Serum Klotho Is Elevated in Patients with Acute Myocardial Infarction and Could Predict Poor In-Hospital Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Subjects
2.2. Definition and Collection of Clinical Data
2.3. Serum Sampling and Measurement of Serum Klotho
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Levels of Serum Klotho Analyzed in Different Groups
3.3. The Correlation between Serum Klotho and Cardiac–Renal Function
3.4. Discrimination Performance of Serum Klotho for Prognosis in ACS Patients
3.5. Risk Factors of Poor Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Franczak, A.; Krzywonos-Zawadzka, A.; Kałużna-Oleksy, M.; Bil-Lula, I. The Biological Role of Klotho Protein in the Development of Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, 5171945. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Imura, A.; Urakawa, I.; Shimada, T.; Murakami, J.; Aono, Y.; Hasegawa, H.; Yamashita, T.; Nakatani, K.; Saito, Y.; et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010, 398, 513–518. [Google Scholar] [CrossRef]
- Sze, L.; Neidert, M.C.; Bernays, R.L.; Zwimpfer, C.; Wiesli, P.; Haile, S.R.; Brändle, M.; Schmid, C. Gender dependence of serum soluble Klotho in acromegaly. Clin. Endocrinol. 2014, 80, 869–873. [Google Scholar] [CrossRef]
- Verde, Z.; González-Moro, J.M.; Chicharro, L.M.; Reinoso-Barbero, L.; Bandrés, F.; Gómez-Gallego, F.; Santiago, C. A Paradox: α-Klotho Levels and Smoking Intensity. Lung 2017, 195, 53–57. [Google Scholar] [CrossRef]
- Nakanishi, K.; Nishida, M.; Harada, M.; Ohama, T.; Kawada, N.; Murakami, M.; Moriyama, T.; Yamauchi-Takihara, K. Klotho-related Molecules Upregulated by Smoking Habit in Apparently Healthy Men: A Cross-sectional Study. Sci. Rep. 2015, 5, 14230. [Google Scholar] [CrossRef]
- Edmonston, D.; Grabner, A.; Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 11–24. [Google Scholar] [CrossRef]
- Hu, M.C.; Moe, O.W. Klotho as a potential biomarker and therapy for acute kidney injury. Nat. Rev. Nephrol. 2012, 8, 423–429. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C.; Moe, O.W. Klotho in Clinical Nephrology: Diagnostic and Therapeutic Implications. Clin. J. Am. Soc. Nephrol. CJASN 2020, 16, 162–176. [Google Scholar] [CrossRef]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Liu, Q.F.; Feng, J.H.; Li, S.S.; Gu, X.X.; Xiong, Y.; Qiang, S.; Ye, J.M. Association of Soluble Klotho Level with Adverse Outcomes in Patients on Maintenance Hemodialysis. Dis. Markers 2020, 2020, 4923970. