure, Case Report, Review of the Biophysics of CCM Function, and AMY-CCM Registry Presentation. J. Clin. Med. 2023, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Samara, M.A.; Feldman, D.S. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat. Rev. Cardiol. 2013, 10, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Cappannoli, L.; Scacciavillani, R.; Rocco, E.; Perna, F.; Narducci, M.L.; Vaccarella, M.; D’aMario, D.; Pelargonio, G.; Massetti, M.; Crea, F.; et al. Cardiac contractility modulation for patient with refractory heart failure: An updated evidence-based review. Heart Fail. Rev. 2021, 26, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Borggrefe, M.; Neuser, H.; Ohlow, M.; Röger, S.; Goette, A.; Remppis, B.A.; Kuck, K.; Najarian, K.B.; Gutterman, D.D.; et al. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2019, 21, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Van Linthout, S.; Spillmann, F.; Klein, O.; Biewener, S.; Remppis, A.; Gutterman, D.; Linke, W.A.; Pieske, B.; Hamdani, N.; et al. Cardiac contractility modulation signals improve exercise intolerance and maladaptive regulation of cardiac key proteins for systolic and diastolic function in HFpEF. Int. J. Cardiol. 2016, 203, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Kuschyk, J.; Nägele, H.; Heinz-Kuck, K.; Butter, C.; Lawo, T.; Wietholt, D.; Roeger, S.; Gutterman, D.; Burkhoff, D.; Rousso, B.; et al. Cardiac contractility modulation treatment in patients with symptomatic heart failure despite optimal medical therapy and cardiac resynchronization therapy (CRT). Int. J. Cardiol. 2019, 277, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Tint, D.; Florea, R.; Micu, S. New Generation Cardiac Contractility Modulation Device—Filling the Gap in Heart Failure Treatment. J. Clin. Med. 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Jones, P.G. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Kuck, K.-H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R.; et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Kherad, B.; Klein, O.; Lipp, A.; Blaschke, F.; Gutterman, D.; Burkhoff, D.; Hamdani, N.; Spillmann, F.; Van Linthout, S. Cardiac contractility modulation: Mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur. J. Heart Fail. 2019, 21, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Rastogi, S.; Gupta, R.C.; Mishra, S.; Sharov, V.G.; Stanley, W.C.; Mika, Y.; Rousso, B.; Burkhoff, D.; Ben-Haim, S.; et al. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. JACC 2007, 49, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Kassiotis, C.; Razeghi, P.; Taegtmeyer, H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail. Rev. 2007, 12, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Goodman, R. A mechanism for stimulation of biosynthesis by electromagnetic fields: Charge transfer in DNA and base pair separation. J. Cell. Physiol. 