Advanced Heart Failure: Therapeutic Options and Challenges in the Evolving Field of Left Ventricular Assist Devices
Abstract
1. Advanced Heart Failure (ADHF)
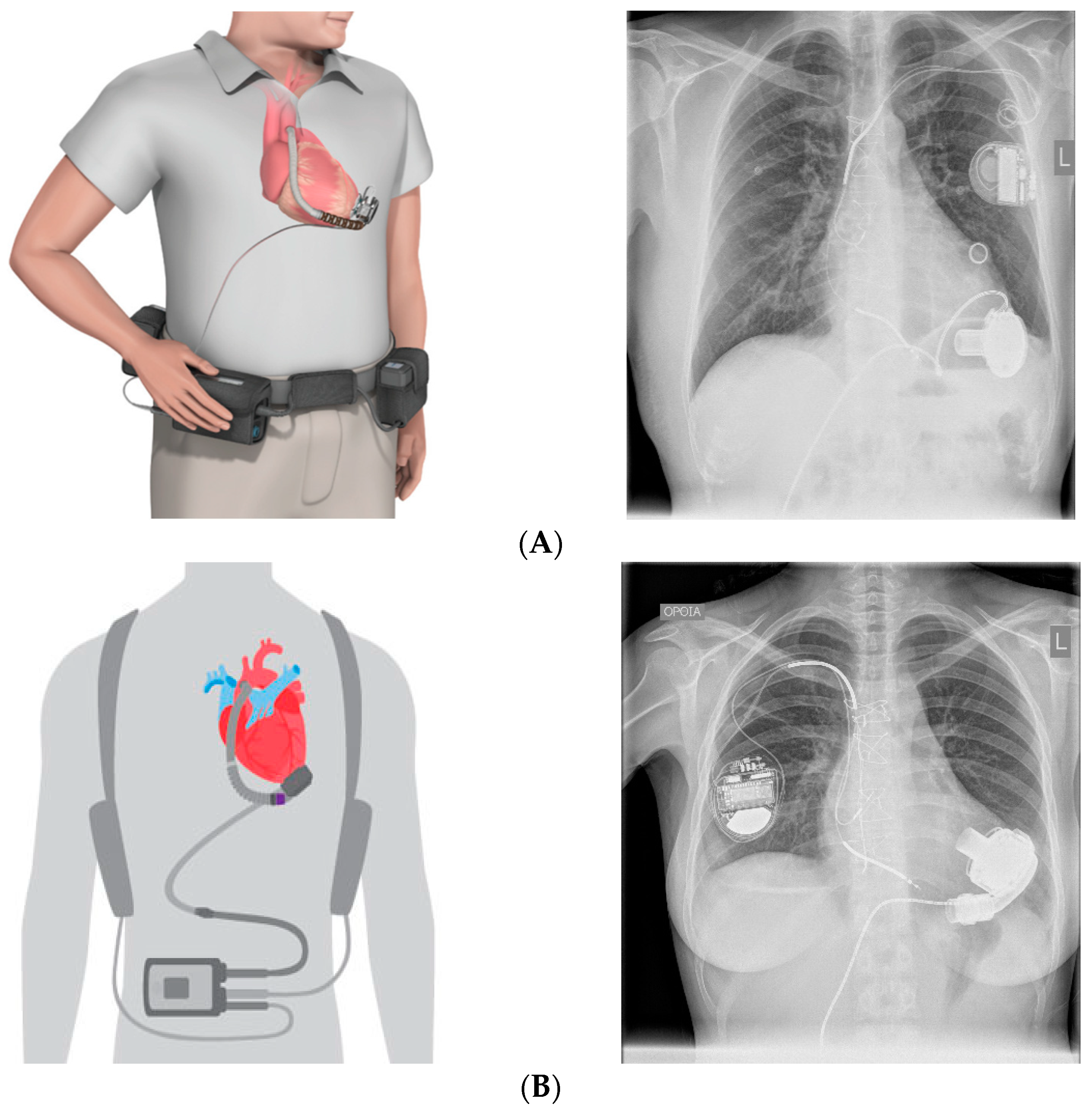
2. Anatomy of the Current LVAD Technology
3. Indications for Durable MCS
4. Patient Selection
5. Referral of Patients to Advanced Heart Failure Centers
6. Considerations for Candidate Selection and Pre- and Post-LVAD Patient Management
6.1. Right Ventricular Failure (RVF)
6.2. Post-Operative Temporary Mechanical RV Support
6.3. Aortic Insufficiency (AI)
6.4. Renal Dysfunction
6.5. Bleeding Risk
6.6. Infection
6.7. Psychosocial Evaluation
6.8. Exercise Training (ET)
6.9. Myocardial Recovery
| Group, Year (Ref. #) | n | HF Etiology | Adjuvant Drug Therapy Protocol | Heart Function Therapy Protocol | LVAD Support Duration (Months) | Cardiac Recovery | Freedom from HF Recurrence After Explantation, Follow-Up Duration |
|---|---|---|---|---|---|---|---|
| US multicentre study, 2020 [108] | 40 | NICM 100% | Yes | Yes | Up to 18 | 50% | 90% and 77%, 1 and 3 yrs respectively |
| US LVAD Working Group, 2007 [120] | 67 | NICM: 55%, ICM: 45% | Not standardized | Yes | 4.5 | NICM: 13.5% ICM: 3.3% | 100%, 6 months |
| Berlin, 2008 and 2010 [121,122] | 188 | NICM: 100% | Not standardized | Yes | 4 | NICM: 19% | 74% and 66%, 3 and 5 yrs, respectively |
| Utah Cardiac Recovery Program, 2016 [123] | 154 | NICM: 60%, ICM: 40% | Not standardized | Yesu | 6 | NICM: 21% ICM: 5% | N/A |
| Montefiore, 2013 [124] | 21 | NICM: 62%, ICM: 38% | Yes | Yes | 9 | NICM: 23% ICM: 0% | 100%, 57 months |
| Gothenburg, 2006 [125] | 18 | NICM: 83%, ICM: 17% | Not standardized | Yes | 7 | NICM: 17% ICM: 0% | 33%, 8 yrs |
| Vancouver, 2011 [126] | 17 | Not reported | Not standardized | Yes | 7 | NICM and ICM: 23% | 100%, 2 yrs |
| Pittsburgh, 2003 [127] | 18 | NICM: 72%, ICM: 28% | Not standardized | Yes | 8 | NICM: 38% ICM: 20% | 67%, 16.5 months |
| Texas Heart Institute, 2003 [128] | 16 | NICM: 75%, ICM: 25% | Yes | Yes | 8 | NICM: 58% ICM: 50% | 78%, 14.3 months |
| US IMAC, 2012 [129] | 14 | NICM: 100% | Not standardized | Yes | 3.5 | NICM: 67% | 87.5%, 17.5 months |
| Harefield, 2006 [103] | 15 | NICM: 100% | Yes | Yes | 11 | NICM: 73% | 100% and 89%, 1 and 4 yrs, respectively |
| Harefield, 2011 [113] | 20 | NICM: 100% | Yes | Yes | 9 | NICM: 60% | 83%, 3 yrs |
| University of Athens, 2007 [130] | 8 | NICM: 100% | Yes | Yes | 7 | NICM: 50% | 100%, 2yrs |
6.10. Complications after LVAD Implantation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lesyuk, W.; Kriza, C.; Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: A systematic review 2004–2016. BMC Cardiovasc. Disord. 2018, 18, 74. [Google Scholar] [CrossRef]
- McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005, 294, 1625–1633. [Google Scholar] [PubMed]
- Costanzo, M.R.; Mills, R.M.; Wynne, J. Characteristics of “Stage D” heart failure: Insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am. Heart J. 2008, 155, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D. Advanced heart failure: Where do we stand? Hell. J. Cardiol. 2018, 59, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, G.; Metra, M.; Lund, L.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Yim, C.; Barrón, Y.; Moore, S.; Murtaugh, C.; Lala, A.; Aldridge, M.; Goldstein, N.; Gelfman, L.P. Hospice Enrollment in Patients with Advanced Heart Failure Decreases Acute Medical Service Utilization. Circ. Heart Fail. 2017, 10, e003–e335. [Google Scholar]
- Rogers, J.; Patel, C.; Mentz, R.; Granger, B.B.; Steinhauser, K.E.; Fiuzat, M.; Adams, P.A.; Speck, A.; Johnson, K.S.; Krishnamoorthy, A.; et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J. Am. Coll. Cardiol. 2017, 70, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Dipchand, A.I.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Yusen, R.D.; Stehlik, J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J. Heart Lung Transplant. 2016, 35, 1158–1169. [Google Scholar] [CrossRef]
