Physician Perceptions of the Safety and Efficacy of GLP-1 Receptor Agonists: Underestimation of Cardiovascular Risk Reduction and Discrepancies with Clinical Evidence
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Data Interpretation
3.1.1. Descriptive Statistics
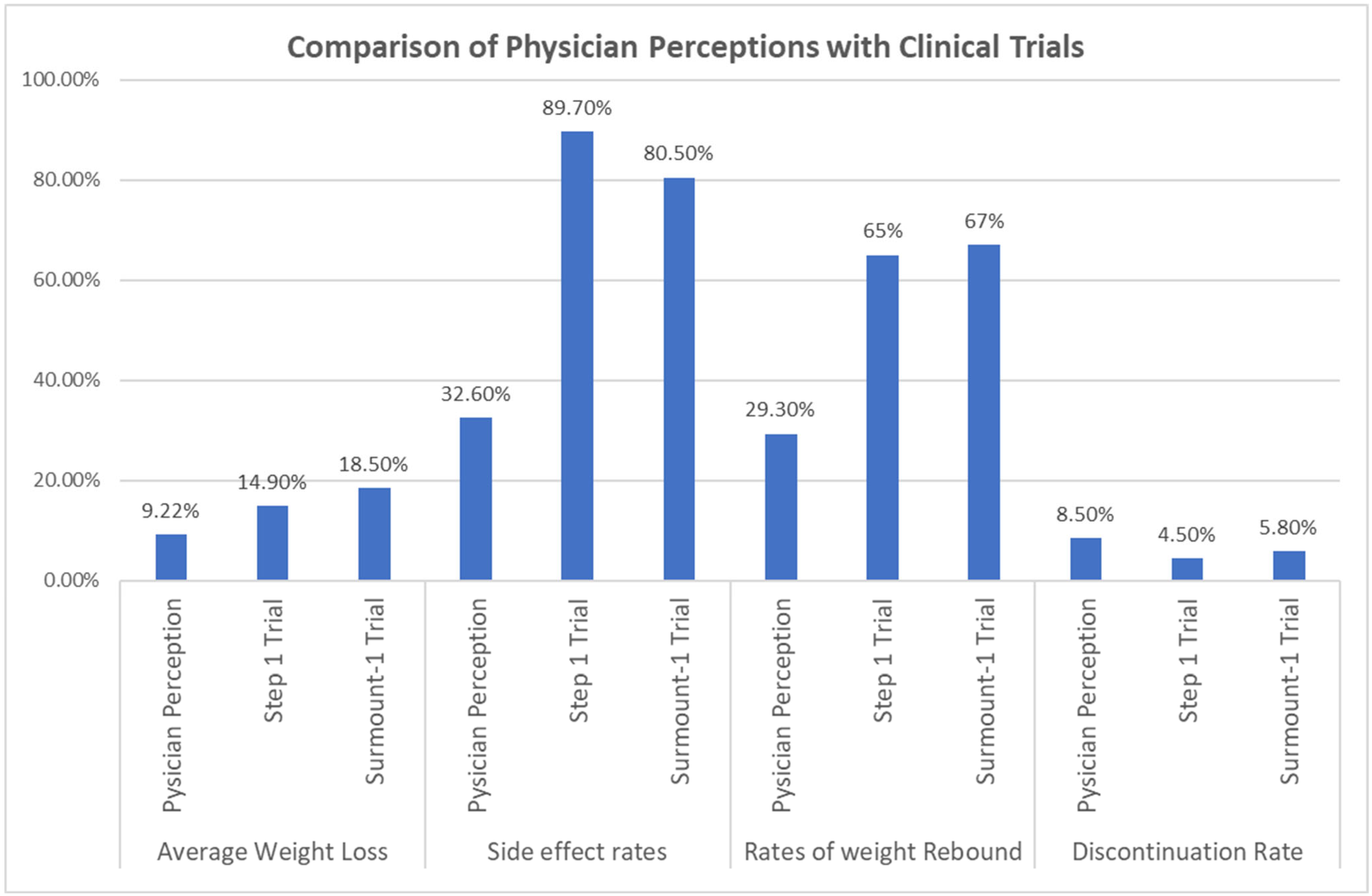
3.1.2. Comparison of Physician Perceptions with Actual Rates in Previous Clinical Trials
3.1.3. Average Weight Loss
3.1.4. Side Effects (%)
3.1.5. Weight Rebound
3.1.6. Discontinuation Rate at 72 Weeks
3.1.7. Cardiovascular Protection and Risk Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-Garcia, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Berger, S.E.; Huggins, G.S.; McCaffery, J.M.; Jacques, P.F.; Lichtenstein, A.H. Change in cardiometabolic risk factors associated with magnitude of weight regain 3 years after a 1-year intensive lifestyle intervention in type 2 diabetes mellitus: The look AHEAD trial. J. Am. Heart Assoc. 2019, 8, e010951. [Google Scholar] [CrossRef]
- Patti, A.M.; Rizvi, A.A.; Giglio, R.V.; Stoian, A.P.; Ligi, D.; Mannello, F. Impact of Glucose-Lowering Medications on Cardiovascular and Metabolic Risk in Type 2 Diabetes. J. Clin. Med. 2020, 9, 912. [Google Scholar] [CrossRef]
- Giglio, R.V.; Stoian, A.P.; Al-Rasadi, K.; Banach, M.; Patti, A.M.; Ciaccio, M.; Rizvi, A.A.; Rizzo, M. Novel Therapeutical Approaches to Managing Atherosclerotic Risk. Int. J. Mol. Sci. 2021, 22, 4633. [Google Scholar] [CrossRef]
- Nikolic, D.; Patti, A.M.; Giglio, R.V.; Chianetta, R.; Castellino, G.; Magán-Fernández, A.; Citarrella, R.; Papanas, N.; Janez, A.; Stoian, A.P.; et al. Liraglutide Improved Cardiometabolic Parameters More in Obese than in Non-obese Patients with Type 2 Diabetes: A Real-World 18-Month Prospective Study. Diabetes Ther. 2022, 13, 453–464. [Google Scholar] [CrossRef]
- Rizzo, M.; Nikolic, D.; Patti, A.M.; Mannina, C.; Montalto, G.; McAdams, B.S.; Rizvi, A.A.; Cosentino, F. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 2814–2821. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Allotta, A.; Bruno, A.; Di Bella, T.; Stoian, A.P.; Ciaccio, M.; Rizzo, M. Effect of Semaglutide on Subclinical Atherosclerosis and Cardiometabolic Compensation: A Real-World Study in Patients with Type 2 Diabetes. Biomedicines 2023, 11, 1362. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S. The evolution of diabetes treatment through the ages: From starvation diets to insulin, incretins, SGLT2-inhibitors and beyond. J. Indian Inst. Sci. 2023, 103, 123–133. [Google Scholar] [CrossRef]