2008, 214, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guan, J.; Jiang, B.; Brenner, D.A.; del Monte, F.; Ward, J.E.; Connors, L.H.; Sawyer, D.B.; Semigran, M.J.; Macgillivray, T.E.; et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38α MAPK pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- Wentink, A.S.; Nillegoda, N.B.; Feufel, J.; Ubartaitė, G.; Schneider, C.P.; Rios, P.D.L.; Hennig, J.; Barducci, A.; Bukau, B. Molecular dissection of amyloid disaggregation by human HSP70. Nature 2020, 587, 483–488. [Google Scholar] [CrossRef] [PubMed]

| General Characteristics | Total |
|---|---|
| Total Number (n) | 10 |
| Age, years, mean [DS] | 76.7 ± 4.8 |
| Male gender, number (%) | 9 (90) |
| BMI (kg/m2) | 24.4 ± 3.4 |
| Years since first diagnosis | 3.1 ± 1.7 |
| HF Hospitalization (WHF) in the last 12 months | 2.0 ± 1.2 |
| Hypertension (%) | 90% |
| Diabetes (%) | 50% |
| Dyslipidemia (%) | 40% |
| Chronic pulmonary disease (%) | 10% |
| Chronic kidney disease (%) | 70% |
| Chronic liver disease (%) | 20% |
| Coronary artery disease (%) | 20% |
| Atrial fibrillation (%) | 50% |
| Aortic Stenosis (%) | 20% |
| General Characteristics | Total |
|---|---|
| ACE-I (%) | 30 |
| ARBs (%) | 0 |
| BBs (%) | 60 |
| Diuretics (%) | 100 |
| CCBs (%) | 10 |
| Antiplatelets (%) | 50 |
| Anticoagulants (%) | 50 |
| Anti-arrhythmics (%) | 30 |
| Vaso-dilators (%) | 0 |
| Digoxin (%) | 0 |
| MRAs (%) | 40 |
| ARNI (%) | 50 |
| SGLUT-2 (%) | 20 |
| Tafamidis (%) | 30 |
| General Characteristics | Total |
|---|---|
| NYHA class | 3.2 ± 0.4 |
| 6MWT (meters) | 264.6 ± 100.1 |
| KCCQs | 42.0 ± 31 |
| NT pro-BNP (pg/mL) | 4300.8 ± 3085.8 |
| eGFR (mL/min/1.73 m2) | 52.0 ± 8.2 |
| General Characteristics | Total |
|---|---|
| EF (%) | 36.5 ± 7.3 |
| iLAV (mL/m2) | 39.7 ± 7.5 |
| LV IVS (mm) | 21.3 ± 3.5 |
| LV PP (mm) | 18 ± 6.7 |
| E/E′ | 11.4 ± 7.7 |
| RV free wall thickness (mm) | 12.0 ± 5.4 |
| TAPSE (mm) | 12.2 ± 2.3 |
| Pericardial effusion (%) | 40 |
| Circumferential mid global strain (%) | 7.5 ± 1.5 |
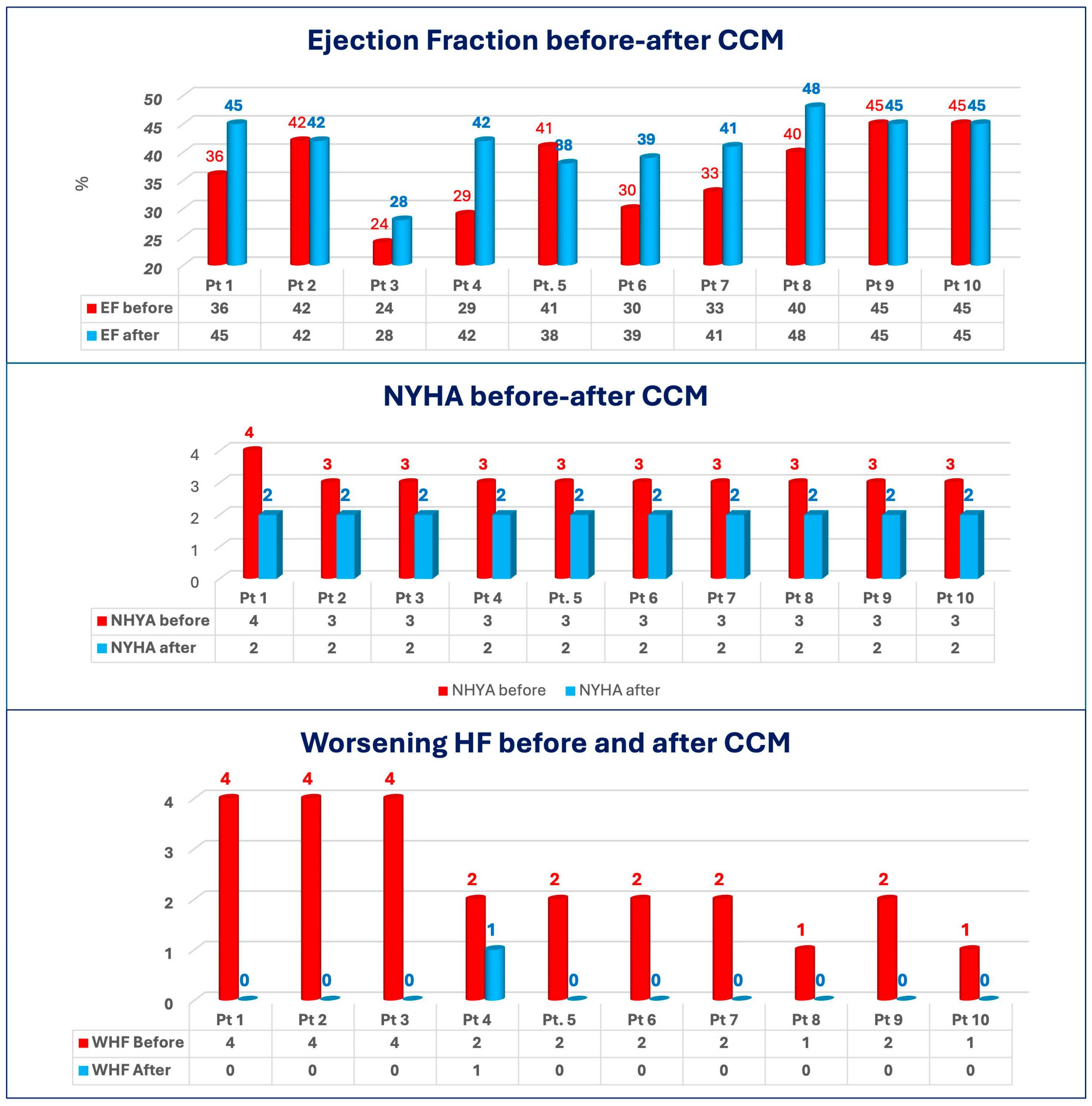
| PRE-CCM | POST-CCM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pts | Sex | Age | ATTR-CM Wild Type (WT) Hereditary (H) | Time to Diagnosis (Months) | NYHA | EF (%) | Tafamidis | WHF Previous 12 Months | WHF After CCM | NYHA | EF (%) | Tafamidis | Follow-Up (Months) |
| 1 | M | 70 | WT | 68 | 4 | 36 | No | 4 | 0 | 2 | 45 | Yes | 42 |
| 2 | M | 82 | WT | 16 | 3 | 42 | No | 4 | 0 | 2 | 42 | Yes | 12 |
| 3 | M | 72 | H | 79 | 3 | 24 | No | 4 | 0 | 2 | 28 | Yes | 12 |