- Khush, K.; Zaroff, J.; Nguyen, J.; Menza, R.; Goldstein, B.A. National Decline in Donor Heart Utilization with Regional Variability: 1995–2010. Am. J. Transplant. 2015, 15, 642–649. [Google Scholar] [CrossRef]
- McLarty, A. Mechanical Circulatory Support and the Role of LVADs in Heart Failure Therapy. Clin. Med. Insights Cardiol. 2015, 9, 1–5. [Google Scholar] [CrossRef]
- Kirklin, J.; Pagani, F.; Kormos, R.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Mehra, M.; Daniel, J.; Goldstein, D.; Cleveland, J.; Cowger, J.A.; Hall, S.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Chuang, J.; et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENT UM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial left ventricular assist device for advanced heart failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef]
- Kormos, R.; Cowger, J.; Pagani, F.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs data base annual report: Evolving indications, outcomes, and scientific partnerships. Ann. Thorac. Surg. 2019, 107, 341–353. [Google Scholar] [CrossRef]
- Mancini, D.; Colombo, P. Left Ventricular Assist Devices. A Rapidly Evolving Alternative to Transplant. J. Am. Coll. Cardiol. 2015, 65, 2542–2555. [Google Scholar]
- Rodriguez, L.; Suarez, E.; Loebe, M.; Bruckner, B.A. Ventricular Assist Devices (VAD) Therapy: New Technology, New Hope? Methodist DeBakey Cardiovasc. J. 2013, 9, 32–37. [Google Scholar] [PubMed]
- Kervan, U.; Kocabeyoglu, S.S.; Emre Sert, D.; Karahan, M.; Temizhan, A.; Demirkan, B.; Akin, Y.; Beyazal, O.F.; Akdi, M.; Catav, Z. Midterm Results of Minimally Invasive Left Thoracotomy Fully Magnetically Levitated Left Ventricular Assist Device Implantation. ASAIO J. 2021, 67, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.; Cowger, J.; Lee, S.; Horstmanshof, D.; Cleveland, J.C., Jr.; Goldstein, D.J.; Mehra, M.R.; Uriel, N.; Salerno, C.T.; Bourque, K.; et al. Outcomes in Smaller Body Size Adults After Heart Mate 3 Left Ventricular Assist Device Implantation. Ann. Thorac. Surg. 2022, 114, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Subramanian, M.; Vader, J. Heart Mate 3 Implantation Through Left Atrial e-PTFE Conduit for Restrictive Cardiomyopathy. Ann. Thorac. Surg. Short Rep. 2023, 1, 191–193. [Google Scholar] [CrossRef]
- McGiffin, D.; Kure, C.; McLean, J.; Marasco, S.; Bergin, P.; Hare, J.L.; Leet, A.; Patel, H.; Zimmet, A.; Rix, J.; et al. The results of a single-center experience with Heart Mate 3 in a biventricular configuration. Heart Lung Transplant. 2020, 40, S193–S200. [Google Scholar] [CrossRef]
- Miller, L.W.; Pagani, F.D.; Russell, S.D.; John, R.; Boyle, A.J.; Aaronson, K.D.; Conte, J.V.; Naka, Y.; Mancini, D.; Delgado, R.M.; et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 2007, 357, 885–896. [Google Scholar] [CrossRef]
- Topkara, V.; Kondareddy, S.; Malik, F.; Wang, I.W.; Mann, D.L.; Ewald, G.A.; Moazami, N. Infectious Complications in Patients with Left Ventricular Assist Device: Etiology and Outcomes in the Continuous-Flow Era. Ann. Thorac. Surg. 2010, 90, 1270–1277. [Google Scholar] [CrossRef]
- Mehra, M.R.; Salerno, C.; Cleveland, J.C.; Pinney, S.; Yuzefpolskaya, M.; Milano, C.A.; Itoh, A.; Goldstein, D.J.; Uriel, N.; Gulati, S.; et al. Health care Resource Use and Cost Implications in the MOMENTUM 3 Long-Term Outcome Study. Circulation 2018, 138, 1923–1934. [Google Scholar] [CrossRef]
- Cheng, A.; Williamitis, C.; Slaughter, M. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: Is there an advantage to pulsatility? Ann. Cardiothorac. Surg. 2014, 3, 573–581. [Google Scholar] [PubMed]
- Medtronic Press Release 2021. Available online: https://www.medtronic.com/content/dam/medtroniccom/global/HCP/Documents/hvad-urgent-medical-device-noticejune-2021.pdf (accessed on 10 June 2021).
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef]
