Management of Non-A Non-B Aortic Dissection: A Narrative Review
Abstract
:1. Introduction
2. Indication for Treatment
- Dissection-associated malperfusion
- Pleural effusion containing blood suggesting rupture
- Contained and/or free aortic rupture
- Persistent pain
- Uncontrollable arterial hypertension
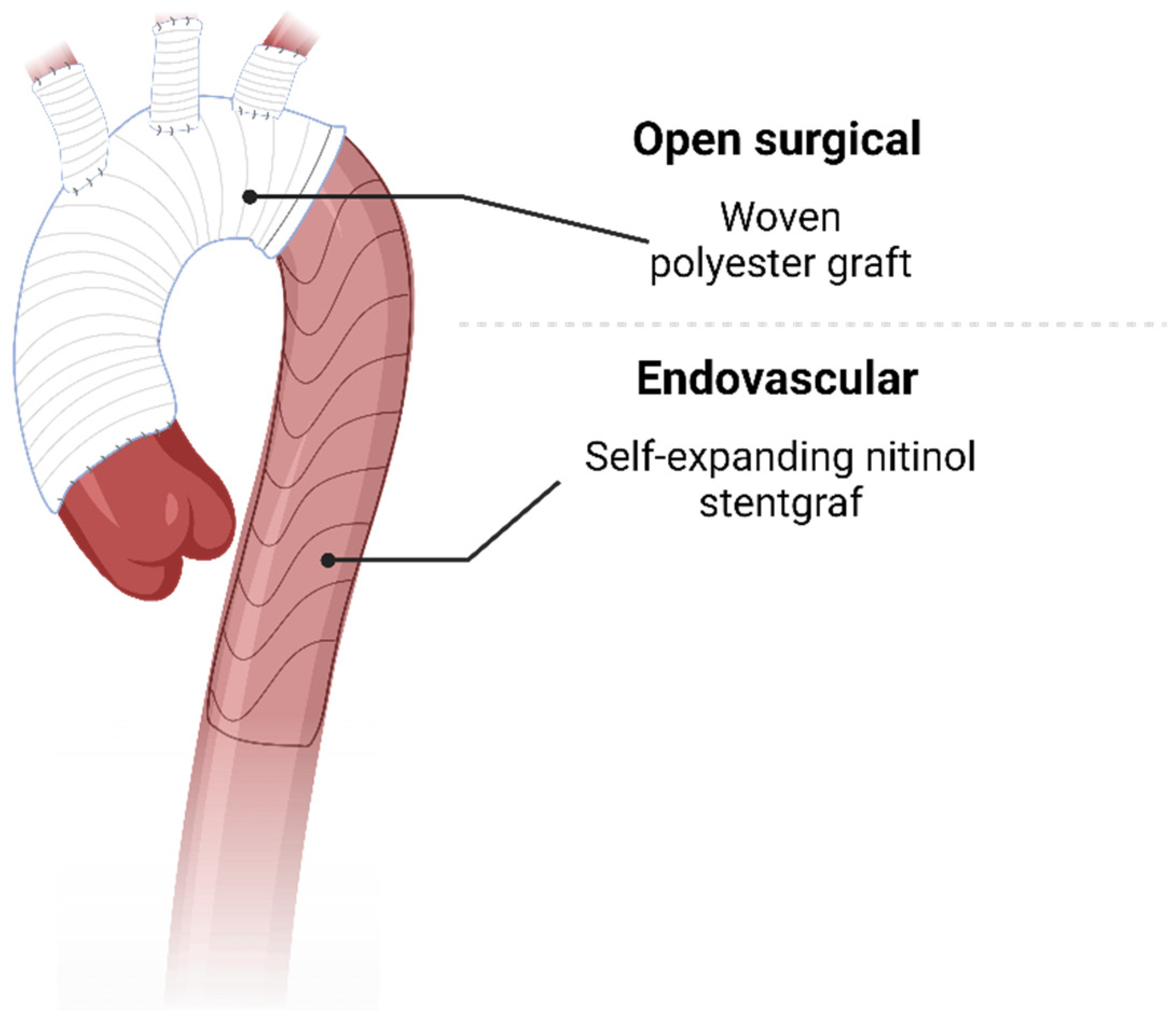
3. Open Treatment
3.1. Frozen Elephant Trunk (FET)
3.2. Conventional Total Arch Replacement
4. Endovascular Treatment
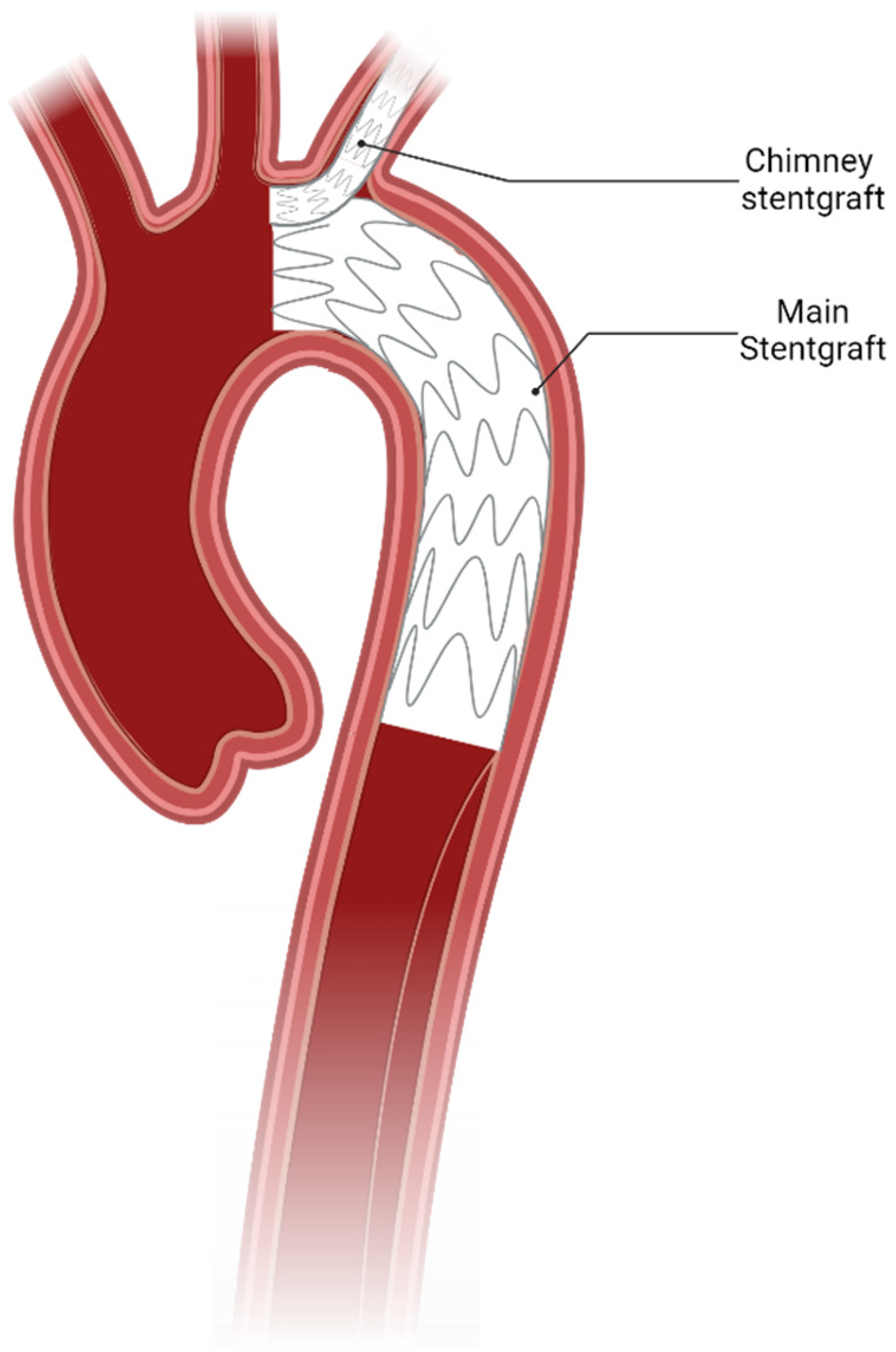
4.1. Carotid Subclavian Bypass/Extrathoracic Transposition
4.2. Total Endovascular Approach
4.3. Notable Mention: Other Hybrid Repair
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TEM | type of dissection, entry site, and malperfusion |
| EACTS/STS | European association of cardiothoracic surgery/Society of thoracic surgeons |
| ESC | European society of cardiology |
| CTA | Computed tomography angiography |
| FET | Frozen elephant trunk |
| SACP | selective antegrade cerebral perfusion |
| dSINE | distal stent graft induced new entry |
| TEVAR | thoracic endovascular aortic repair |
| PTFE | polytetrafluoroethylene |
References
- Pasic, M.; Knollman, F.; Hetzer, R. Isolated non-A, non-B dissection of the aortic arch. N. Engl. J. Med. 1999, 341, 1775. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Grabenwoger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardiothorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Riviere, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Rylski, B.; Czerny, M.; Baier, A.L.M.; Kreibich, M.; Siepe, M.; Beyersdorf, F. Aortic dissection reconsidered: Type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Rylski, B.; Perez, M.; Beyersdorf, F.; Reser, D.; Kari, F.A.; Siepe, M.; Czerny, M. Acute non-A non-B aortic dissection: Incidence, treatment and outcome. Eur. J. Cardiothorac. Surg. 2017, 52, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, S.; de Beaufort, H.W.L.; Tolenaar, J.L.; Bavaria, J.E.; Desai, N.D.; Di Eusanio, M.; Di Bartolomeo, R.; Peterson, M.D.; Ehrlich, M.; Evangelista, A.; et al. Acute aortic dissections with entry tear in the arch: A report from the International Registry of Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2019, 157, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, P.P.; Wagner, M. Acute non-A-non-B aortic dissection: Surgical or conservative approach? Eur. J. Cardiothorac. Surg. 2016, 49, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Maier, A.; Kletzer, J.; Schlett, C.L.; Kondov, S.; Czerny, M.; Rylski, B.; Kreibich, M. Radiographic complicated and uncomplicated descending aortic dissections: Aortic morphological differences by CT angiography and risk factor analysis. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Yamamoto, H.; Takagi, D.; Kadohama, T.; Yamaura, G.; Kiryu, K.; Igarashi, I. Aortic remodeling, reintervention, and survival after zone 0 arch repair with frozen elephant trunks for acute type A aortic dissection: Midterm results. JTCVS Tech. 2022, 14, 29–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, Y.; Minatoya, K.; Oda, T.; Itonaga, T.; Seike, Y.; Tanaka, H.; Sasaki, H.; Kobayashi, J. Surgical outcomes for acute type A aortic dissection with aggressive primary entry resection. Eur. J. Cardiothorac. Surg. 2016, 50, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Chikvatia, S.; Siepe, M.; Kondov, S.; Meissl, D.; Gottardi, R.; Rylski, B.; Czerny, M.; Kreibich, M. Concomitant aortic root replacement during frozen elephant trunk implantation does not increase perioperative risk. Eur. J. Cardiothorac. Surg. 2023, 63, ezad053. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Kreibich, M.; Rylski, B.; Schibilsky, D.; Pooth, J.S.; Fagu, A.; Zimmer, E.; Pingpoh, C.; Beyersdorf, F.; Czerny, M.; et al. Composition of the surgical team in aortic arch surgery-a risk factor analysis. Eur. J. Cardiothorac. Surg. 2022, 62, ezac171. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Kreibich, M.; Rylski, B.; Morlock, J.; Kondov, S.; Scheumann, J.; Kari, F.A.; Staier, K.; Maier, S.; Beyersdorf, F.; et al. Evaluation of myocardial injury, the need for vasopressors and inotropic support in beating-heart aortic arch surgery. J. Cardiovasc. Surg. 2020, 61, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Papantchev, V.; Stoinova, V.; Aleksandrov, A.; Todorova-Papantcheva, D.; Hristov, S.; Petkov, D.; Nachev, G.; Ovtscharoff, W. The role of Willis circle variations during unilateral selective cerebral perfusion: A study of 500 circles. Eur. J. Cardiothorac. Surg. 2013, 44, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Siepe, M.; Berger, T.; Kondov, S.; Morlock, J.; Pingpoh, C.; Beyersdorf, F.; Rylski, B.; Czerny, M. The Frozen Elephant Trunk Technique for the Treatment of Type B and Type Non-A Non-B Aortic Dissection. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, K.; Dell’Aquila, A.M.; Motekallemi, A.; Oberhuber, A.; Schafers, J.F.; Marchiori, E.; Weber, R.; Martens, S.; Rukosujew, A. The frozen elephant trunk technique in acute aortic dissection: The ultimate solution? An institutional experience. Front. Cardiovasc. Med. 2024, 11, 1330033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, T.; Kreibich, M.; Mueller, F.; Breurer-Kellner, L.; Rylski, B.; Kondov, S.; Schrofel, H.; Pingpoh, C.; Beyersdorf, F.; Siepe, M.; et al. Risk factors for stroke after total aortic arch replacement using the frozen elephant trunk technique. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 865–871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wisniewski, K.; Motekallemi, A.; Dell’Aquila, A.M.; Oberhuber, A.; Schaefers, J.F.; Ibrahim, A.; Martens, S.; Rukosujew, A. Single-Center Experience With the Thoraflex Hybrid Prosthesis: Indications, Implantation Technique and Results. Front. Cardiovasc. Med. 2022, 9, 924838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rylski, B.; Blanke, P.; Beyersdorf, F.; Desai, N.D.; Milewski, R.K.; Siepe, M.; Kari, F.A.; Czerny, M.; Carrel, T.; Schlensak, C.; et al. How does the ascending aorta geometry change when it dissects? J. Am. Coll. Cardiol. 2014, 63, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Berger, T.; Morlock, J.; Kondov, S.; Scheumann, J.; Kari, F.A.; Rylski, B.; Siepe, M.; Beyersdorf, F.; Czerny, M. The frozen elephant trunk technique for the treatment of acute complicated Type B aortic dissection. Eur. J. Cardiothorac. Surg. 2018, 53, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Eggebrecht, H.; Rousseau, H.; Mouroz, P.R.; Janosi, R.A.; Lescan, M.; Schlensak, C.; Bockler, D.; Ante, M.; Weijde, E.V.; et al. Distal Stent Graft-Induced New Entry After TEVAR or FET: Insights Into a New Disease From EuREC. Ann. Thorac. Surg. 2020, 110, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Qi, R.; Zhong, Y.; Chen, S.; Liu, H.; Guo, R.; Ge, Y.; Sun, L.; Zhu, J. Early and Long-Term Follow-Up for Chronic Type B and Type Non-A Non-B Aortic Dissection Using the Frozen Elephant Trunk Technique. Front. Cardiovasc. Med. 2021, 8, 714638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Yang, F.; Chen, L.; Xie, E.; Su, S.; Liu, Y.; Geng, Q.; Fan, R.; Li, J.; Luo, J. Management and Outcomes of Non-A Non-B Aortic Dissection. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Kreibich, M.; Morlock, J.; Kondov, S.; Scheumann, J.; Kari, F.A.; Rylski, B.; Siepe, M.; Beyersdorf, F.; Czerny, M. True-lumen and false-lumen diameter changes in the downstream aorta after frozen elephant trunk implantation. Eur. J. Cardiothorac. Surg. 2018, 54, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Berger, T.; Rylski, B.; Chen, Z.; Beyersdorf, F.; Siepe, M.; Czerny, M. Aortic reinterventions after the frozen elephant trunk procedure. J. Thorac. Cardiovasc. Surg. 2020, 159, 392–399.e1. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Bunte, D.; Berger, T.; Votsch, A.; Rylski, B.; Krombholz-Reindl, P.; Chen, Z.; Morlock, J.; Beyersdorf, F.; Winkler, A.; et al. Distal Stent Graft-Induced New Entries After the Frozen Elephant Trunk Procedure. Ann. Thorac. Surg. 2020, 110, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Graap, M.; Rylski, B.; Fagu, A.; Gottardi, R.; Walter, T.; Discher, P.; Hagar, M.T.; Kondov, S.; Czerny, M.; et al. Distal Aortic Failure Following the Frozen Elephant Trunk Procedure for Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 911548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, T.; Kreibich, M.; Rylski, B.; Kondov, S.; Fagu, A.; Beyersdorf, F.; Siepe, M.; Czerny, M. The 3-step approach for the treatment of multisegmental thoraco-abdominal aortic pathologies. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 269–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreibich, M.; Siepe, M.; Berger, T.; Kondov, S.; Morlock, J.; Pingpoh, C.; Beyersdorf, F.; Rylski, B.; Czerny, M. Downstream thoracic endovascular aortic repair following zone 2, 100-mm stent graft frozen elephant trunk implantation. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 1141–1146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, T.; Berger, T.; Kondov, S.; Gottardi, R.; Benk, J.; Rylski, B.; Czerny, M.; Kreibich, M. Postoperative In-Stent Thrombus Formation Following Frozen Elephant Trunk Total Arch Repair. Front. Cardiovasc. Med. 2022, 9, 921479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Sayed Ahmad, A.; Silaschi, M.; Borger, M.; Seidiramool, V.; Hamiko, M.; Leontyev, S.; Zierer, A.; Doss, M.; Etz, C.D.; Benedikt, P.; et al. The Frozen Elephant Technique Using a Novel Hybrid Prosthesis for Extensive Aortic Arch Disease: A Multicentre Study. Adv. Ther. 2023, 40, 1104–1113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Folkmann, S.; Arnold, Z.; Geisler, D.; Lenz, V.; Miosga, D.; Harrer, M.; Trnka, H.; Eller, R.; Aschacher, T.; Winkler, B.; et al. First-in-men experience with a novel frozen elephant trunk prosthesis featuring an endovascular side branch for left subclavian artery connection. Eur. J. Cardiothorac. Surg. 2024, 66, ezae302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Carino, D.; Singh, M.; Molardi, A.; Agostinelli, A.; Goldoni, M.; Pacini, D.; Nicolini, F. Non-A non-B aortic dissection: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2019, 55, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kosiorowska, M.; Berezowski, M.; Widenka, K.; Kreibich, M.; Beyersdorf, F.; Czerny, M.; Rylski, B. Non-A non-B acute aortic dissection with entry tear in the aortic arch. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 878–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nucera, M.; Kreibich, M.; Yildiz, M.; Berger, T.; Kolb, R.K.; Kondov, S.; Kunzmann, S.; Rylski, B.; Makaloski, V.; Siepe, M.; et al. Endovascular aortic repair in patients with Marfan and Loeys-Dietz syndrome is safe and durable when employed by a multi-disciplinary aortic team. Eur. J. Cardiothorac. Surg. 2024, 65, ezae069. [Google Scholar] [CrossRef] [PubMed]
- Le Huu, A.; Olive, J.K.; Cekmecelioglu, D.; Chatterjee, S.; Amarasekara, H.S.; Green, S.Y.; Coselli, J.S.; Preventza, O. Endovascular therapy for patients with heritable thoracic aortic disease. Ann. Cardiothorac. Surg. 2022, 11, 31–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morasch, M.D. Technique for subclavian to carotid transposition, tips, and tricks. J. Vasc. Surg. 2009, 49, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Voigt, S.L.; Bishawi, M.; Ranney, D.; Yerokun, B.; McCann, R.L.; Hughes, G.C. Outcomes of carotid-subclavian bypass performed in the setting of thoracic endovascular aortic repair. J. Vasc. Surg. 2019, 69, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Mylonas, S.; Majd, P.; Brunkwall, J.S. A current systematic evaluation and meta-analysis of chimney graft technology in aortic arch diseases. J. Vasc. Surg. 2017, 66, 1602–1610.E2. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, W.; Schlosser, F.J.; Moll, F.L.; Sumpio, B.E.; Muhs, B.E. Thoracic endovascular aortic repair with the chimney graft technique. J. Vasc. Surg. 2013, 58, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Burysz, M.; Milnerowicz, A.; Bartus, K.; Litwinowicz, R. Total endovascular repair of an aortic arch using a triple-branched graft in acute non-A non-B aortic dissection. Kardiol. Pol. 2023, 81, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, Q.; Wu, W.; Dai, X.; Fang, G.; Xie, X.; Chen, L. Triple-Branched Stent Graft Implantation for Acute Non-A-non-B Aortic Dissection. Ann. Thorac. Surg. 2023, 115, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Spath, P.; Stana, J.; Marazzi, G.; Peterss, S.; Fernandez-Prendes, C.; Tsilimparis, N. Emergent physician modified carotid fenestrated TEVAR for the treatment of a complicated acute type nonA-nonB aortic dissection with undetected multiorgan malperfusion. J. Cardiovasc. Surg. 2023, 64, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Weigang, E.; Sodeck, G.; Schmidli, J.; Antona, C.; Gelpi, G.; Friess, T.; Klocker, J.; Szeto, W.Y.; Moeller, P.; et al. Targeting landing zone 0 by total arch rerouting and TEVAR: Midterm results of a transcontinental registry. Ann. Thorac. Surg. 2012, 94, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wallen, T.; Carter, T.; Habertheuer, A.; Badhwar, V.; Jacobs, J.P.; Yerokun, B.; Wallace, A.; Milewski, K.; Szeto, W.Y.; Bavaria, J.E.; et al. National Outcomes of Elective Hybrid Arch Debranching with Endograft Exclusion versus Total Arch Replacement Procedures: Analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Aorta 2021, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Zhu, C.; Zheng, X.; Wang, T.; Xu, R.; Zhu, Z.; Li, D.; Piao, H.; Li, B.; Wang, Y.; et al. A New Aortic Arch Inclusion Technique With Frozen Elephant Trunk for Type A Aortic Dissection. Ann. Surg. 2020, 271, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, B.; Xi, S.; Li, Z.; Zhu, Z.; Jin, Z.; Yang, F.; Liu, L. Experience with aortic arch inclusion technique using artificial blood vessel for type A aortic dissection: An application study. J. Cardiothorac. Surg. 2024, 19, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Piao, H.; Wang, Y.; Li, B.; Zhu, Z.; Wang, T.; Liu, K. Early outcomes with a hybrid technique for repair of a non-A non-B aortic dissection. J. Thorac. Cardiovasc. Surg. 2022, 163, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kletzer, J.; Kondov, S.; Dimov, A.; Werdecker, V.; Czerny, M.; Kreibich, M.; Berger, T. Management of Non-A Non-B Aortic Dissection: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 1. https://doi.org/10.3390/jcdd12010001
Kletzer J, Kondov S, Dimov A, Werdecker V, Czerny M, Kreibich M, Berger T. Management of Non-A Non-B Aortic Dissection: A Narrative Review. Journal of Cardiovascular Development and Disease. 2025; 12(1):1. https://doi.org/10.3390/jcdd12010001
Chicago/Turabian StyleKletzer, Joseph, Stoyan Kondov, Aleksandar Dimov, Victoria Werdecker, Martin Czerny, Maximilian Kreibich, and Tim Berger. 2025. "Management of Non-A Non-B Aortic Dissection: A Narrative Review" Journal of Cardiovascular Development and Disease 12, no. 1: 1. https://doi.org/10.3390/jcdd12010001
APA StyleKletzer, J., Kondov, S., Dimov, A., Werdecker, V., Czerny, M., Kreibich, M., & Berger, T. (2025). Management of Non-A Non-B Aortic Dissection: A Narrative Review. Journal of Cardiovascular Development and Disease, 12(1), 1. https://doi.org/10.3390/jcdd12010001