- Zinman, B.; Lachin, J.M.; Inzucchi, S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2016, 374, 1094. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Packer, M. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2022, 386, e57. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; Le Roux, C.W.; Ortiz, R.V.; Jensen, C.B.; et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Bhatt, D.L.; Buse, J.B.; Del Prato, S.; Kahn, S.E.; Lincoff, A.M.; McGuire, D.K.; Nauck, M.A.; Nissen, S.E.; Sattar, N.; et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am. Heart J. 2024, 267, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Thank you for your participation in our survey of medical professionals’ perceptions and experiences with novel weight loss medications. Your anonymous responses will contribute to a broader understanding of how these weight loss treatments may be used in clinical practice. |
| Please answer the following questions based on your estimation or clinical experience: |
| Have you ever prescribed any of the following medications: semaglutide (Ozempic, Wegovy), tirzepatide (Mounjaro, Zepbound)? |
| In your estimation, what is the average percentage of weight loss in patients taking these weight loss medications? |
| In your estimation, what percentage of patients achieve at least 5% weight loss while on these medications? |
| In your estimation, what percentage of patients achieve at least 20% weight loss while on these medications? |
| Do you believe these medications provide secondary cardiovascular prevention benefits in overweight diabetic patients with established cardiovascular disease? |
| Do you believe these medications provide secondary cardiovascular prevention benefits in overweight non-diabetic patients with established cardiovascular disease? |
| In your estimation, what percentage of patients experience any side effects from these weight loss medications? |
| Please estimate the percentage of patients that experience nausea: |
| Please estimate the percentage of patients that experience diarrhea: |
| What is your estimation of the rate at which patients discontinue the use of weight loss medications due to any reason? |
| In patients who interrupt or stop using weight loss medication, what is your estimation of the percentage of weight that is regained? |
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 60 (49.2) |
| Female | 62 (50.8) |
| Age (years), Mean | 41.6 |
| Have you prescribed these agents? | |
| No | 67 (54.9) |
| Yes | 55 (45.1) |
| * CV Protection in diabetics | |
| No | 63 (51.6) |
| Yes | 59 (48.4) |
| CV reduction in non-diabetics | |
| No | 74 (60.7) |
| Yes | 48 (39.3) |
| Average weight loss (%), Mean ± SD | 9.22 ± 4.04 |
| At least 5% weight loss (%), Mean ± SD | 49.30 ± 18.39 |
| At least 20% weight loss (%), Mean ± SD | 18.81 ± 13.74 |
| Any side effect (%), Mean ± SD | 32.62 ± 15.96 |
| Nausea (%), Mean ± SD | 18.36 ± 11.12 |
| Diarrhea (%), Mean ± SD | 7.50 ± 4.95 |
| D/C rate at 72 weeks (%), Mean ± SD | 8.59 ± 5.85 |
| Weight rebound (%), Mean ± SD | 29.26 ± 20.58 |
| Comparison | Physician Mean % (SD, n) | Reference Mean % (SD, n) | t-Value | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|---|
| Average weight loss | |||||