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Beom, J.H.; Kim, J.H.; Woo, J.S.; Park, I.; Chung, S.P.; Chung, Y.E.; You, J.S. Recombinant Klotho Protein Ameliorates Myocardial Ischemia/Reperfusion Injury by Attenuating Sterile Inflammation. Biomedicines 2022, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Gillings, N.; Flores, B.; Takahashi, M.; Kuro, O.M.; Moe, O.W. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017, 91, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Glassford, N.J.; Schneider, A.G.; Xu, S.; Eastwood, G.M.; Young, H.; Peck, L.; Venge, P.; Bellomo, R. The nature and discriminatory value of urinary neutrophil gelatinase-associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med. 2013, 39, 1714–1724. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Udell, J.A.; Morrow, D.A.; Jarolim, P.; Kuder, J.F.; Solomon, S.D.; Pfeffer, M.A.; Braunwald, E.; Sabatine, M.S. Klotho, fibroblast growth factor-23, and the renin-angiotensin system—An analysis from the PEACE trial. Eur. J. Heart Fail. 2019, 21, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Chen, C.; Ge, J.; Li, M.; Zhang, Y.; Liu, H.; Song, B. Association between serum Klotho concentration and heart failure in adults, a cross-sectional study from NHANES 2007–2016. Int. J. Cardiol. 2023, 370, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Sanchez-Delgado, G.; García-Lario, J.V.; Castillo, M.J.; Ruiz, J.R. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging 2020, 12, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, S.; Sun, Q.W.; Zhang, B.; Ullah, M.; Sun, Z. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ. Res. 2021, 128, 492–507. [Google Scholar] [CrossRef]
- Taneike, M.; Nishida, M.; Nakanishi, K.; Sera, F.; Kioka, H.; Yamamoto, R.; Ohtani, T.; Hikoso, S.; Moriyama, T.; Sakata, Y.; et al. Alpha-Klotho is a novel predictor of treatment responsiveness in patients with heart failure. Sci. Rep. 2021, 11, 2058. [Google Scholar] [CrossRef]
- Poelzl, G.; Ghadge, S.K.; Messner, M.; Haubner, B.; Wuertinger, P.; Griesmacher, A.; Doerler, J.; Ensinger, C.; Ulmer, H.; Zaruba, M.M. Klotho is upregulated in human cardiomyopathy independently of circulating Klotho levels. Sci. Rep. 2018, 8, 8429. [Google Scholar] [CrossRef]
- Chen, P.; Tang, Y.; Luo, L.; Chen, H.; He, X. Lower serum Klotho level and higher systemic immune-inflammation index: An inverse correlation. BMC Geriatr. 2023, 23, 650. [Google Scholar] [CrossRef]
- Liu, F.; Wu, S.; Ren, H.; Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011, 13, 254–262. [Google Scholar] [CrossRef]