- Pya, Y.; Maly, J.; Bekbossynova, M.; Salov, R.; Schueler, S.; Meyns, B.; Kassif, Y.; Massetti, M.; Zilbershlag, M.; Netuka, I. First human use of a wireless coplanar energy transfer coupled with a continuous-flow left ventricular assist device. J. Heart Lung Transplant. 2019, 38, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Rogers, J.G. Left ventricular assist device therapy in advanced heart failure: Patient selection and outcomes. Eur. J. Heart Fail. 2017, 19, 595–602. [Google Scholar] [CrossRef]
- Mehra, M.; Canter, C.; Hannan, M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A10-year update. J. Heart Lung Transplant. 2017, 35, S1–S23. [Google Scholar] [CrossRef]
- Saeed, D.; Feldman, D.; Banayosy, A.E.; Birks, E.; Blume, E.; Cowger, J.; Hayward, C.; Jorde, U.; Kremer, J.; MacGowan, G.; et al. The 2023 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: A 10-Year Update. J. Heart Lung Transplant. 2023, 42, e1–e222. [Google Scholar] [CrossRef] [PubMed]
- Frazier, O.H.; Rose, E.A.; Oz, M.C.; Dembitsky, W.; McCarthy, P.; Radovancevic, B.; Poirier, V.L.; Dasse, K.A. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting Heart transplantation. J. Thorac. Cardiovasc. Surg. 2001, 122, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Mehra, M.R.; Pfeffer, M.A. Oxford Textbook of Advanced Heart Failure and Cardiac Transplantation. Oxford Textbooks in Cardiology; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Teuteberg, J.J.; Stewart, G.C.; Jessup, M.; Kormos, R.L.; Sun, B.; Frazier, O.H.; Naftel, D.C.; Stevenson, L.W. Implant strategies changeover time and impact outcomes: Insights from the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). JACC Heart Fail. 2013, 1, 369–378. [Google Scholar] [CrossRef]
- Mody, K.; Duong, J.; Dionizovik-Dimanovski, M. Five-Fold Increase in Antibody-Mediated Rejection (AMR) Post Heart Transplant in Patients Developing Allosensitization During Left Ventricular Assist Device Support (LVAD). J. Heart Lung Transplant. 2014, 32, S100. [Google Scholar] [CrossRef]
- Alba, A.; McDonald, M.; Rao, V.; Ross, H.J.; Delgado, D.H. The effect of ventricular assist devices on the long-term post-transplant outcomes: A systematic review of observational studies. Eur. J. Heart Fail. 2011, 13, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Civitello, A.; Frazier, O. Mechanical Circulatory Support for Advanced Heart Failure. A Texas Heart Institute/Baylor College of Medicine Approach; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.; Rogers, J.; Milano, C.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M., 3rd; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef]
- Alba, A.C.; Rao, V.; Ross, H.J.; Ross, H.J.; Delgado, D.H. Usefulness of the NTERMACS scale to predict outcomes after mechanical assist device implantation. J. Heart Lung Transplant. 2009, 28, 827–833. [Google Scholar] [CrossRef]
- Boyle, A.J.; Ascheim, D.D.; Russo, M.J.; Kormos, R.L.; John, R.; Naka, Y.; Gelijns, A.C.; Hong, K.N.; Teuteberg, J.J. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J. Heart Lung Transplant. 2011, 30, 402–407. [Google Scholar] [CrossRef]
- Jorde, U.P.; Kushwaha, S.S.; Tatooles, A.J.; Naka, Y.; Bhat, G.; Long, J.W.; Horstmanshof, D.A.; Kormos, R.L.; Teuteberg, J.J.; Slaughter, M.S.; et al. Results of the destination therapy post-Food and Drug Administration approval study with a continuous flow left ventricular assist device: A prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support). J. Am. Coll. Cardiol. 2014, 63, 1751–1757. [Google Scholar] [CrossRef]
- Estep, J.D.; Starling, R.C.; Horstmanshof, D.A.; Milano, C.A.; Selzman, C.H.; Shah, K.B.; Loebe, M.; Moazami, N.; Long, J.W.; Stehlik, J.; et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory Heart failure patients: Results from the ROADMAP study. J. Am. Coll. Cardiol. 2015, 66, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Starling, R.C.; Rogers, J.G.; Horstmanshof, D.A.; Long, J.W.; Kasirajan, V.; Stehlik, J.; Chuang, J.; Farrar, D.J.; Estep, J.D. Left ventricular assist devices versus medical management in ambulatory heart failure patients: Analysis of INTERMACS profiles 4 and 5 to 7 from the ROADMAP study. J. Heart Lung Transplant. 2018, 37, 706–714. [Google Scholar] [CrossRef]