| Physicians’ vs. STEP 1 trial * | −9.22 (4.038, 122) | −14.9 (NR, 1306) | −15.533 | <0.001 | −5.679 (−6.40–−4.95) |
| Physicians’ vs. SURMOUNT-1 trial * | −9.22 (4.038, 122) | −18.5 (NR, 1896) | −25.380 | <0.001 | −9.279 (−10.00–−8.55) |
| Rate of side effects | |||||
| Physicians’ vs. STEP 1 trial * | 32.62 (15.957, 122) | 89.7 (15.95, 1306) | −39.508 | <0.001 | −57.077 (−59.94–−54.22) |
| Physicians’ vs. SURMOUNT-1 trial * | 32.62 (15.957, 122) | 80.5 (15.95, 1896) | −33.140 | <0.001 | −47.877 (−50.74–−45.02) |
| % Weight regained | |||||
| Physicians’ vs. STEP 4 trial * | 29.26 (20.577, 122) | 65 (15, 268) | −19.183 | <0.001 | −35.738 (−39.43–−32.05) |
| Physicians’ vs. SURMOUNT-4 trial * | 29.26 (20.577, 122) | 67 (15, 335) | −20.257 | <0.001 | −37.738 (−41.43–−34.05) |
| Discontinuation rate | |||||
| Physicians’ vs. STEP 1 trial * | 8.59 (5.855, 121) | 4.5 (NR, 1306) | 7.678 | <0.001 | 4.087 (3.03–5.14) |
| Physicians’ vs. SURMOUNT-1 trial * | 8.59 (5.855, 121) | 5.8 (6.2, 1896) | 5.236 | <0.001 | 2.787 (1.73–3.84) |
| Comparison | Observed Successes/Trials | Proportion (95% CI) | Test Value | Z Score | One-Tailed p-Value |
|---|---|---|---|---|---|
| CV protection in diabetics | 59/122 | 0.484 (0.397–0.571) | 0.5 | −0.362 | 0.359 |
| CV reduction in non-diabetics * | 48/122 | 0.393 (0.311–0.482) | 0.5 | −2.354 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, S.; Srivastava, P.K.; Attaluri, J.; Nayeri, R.; Chatterjee, D.; Patel, J.; Nsair, A.; Budoff, M.; Nayeri, A. Physician Perceptions of the Safety and Efficacy of GLP-1 Receptor Agonists: Underestimation of Cardiovascular Risk Reduction and Discrepancies with Clinical Evidence. J. Cardiovasc. Dev. Dis. 2025, 12, 19. https://doi.org/10.3390/jcdd12010019
Krishnan S, Srivastava PK, Attaluri J, Nayeri R, Chatterjee D, Patel J, Nsair A, Budoff M, Nayeri A. Physician Perceptions of the Safety and Efficacy of GLP-1 Receptor Agonists: Underestimation of Cardiovascular Risk Reduction and Discrepancies with Clinical Evidence. Journal of Cardiovascular Development and Disease. 2025; 12(1):19. https://doi.org/10.3390/jcdd12010019
Chicago/Turabian StyleKrishnan, Srikanth, Pratyaksh K. Srivastava, Jayram Attaluri, Rebecca Nayeri, Dhananjay Chatterjee, Jay Patel, Ali Nsair, Matthew Budoff, and Arash Nayeri. 2025. "Physician Perceptions of the Safety and Efficacy of GLP-1 Receptor Agonists: Underestimation of Cardiovascular Risk Reduction and Discrepancies with Clinical Evidence" Journal of Cardiovascular Development and Disease 12, no. 1: 19. https://doi.org/10.3390/jcdd12010019
APA StyleKrishnan, S., Srivastava, P. K., Attaluri, J., Nayeri, R., Chatterjee, D., Patel, J., Nsair, A., Budoff, M., & Nayeri, A. (2025). Physician Perceptions of the Safety and Efficacy of GLP-1 Receptor Agonists: Underestimation of Cardiovascular Risk Reduction and Discrepancies with Clinical Evidence. Journal of Cardiovascular Development and Disease, 12(1), 19. https://doi.org/10.3390/jcdd12010019