- Mao, Q.; Deng, M.; Zhao, J.; Zhou, D.; Chen, M.; Liu, Q.; Xu, S.; Zhao, X. Low serum Klotho reflects senile inflammation in middle-aged and elderly patients with coronary atherosclerosis. Cytokine 2023, 167, 156213. [Google Scholar] [CrossRef] [PubMed]
- Junho, C.V.; Gonzalez-Lafuente, L.; Neres-Santos, R.S.; Navarro-García, J.A.; Rodriguez-Sanchez, E.; Ruiz-Hurtado, G.; Carneiro-Ramos, M.S. Klotho relieves inflammation and exerts a cardioprotective effect during renal ischemia/reperfusion-induced cardiorenal syndrome. Biomed. Pharmacother. 2022, 153, 113515. [Google Scholar] [CrossRef]
- Ciliberti, G.; Guerra, F.; Pizzi, C.; Merlo, M.; Zilio, F.; Bianco, F.; Mancone, M.; Zaffalon, D.; Gioscia, R.; Bergamaschi, L.; et al. Characteristics of patients with recurrent acute myocardial infarction after MINOCA. Prog. Cardiovasc. Dis. 2023, 81, 42–47. [Google Scholar] [CrossRef]
| Variables | Total (n = 349) | In-Hospital Death Group (n = 12) | Discharge Group (n = 337) | p Value |
|---|---|---|---|---|
| Demography | ||||
| Age (years) | 64 (54, 75) | 80 (75, 86) | 64 (54, 74) | <0.001 |
| Male (%) | 265 (75.7) | 9 (75.0) | 256 (75.7) | 0.590 |
| Hypertension (%) | 211 (60.3) | 10 (83.3) | 201 (59.5) | 0.135 |
| Diabetes mellitus (%) | 105 (30.0) | 2 (16.7) | 103 (30.5) | 0.522 |
| Prior MI (%) | 50 (14.3) | 3 (25.0) | 47 (13.9) | 0.390 |
| CHF (%) | 4 (1.1) | 1 (8.3) | 3 (0.9) | 0.131 |
| CKD (%) | 34 (9.7) | 3 (25.0) | 31 (9.2) | 0.100 |
| Cerebral infarction (%) | 47 (13.4) | 4 (33.3) | 43 (12.7) | 0.063 |
| Previous PCI (%) | 46 (13.1) | 1 (8.3) | 45 (13.3) | 0.616 |
| Previous CABG (%) | 8 (2.3) | 1 (8.3) | 7 (2.1) | 0.246 |
| Chronic lung diseases (%) | 10 (2.9) | 1 (8.3) | 9 (2.7) | 0.298 |
| Tumor (%) | 19 (5.4) | 2 (16.7) | 17 (5.0) | 0.133 |
| Dislipidemia (%) | 75 (21.4) | 0 (0.0) | 75 (22.2) | 0.077 |
| Clinical presentation | ||||
| STEMI (%) | 209 (59.7) | 6 (50.0) | 203 (60.1) | 0.555 |
| Extensive anterior myocardial infarction (%) | 55 (15.7) | 5 (41.7) | 50 (14.8) | 0.026 |
| Killip classification ≥ III stage (%) | 27 (7.7) | 9 (75.0) | 18 (5.3) | <0.001 |
| Heart rate (bpm) | 76 (68, 89) | 110 (95, 124) | 76 (68, 87) | <0.001 |
| Systolic pressure (mmHg) | 124 ± 27 | 124 ± 44 | 124 ± 27 | 0.975 |
| Diastolic pressure (mmHg) | 72 ± 16 | 72 ± 27 | 71 ± 15 | 0.945 |
| Cardiogenic shock (%) | 15 (4.3) | 4 (33.3) | 11 (3.3) | 0.001 |
| Ventricular tachycardia (%) | 20 (5.7) | 0 (0.0) | 20 (5.9) | 0.385 |
| Ventricular fibrillation (%) | 17 (4.9) | 4 (33.3) | 13 (3.8) | 0.002 |