| 4 | M | 76 | WT | 88 | 3 | 29 | Yes | 2 | 1 | 2 | 42 | Yes | 12 |
| 5 | M | 83 | WT | 16 | 3 | 41 | No | 2 | 0 | 2 | 38 | Yes | 12 |
| 6 | M | 78 | WT | 22 | 3 | 30 | Yes | 2 | 0 | 2 | 39 | Yes | 12 |
| 7 | M | 57 | WT | 18 | 3 | 33 | No | 2 | 0 | 2 | 41 | Yes | 12 |
| 8 | M | 85 | WT | 34 | 3 | 40 | No | 1 | 0 | 2 | 48 | Yes | 34 |
| 9 | M | 77 | WT | 58 | 3 | 45 | No | 2 | 0 | 2 | 45 | Yes | 39 |
| 10 | M | 81 | WT | 28 | 3 | 45 | Yes | 1 | 0 | 2 | 45 | Yes | 19 |
| Parameter | Delta |
|---|---|
| EF (%) | +4.8 ± 6.1 |
| E/E′ (%) | −11.1 ± 22.7 |
| TAPSE (%) | +5.3 ± 10.3 |
| NYHA class | −1.2 ± 0.4 |
| 6MWT (%) | +31.3 ± 53.3 |
| KCCQs (%) | +121.0 ± 252.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchese, P.; Gennaro, F.; Mazzotta, G.; Grossi, P.; Cocchiara, L.; Guarracini, S.; Mazzocchetti, L.; Ziacchi, M.; Biffi, M.; Magnano, R.; et al. Efficacy of Contractility Modulation Therapy in Patients with Transthyretin Amyloid Cardiomyopathy, Mildly Reduced to Reduced EF and NYHA III and IV: A Multicentric, Prospective Pilot Study for AMY-CCM Registry. J. Cardiovasc. Dev. Dis. 2025, 12, 380. https://doi.org/10.3390/jcdd12100380
Marchese P, Gennaro F, Mazzotta G, Grossi P, Cocchiara L, Guarracini S, Mazzocchetti L, Ziacchi M, Biffi M, Magnano R, et al. Efficacy of Contractility Modulation Therapy in Patients with Transthyretin Amyloid Cardiomyopathy, Mildly Reduced to Reduced EF and NYHA III and IV: A Multicentric, Prospective Pilot Study for AMY-CCM Registry. Journal of Cardiovascular Development and Disease. 2025; 12(10):380. https://doi.org/10.3390/jcdd12100380
Chicago/Turabian StyleMarchese, Procolo, Francesca Gennaro, Giovanni Mazzotta, Pierfrancesco Grossi, Luigi Cocchiara, Stefano Guarracini, Lorenzo Mazzocchetti, Matteo Ziacchi, Mauro Biffi, Roberta Magnano, and et al. 2025. "Efficacy of Contractility Modulation Therapy in Patients with Transthyretin Amyloid Cardiomyopathy, Mildly Reduced to Reduced EF and NYHA III and IV: A Multicentric, Prospective Pilot Study for AMY-CCM Registry" Journal of Cardiovascular Development and Disease 12, no. 10: 380. https://doi.org/10.3390/jcdd12100380
APA StyleMarchese, P., Gennaro, F., Mazzotta, G., Grossi, P., Cocchiara, L., Guarracini, S., Mazzocchetti, L., Ziacchi, M., Biffi, M., Magnano, R., Di Marco, M., Ruzzolini, M., Bisignani, A., Bianco, M., Garrone, P., Grosso Marra, W., Cannillo, M., Lavalle, C., Masarone, D., & Chimenti, C. (2025). Efficacy of Contractility Modulation Therapy in Patients with Transthyretin Amyloid Cardiomyopathy, Mildly Reduced to Reduced EF and NYHA III and IV: A Multicentric, Prospective Pilot Study for AMY-CCM Registry. Journal of Cardiovascular Development and Disease, 12(10), 380. https://doi.org/10.3390/jcdd12100380