- Ambardekar, A.; Kittleson, M.; Palardy, M.; Mountis, M.M.; Forde-McLean, R.C.; DeVore, A.D.; Pamboukian, S.V.; Thibodeau, J.T.; Teuteberg, J.J.; Cadaret, L.; et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. Heart Lung Transplant. 2019, 38, 418–419. [Google Scholar] [CrossRef]
- Allen, L.A.; McIlvennan, C.K.; Thompson, J.S.; Dunlay, S.M.; LaRue, S.J.; Lewis, E.F.; Patel, C.B.; Blue, L.; Fairclough, D.L.; Leister, E.C.; et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: The DECIDE-LVAD randomized clinical trial. JAMA Intern. Med. 2018, 178, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Januzzi, J.L., Jr.; Allen, L.A.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Jessup, M.; Lindenfeld, J.; Maddox, T.M.; et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2018, 71, 201–230. [Google Scholar]
- Lampert, B.; Teuteberg, J. Right ventricular failure after left ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Akintoye, E.; Mohsen, A.; Inampudi, C.; Briasouli, A.; Asleh, R.; Alvarez, P. Trends in utilization, mortality, major complications and cost after total artificial heart implantation in the United States(2009–2015). Hell. J. Cardiol. 2020, 61, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.C.; Topkara, V.K.; Mercando, M.; Kay, J.; Kruger, K.H.; Aboodi, M.S.; Oz, M.C.; Naka, Y. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J. Heart Lung Transplant. 2006, 25, 1–6. [Google Scholar] [CrossRef]
- Pettinari, M.; Jacobs, S.; Rega, F.; Verbelen, T.; Droogne, W.; Meyns, B. Are right ventricular risk scores useful? Eur. J. CardioThoracic Surg. 2012, 42, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.G.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The right ventricular failure risk score. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Bellavia, D.; Iacovoni, A.; Scardulla, C.; Moja, L.; Pilato, M.; Kushwaha, S.S.; Senni, M.; Clemenza, F.; Agnese, V.; Falletta, C.; et al. Prediction of right ventricular failure after ventricular assist device implant: Systematic review and meta-analysis of observational studies. Eur. J. Heart Fail. 2017, 19, 926–946. [Google Scholar] [CrossRef]
- Fitzpatrick, J.R., 3rd; Frederick, J.R.; Hsu, V.M.; Kozin, E.D.; O’Hara, M.L.; Howell, E.; Dougherty, D.; McCormick, R.C.; Laporte, C.A.; Cohen, J.E.; et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J. Heart Lung Transplant. 2008, 27, 1286–1292. [Google Scholar] [PubMed]
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Renlund, D.G.; et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am. J. Cardiol. 2010, 105, 1030. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.D.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with Heart Mate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, Y.; McCarthy, P.M.; Smedira, N.G.; Banbury, M.K.; Navia, J.L.; Feng, J.; Hsu, A.P.; Yeager, M.L.; Buda, T.; Hoercher, K.J.; et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation 2002, 106, 192–202. [Google Scholar] [CrossRef]
- Puwanant, S.; Hamilton, K.K.; Klodell, C.T.; Hill, J.A.; Schofield, R.S.; Cleeton, T.S.; Pauly, D.F.; Aranda, J.M., Jr. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J. Heart Lung Transplant. 2008, 27, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Vivo, R.P.; Cordero-Reyes, A.M.; Qamar, U.; Garikipati, S.; Trevino, A.R.; Aldeiri, M.; Loebe, M.; Bruckner, B.A.; Torre-Amione, G.; Bhimaraj, A.; et al. Increased right-to-left ventricle diameter ratio is a strong predictor of right ventricular failure after left ventricular assist device. J. Heart Lung Transplant. 2013, 32, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Seetha Rammohan, H.R.; Gertz, Z.M.; Rame, J.E.; Woo, Y.J.; Kirkpatrick, J.N. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J. Card. Fail. 2013, 19, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Righini, F.M.; Focardi, M.; Lunghetti, S.; Bernazzali, S.; Marchetti, L.; Biagioli, B.; Galderisi, M.; Maccherini, M.; et al. Speckle tracking echocardiography as a new technique to evaluate right ventricular function in patients with left ventricular assist device therapy. J. Heart Lung Transplant. 2013, 32, 424–430. [Google Scholar] [CrossRef]