| Atrial fibrillation (%) | 27 (7.7) | 3 (25.0) | 24 (7.1) | 0.056 |
| Atrioventricular block (%) | 10 (2.9) | 0 (0.0) | 10 (3.0) | 0.545 |
| Smokers (%) | 214 (61.3) | 4 (33.3) | 210 (62.7) | 0.042 |
| Contrast volume (mL) | 200 (100, 200) | 0 (0, 100) | 200 (100, 205) | 0.003 |
| Laboratory tests | ||||
| Hemoglobin (g/L) | 133 (118, 144) | 116 (105, 133) | 133 (119, 144) | 0.058 |
| WBC (×109/L) | 8.9 (6.9, 11.5) | 12.3 (9.1, 16.1) | 8.8 (6.9, 11.4) | 0.027 |
| PLT (×109/L) | 197 (162, 243) | 195 (116, 252) | 197 (163, 243) | 0.484 |
| SCr in admission (μmol/L) | 76.0 (63.0, 90.0) | 103.5 (84.0, 172.0) | 75.0 (62.5, 88.0) | <0.001 |
| eGFR (mL/min × 1.73 m2) | 88.54 (68.62, 100.07) | 41.87 (26.38, 68.62) | 89.35 (70.24, 100.81) | <0.001 |
| BUN (mmol/l) | 5.72 (4.42, 7.38) | 10.74 (8.10, 15.35) | 5.67 (4.38, 7.06) | <0.001 |
| TNI (ng/mL) | 11.1 (2.47, 47.37) | 15.2 (1.5, 25.1) | 10.9 (2.5, 49.4) | 0.700 |
| CK-MB (U/L) | 42.4 (8.1, 188.7) | 25.1 (12.4, 80.0) | 43.3 (8.0, 190.6) | 0.358 |
| BNP (pg/mL) | 195 (82, 409) | 1129 (350, 2917) | 190 (81, 360) | 0.001 |
| FBG (mmol/L) | 6.50 (5.32, 8.54) | 10.2 (7.3, 12.3) | 6.4 (5.3, 8.4) | 0.012 |
| Albumin (g/L) | 38.0 (35.0, 40.9) | 32.9 (28.4, 36.5) | 38.1 (35.0, 41.0) | 0.002 |
| CHO (mmol/L) | 4.30 ± 1.14 | 3.65 ± 0.63 | 4.32 ± 1.14 | 0.053 |
| TG (mmol/L) | 1.48 (1.08, 2.17) | 1.17 (0.76, 1.45) | 1.49 (1.08, 2.20) | 0.051 |
| LDL (mmol/L) | 2.69 ± 0.94 | 2.03 ± 0.60 | 2.71 ± 0.94 | 0.017 |
| HDL (mmol/L) | 0.97 (0.82, 1.15) | 0.95 (0.68, 1.08) | 0.97 (0.83, 1.15) | 0.198 |
| LVEF (%) | 62 (56, 68) | 53 (40, 65) | 63 (56, 68) | 0.030 |
| Therapies | ||||
| Use of furosemide (mg/d) | 0 (0, 20) | 140 (40, 285) | 0 (0, 20) | <0.001 |
| Intravenous isosorbide dinitrate (%) | 229 (65.4) | 9 (75.0) | 220 (65.1) | 0.555 |
| Non-use of ACEI/ARB (%) | 159 (45.4) | 11 (91.7) | 148 (43.8) | 0.001 |
| Non-use of β-blockers (%) | 79 (22.6) | 4 (33.3) | 75 (22.2) | 0.479 |
| Non-use of statins (%) | 21 (6.0) | 4 (33.3) | 17 (5.0) | 0.003 |
| Vasoactive medication (%) | 56 (16.0) | 6 (50.0) | 50 (14.8) | 0.006 |
| IABP (%) | 16 (4.6) | 1 (8.3) | 15 (4.4) | 0.435 |
| In-hospital PCI (%) | 220 (62.9) | 0 (0.0) | 220 (65.0) | 0.000 |
| In-hospital CABG (%) | 24 (6.9) | 1 (8.3) | 23 (6.8) | 0.580 |
| In-hospital temporary pacemaker (%) | 1 (0.3) | 0 (0.0) | 1 (0.3) | 0.966 |
| In-hospital mechanical ventilation (%) | 21 (6.0) | 3 (25.0) | 18 (5.3) | 0.029 |
| Thrombolysis therapy (%) | 5 (1.4) | 0 (0.0) | 5 (1.5) | 0.671 |
| Biomarker | ||||
| Klotho (pg/mL) | 477.8 (89.6, 589.8) | 721.7 (597.0, 1007.1) | 470.7 (85.8, 582.4) | <0.001 |
| Klotho (pg/mL) | Smokers | Non-smokers | p value |
| 458.8 (75.0, 561.1) | 509.8 (182.3, 640.0) | 0.011 | |
| Male | Female | 0.009 | |
| 458.8 (78.1, 575.4) | 518.1 (277.2, 644.9) | ||
| AMI | UA | 0.035 | |
| 479.8 (93.4, 590.9) | 233.8 (61.6, 477.8) | ||
| Discharge group | In-hospital death group | <0.001 | |
| 468.3 (85.8, 582.4) | 721.7 (567.0, 1007.1) |
| Klotho | ||
|---|---|---|
| r | p Value | |
| Heart rate | 0.111 | 0.039 |
| Killip classification | 0.191 | 0.000 |
| TNI | 0.053 | 0.323 |
| BNP | 0.237 | 0.000 |
| LVEF | 0.001 | 0.979 |
| BUN | 0.015 | 0.785 |
| SCr | 0.069 | 0.197 |
| eGFR | −0.102 | 0.056 |
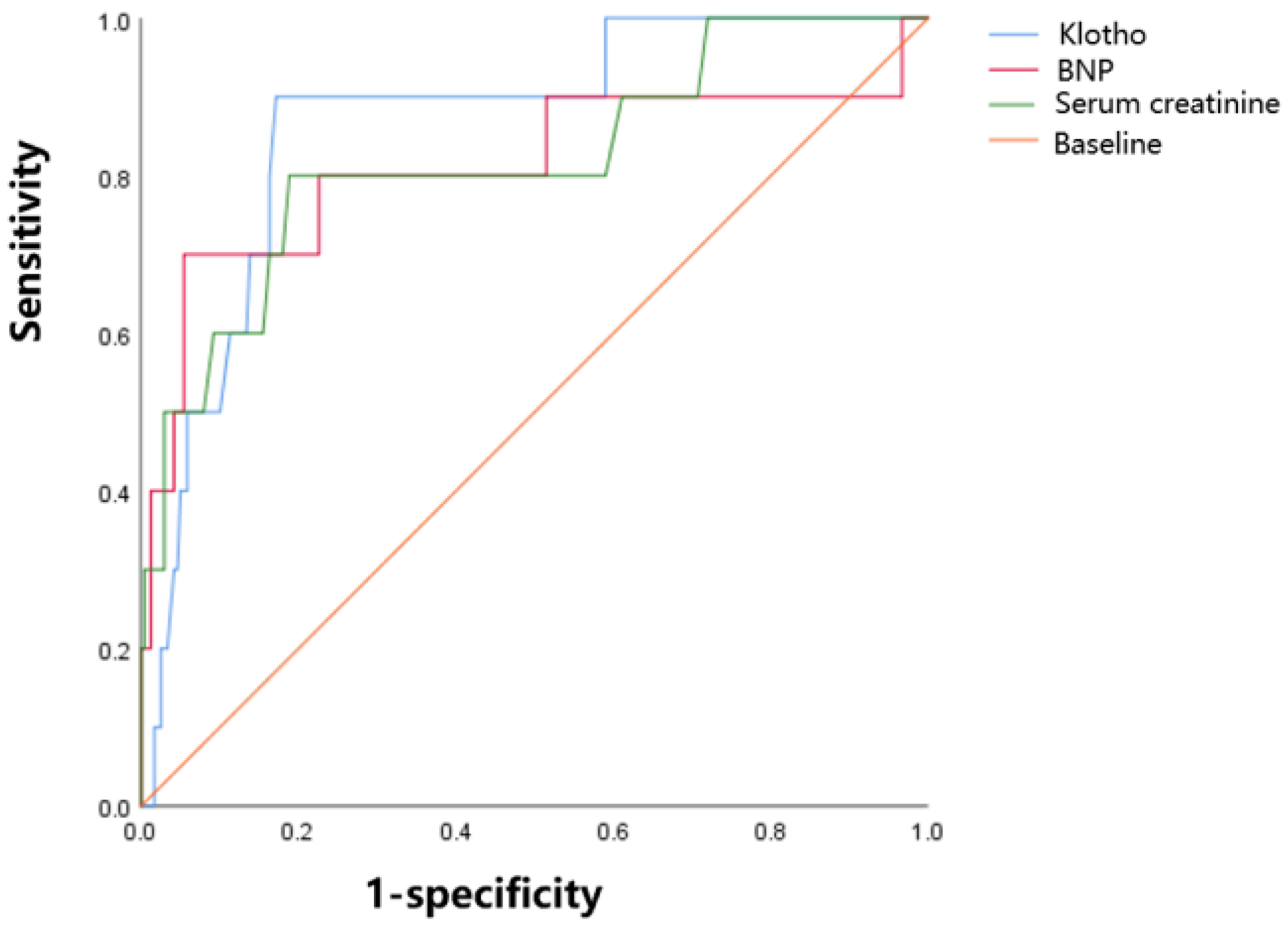
| AUC | 95% CI | p Value | Cut-Off Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| SCr (μmol/L) | 0.819 | 0.664–0.975 | 0.001 | 94.5 | 0.800 | 0.812 |
| BNP (pg/mL) | 0.812 | 0.624–0.999 | 0.001 | 967 | 0.700 | 0.946 |
| Klotho (pg/mL) | 0.865 | 0.761–0.969 | 0.000 | 645.0 | 0.900 | 0.828 |
| Correlates | OR | 95% CI | p Value |
|---|---|---|---|
| Age (≥78 years old) | 8.169 | 1.199–55.672 | 0.032 |
| Heart rate (≥90 bpm) | 12.107 | 1.617–90.658 | 0.015 |
| Killip classification (≥ 3 grade) | 16.590 | 3.037–90.608 | 0.000 |
| Serum creatinine (≥93.5 μmol/L) | 2.707 | 0.426–17.213 | 0.291 |
| Klotho (>645.0 pg/mL) | 6.017 | 1.108–35.555 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Huang, W.; Cao, L.; Yang, F.; Chi, C.; Zhu, J. Serum Klotho Is Elevated in Patients with Acute Myocardial Infarction and Could Predict Poor In-Hospital Prognosis. J. Cardiovasc. Dev. Dis. 2024, 11, 292. https://doi.org/10.3390/jcdd11090292
Pei Y, Huang W, Cao L, Yang F, Chi C, Zhu J. Serum Klotho Is Elevated in Patients with Acute Myocardial Infarction and Could Predict Poor In-Hospital Prognosis. Journal of Cardiovascular Development and Disease. 2024; 11(9):292. https://doi.org/10.3390/jcdd11090292
Chicago/Turabian StylePei, Yuanyuan, Wenfeng Huang, Lingjie Cao, Fengtao Yang, Cheng Chi, and Jihong Zhu. 2024. "Serum Klotho Is Elevated in Patients with Acute Myocardial Infarction and Could Predict Poor In-Hospital Prognosis" Journal of Cardiovascular Development and Disease 11, no. 9: 292. https://doi.org/10.3390/jcdd11090292
APA StylePei, Y., Huang, W., Cao, L., Yang, F., Chi, C., & Zhu, J. (2024). Serum Klotho Is Elevated in Patients with Acute Myocardial Infarction and Could Predict Poor In-Hospital Prognosis. Journal of Cardiovascular Development and Disease, 11(9), 292. https://doi.org/10.3390/jcdd11090292