- Cameli, M.; Sparla, S.; Focardi, M.; Righini, F.M.; Solari, M.; Alvino, F.; Lisi, M.; D’Ascenzi, F.; Bernazzali, S.; Tsioulpas, C.; et al. Evaluation of Right Ventricular Function in the Management of Patients Referred for Left Ventricular Assist Device Therapy. Transplant. Proc. 2015, 47, 2166–2168. [Google Scholar] [CrossRef]
- Ntalianis, A.; Kapelios, C.J.; Kanakakis, J.; Repasos, E.; Pantsios, C.; Nana, E.; Kontogiannis, C.; Malliaras, K.; Tsamatsoulis, M.; Kaldara, E.; et al. Prolonged intra-aortic balloon pump support in biventricular heart failure induces right ventricular reverse remodeling. Int. J. Cardiol. 2015, 192, 38. [Google Scholar] [CrossRef]
- Tanaka, A.; Tuladhar, S.M.; Onsager, D.; Asfaw, Z.; Ota, T.; Juricek, C.; Lahart, M.; Lonchyna, V.A.; Kim, G.; Fedson, S.; et al. The subclavian intraaortic balloon pump: A compelling bridge device for advanced heart failure. Ann. Thorac. Surg. 2015, 100, 2151–2152. [Google Scholar] [CrossRef]
- Bonios, M.J.; Armenis, I.; Kogerakis, N.; Thodou, A.; Fragoulis, S.; Georgiadou, P.; Leontiadis, E.; Chamogeorgakis, T.; Drakos, S.G.; Adamopoulos, S. Prospective Phenotyping of Right Ventricle Function Following Intra-Aortic Balloon Pump Counterpulsation in Left Ventricular Assist Device Candidates: Outcomes and Predictors of Response. ASAIO J. 2023, 69, 215–222. [Google Scholar] [CrossRef]
- Chamogeorgakis, T.; Toumpoulis, I.; Bonios, M.J.; Lanfear, D.; Williams, C.; Koliopoulou, A.; Cowger, J. Treatment Strategies and Outcomes of Right Ventricular Failure Post Left Ventricular Assist Device Implantation: An INTERMACS Analysis. ASAIO J. 2023. ahead of print. [Google Scholar] [CrossRef]
- Nair, N. Use of machine learning techniques to identify risk factors for RV failure in LVAD patients. Front. Cardiovasc. Med. 2022, 9, 848789. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Morris, D.L.; Tang, D.; Batsides, G.; Kirtane, A.; Hanson, I.; Meraj, P.; Kapur, N.K.; O’Neill, W. Outcomes of patients with right ventricular failure requiring short-term hemodynamic support with the Impella RP device. J. Heart Lung Transplant. 2018, 37, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, A.; Baran, D.; Stelling, K.; Cowger, J.A.; Salerno, C.T. Outcomes with the Tandem Protek Duo Dual-Lumen Percutaneous Right Ventricular Assist Device. ASAIO J. 2018, 64, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Kourek, C.; Nanas, S.; Kotanidou, A.; Raidou, V.; Dimopoulou, M.; Adamopoulos, S.; Karabinis, A.; Dimopoulos, S. Modalities of Exercise Training in Patients with Extracorporeal Membrane Oxygenation Support. J. Cardiovasc. Dev. Dis. 2022, 9, 34. [Google Scholar] [CrossRef]
- Bonios, M.J.; Selzman, C.H.; Gilbert, E.M.; McKellar, S.H.; Koliopoulou, A.; Strege, J.L.; Nativi, J.N.; Fang, J.C.; Stehlik, J.; Drakos, S.G. Exertional Angina Due To Fused Aortic Bioprosthesis During Left Ventricular Assist Device Support: Two Cases and Review of the Literature. ASAIO J. 2017, 63, e6–e9. [Google Scholar] [CrossRef]
- John, R.; Mantz, K.; Eckman, P.; Rose, A.; May-Newman, K. Aortic valve pathophysiology during left ventricular assist device support. J. Heart Lung Transplant. 2010, 29, 1321–1329. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Pagani, F.D.; Rogers, J.G.; Miller, L.W.; Sun, B.; Russell, S.D.; Starling, R.C.; Chen, L.; Boyle, A.J.; Chillcott, S.; et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010, 29, S1–S39. [Google Scholar] [CrossRef]
- Jorde, U.; Uriel, N.; Nahumi, N.; Bejar, D.; Gonzalez-Costello, J.; Thomas, S.S.; Han, J.; Morrison, K.A.; Jones, S.; Kodali, S.; et al. Prevalence, Significance, and Management of Aortic Insufficiency in Continuous Flow Left Ventricular Assist Device Recipients. Circ. Heart Fail. 2014, 7, 310–319. [Google Scholar] [CrossRef]
- Potapov, E.; Antonides, C.; Crespo-Leiro, M.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Damman, D.K.; Testani, J.M. The kidney in heart failure: An update. Eur. Heart J. 2015, 36, 1437–1444. [Google Scholar] [CrossRef]
- Hasin, T.; Topilsky, Y.; Schirger, J.A.; Li, Z.; Zhao, Y.; Boilson, B.A.; Clavell, A.L.; Rodeheffer, R.J.; Frantz, R.P.; Edwards, B.S.; et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J. Am. Coll. Cardiol. 2012, 59, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Topkara, V.K.; Coromilas, E.J.; Garan, A.R.; Li, R.C.; Castagna, F.; Jennings, D.L.; Yuzefpolskaya, M.; Takeda, K.; Takayama, H.; Sladen, R.N.; et al. Preoperative Proteinuria and Reduced Glomerular Filtration Rate Predicts Renal Replacement Therapy in Patients Supported With Continuous-Flow Left Ventricular Assist Devices. Circ. Heart Fail. 2016, 9, e002897. [Google Scholar] [CrossRef] [PubMed]
- Sandner, S.E.; Zimpfer, D.; Zrunek, P.; Rajek, A.; Schima, H.; Dunkler, D.; Grimm, M.; Wolner, E.; Wieselthaler, G.M. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann. Thorac. Surg. 2009, 87, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Daimee, U.A.; Wang, M.; Papernov, A.; Sherazi, S.; McNitt, S.; Vidula, H.; Chen, L.; Alexis, J.D.; Kutyifa, V. Renal Function Changes Following Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2017, 120, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, J.M.; Arnaoutakis, G.J.; Allen, J.G.; Weiss, E.S.; Patel, N.D.; Russell, S.D.; Shah, A.S.; Conte, J.V. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann. Thorac. Surg. 2011, 91, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Demirozu, Z.T.; Radovancevic, R.; Hochman, L.F.; Gregoric, I.D.; Letsou, G.V.; Kar, B.; Bogaev, R.C.; Frazier, O.H. Arteriovenous maformation and gastrointestinal bleeding in patients with the Heart-Mate IIl left ventricular assist device. J. Heart Lung Transplant. 2011, 30, 849–853. [Google Scholar] [PubMed]
- Shah, P.; Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Bleeding and thrombosis associated with ventricular assist device therapy. J. Heart Lung Transplant. 2017, 36, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.Y.; Ruckel, S.; Kfoury, A.G.; McKellar, S.H.; Taleb, I.; Gilbert, E.M.; Nativi-Nicolau, J.; Stehlik, J.; Reid, B.B.; Koliopoulou, A.; et al. Novel Model to Predict Gastrointestinal Bleeding During Left Ventricular Assist Device Support. Circ. Heart Fail. 2018, 11, e005267. [Google Scholar] [CrossRef]
- Connors, J.M. Hematologic disorders and continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2014, 33, 1114–1116. [Google Scholar] [CrossRef]
- Mehra, M.R.; Netuka, I.; Uriel, N.; Katz, J.N.; Pagani, F.D.; Jorde, U.P.; Gustafsson, F.; Connors, J.M.; Ivak, P.; Cowger, J.; et al. Aspirin and Hemocompatibility Events With a Left Ventricular Assist Device in Advanced Heart Failure: TheARIES-HM3 Randomized Clinical Trial. JAMA 2023, 330, 2171–2181. [Google Scholar] [CrossRef]
- Hannan, M.M.; Husain, S.; Mattner, F.; Danziger-Isakov, L.; Drew, R.J.; Corey, G.R.; Schueler, S.; Holman, W.L.; Lawler, L.P.; Gordon, S.M.; et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J. Heart Lung Transplant. 2011, 30, 375–384. [Google Scholar] [CrossRef]
- Trachtenberg, B.; Cordero-Reyes, A.; Elias, B.; Loebe, M. A Review of Infections in Patients with Left Ventricular Assist Devices: Prevention, Diagnosis and Management. Methodist DeBakey Cardiovasc. J. 2015, 11, 28–32. [Google Scholar] [CrossRef]
- Acharya, M.; Som, R.; Tsui, S. What is the optimum antibiotic prophylaxis in patients undergoing implantation of a left ventricular assist device? Interdiscip. CardioVascular Thorac. Surg. 2012, 14, 209–214. [Google Scholar] [CrossRef]
- Sims, D.B.; Uriel, N.; Gonzalez-Costello, J.; Deng, M.C.; Restaino, S.W.; Farr, M.A.; Takayama, H.; Mancini, D.M.; Naka, Y.; Jorde, U.P. Human immunodeficiency virus infection and left ventricular assist devices: A case series. J. Heart Lung Transplant. 2011, 30, 1060–1064. [Google Scholar] [CrossRef]
- Pendyal, A.; Gelow, J. Hepatitis C Virus Infection Does Not Impact Survival Following Continuous-Flow Left Ventricular Assist Device Implantation. J. Heart Lung Transplant. 2017, 36, S28. [Google Scholar] [CrossRef]
- Eshelman, A.K.; Mason, S.; Nemeh, H.; Williams, C. LVAD destination therapy: Applying what we know about psychiatric evaluation and management from cardiac failure and transplant. Heart Fail. Rev. 2009, 14, 21–28. [Google Scholar] [CrossRef]
- Cupples, S.; Des, M.A.; Grady, K.L.; De Geest, S.; Dobbels, F.; Lanuza, D.; Paris, W. Report of the Psychosocial Outcomes Work group of the Nursing and Social Sciences Council of the International Society for Heart and Lung Transplantation: Present status of research on psychosocial outcomes in cardiothoracic transplantation: Review and recommendations for the field. J. Heart Lung Transplant. 2006, 25, 716–725. [Google Scholar]
- Tasoulis, A.; Tzanis, G.; Vasileiadis, I.; Dimopoulos, S.; Karatzanos, E.; Nanas, S.; Charitos, C. Effects of left ventricular assist device implantation on respiratory drive. Health Res. J. 2019, 5, 77–83. [Google Scholar] [CrossRef]
- Dimopoulos, S.K.; Drakos, S.G.; Terrovitis, J.V.; Tzanis, G.S.; Nanas, S.N. Improvement in respiratory muscle dysfunction with continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2010, 29, 906–908. [Google Scholar] [CrossRef]
- Dimopoulos, S.; Diakos, N.; Tseliou, E.; Tasoulis, A.; Mpouchla, A.; Manetos, C.; Katsaros, L.; Drakos, S.; Terrovitis, J.; Nanas, S. Chronotropic incompetence and abnormal heart rate recovery early after left ventricular assist device implantation. Pacing Clin. Electrophysiol. 2011, 34, 1607–1614. [Google Scholar] [CrossRef]
- Hayes, K.; Leet, A.S.; Bradley, S.J.; Holland, A.E. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: A preliminary randomized controlled trial. J. Heart Lung Transplant. 2012, 31, 729–734. [Google Scholar] [CrossRef]
- Kerrigan, D.J.; Williams, C.T.; Ehrman, J.K.; Saval, M.A.; Bronsteen, K.; Schairer, J.R.; Swaffer, M.; Brawner, C.A.; Lanfear, D.E.; Selektor, Y.; et al. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: The Rehab-VAD randomized controlled trial. JACC Heart Fail. 2014, 2, 653–659. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Corrà, U.; Laoutaris, I.D.; Pistono, M.; Agostoni, P.G.; Coats, A.J.S.; Crespo Leiro, M.G.; Cornelis, J.; Davos, C.H.; Filippatos, G.; et al. Exercise training in patients with ventricular assist devices: A review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 3–13. [Google Scholar]
- Laoutaris, I.D.; Dritsas, A.; Adamopoulos, S.; Manginas, A.; Gouziouta, A.; Kallistratos, M.S.; Koulopoulou, M.; Voudris, V.; Cokkinos, D.V.; Sfirakis, P. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term post implantation. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 33–40. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Gouziouta, A.; Mantzouratou, P.; Laoutaris, I.D.; Dritsas, A.; Cokkinos, D.V.; Mourouzis, I.; Sfyrakis, P.; Iervasi, G.; Pantos, C. Thyroid hormone signaling is altered in response to physical training in patients with end-stage heart failure and mechanical assist devices: Potential physiological consequences? Interdiscip. CardioVascular Thorac. Surg. 2013, 17, 664–668. [Google Scholar]
- Bobenko, A.; Schoenrath, F.; Knierim, J.H.; Friede, T.; Verheyen, N.; Mehra, M.R.; Haykowsky, M.; Herrmann-Lingen, C.; Duvinage, A.; Pieske-Kraigher, E.; et al. Exercise training in patients with a left ventricular assist device (Ex-VAD): Rationale and design of a multicenter, prospective, assessor-blinded, randomized, controlled trial. Eur. J. Heart Fail. 2019, 21, 1152–1159. [Google Scholar] [CrossRef]
- Birks, E.J.; Tansley, P.D.; Hardy, J.; George, R.S.; Bowles, C.T.; Burke, M.; Banner, N.R.; Khaghani, A.; Yacoub, M.H. Left ventricular assist device and drug therapy for the reversal of heart failure. N. Engl. J. Med. 2006, 355, 1873–1884. [Google Scholar] [CrossRef]
- Drakos, S.G.; Kfoury, A.G.; Stehlik, J.; Selzman, C.H.; Reid, B.B.; Terrovitis, J.V.; Nanas, J.N.; Li, D.Y. Bridge to recovery: Understanding the disconnect between clinical and biological outcomes. Circulation 2012, 126, 230–241. [Google Scholar] [CrossRef]
- Drakos, S.; Pagani, F.; Lundberg, M.; Baldwin, J.T. Advancing the Science of Myocardial Recovery With Mechanical Circulatory Support. A Working Group of the National, Heart, Lung, and Blood Institute. JACC Basic Transl. Sci. 2017, 2, 335–340. [Google Scholar] [CrossRef]
- Birks, E.J.; George, R.S.; Firouzi, A.; Wright, G.; Bahrami, T.; Yacoub, M.H.; Khaghani, A. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J. Thorac. Cardiovasc. Surg. 2012, 144, 190–196. [Google Scholar] [CrossRef]
- Jakovljevic, D.; Yacoub, M.; Schueler, S.; MacGowan, G.A.; Velicki, L.; Seferovic, P.M.; Hothi, S.; Tzeng, B.H.; Brodie, D.A.; Birks, E.; et al. Left Ventricular Assist Device as a Bridge to Recovery for Patients with Advanced Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Birks, E.; Drakos, S.; Lowes, B.D.; Selzman, C.H.; Starling, R.C.; Trivedi, J.; Slaughter, M.S.; Alturi, P.; Goldstein, D.; Patel, S.; et al. A Prospective Multicentre Study of Myocardial Recovery Using Left Ventricular Assist Devices (Remission from Stage D Heart Failure: RESTAGE-HF): Medium Term and Primary End point Results. Circulation 2020, 142, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- Topkara, V.K.; Garan, A.R.; Fine, B.; Godier-Furnémont, A.F.; Breskin, A.; Cagliostro, B.; Yuzefpolskaya, M.; Takeda, K.; Takayama, H.; Mancini, D.M.; et al. Myocardial Recovery in Patients Receiving Contemporary Left Ventricular Assist Devices. Results From the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). Circ. Heart Fail. 2016, 9, e003157. [Google Scholar] [CrossRef] [PubMed]
- Wever-Pinzon, O.; Drakos, S.G.; McKellar, S.H.; Horne, B.D.; Caine, W.T.; Kfoury, A.G.; Li, D.Y.; Fang, J.C.; Stehlik, J.; Selzman, C.H. Cardiac Recovery During Long-Term Left Ventricular Assist Device Support. J. Am. Coll. Cardiol. 2016, 68, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Psotka, M.; Taleb, I.; Alharethi, R.; Shams, M.A.; Wever-Pinzon, O.; Yin, M.; Latta, F.; Stehlik, J.; Fang, J.C.; et al. Framework to Classify Reverse Cardiac Remodeling With Mechanical Circulatory Support: The Utah-Inova Stages. Circ. Heart Fail. 2021, 14, e007991. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Maybaum, S.; MacGillivray, T.E.; Moore, S.A.; Bogaev, R.; Farrar, D.J.; Frazier, O.H. Young patients with non-ischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. J. Card. Fail. 2012, 18, 392–395. [Google Scholar] [CrossRef]
- Birks, E.J.; George, R.S.; Hedger, M.; Bahrami, T.; Wilton, P.; Bowles, C.T.; Webb, C.; Bougard, R.; Amrani, M.; Yacoub, M.H.; et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation 2011, 123, 381–390. [Google Scholar] [CrossRef]
- Bonios, M.J.; Koliopoulou, A.; Wever-Pinzon, O.; Taleb, I.; Stehlik, J.; Xu, W.; Wever-Pinzon, J.; Catino, A.; Kfoury, A.G.; Horne, B.D.; et al. Cardiac Rotational Mechanics As a Predictor of Myocardial Recovery In Heart Failure Patients Undergoing Chronic Mechanical Circulatory Support: A Pilot Study. Circ. Cardiovasc. Imaging 2018, 11, e007117. [Google Scholar] [CrossRef]
- Diakos, N.; Taleb, I.; Kyriakopoulos, C.; Shah, K.S.; Javan, H.; Richins, T.J.; Yin, M.Y.; Yen, C.G.; Dranow, E.; Bonios, M.J.; et al. Circulating and Myocardial Cytokines Predict Cardiac Structural and Functional Improvement in Patients With Heart Failure Undergoing Mechanical Circulatory Support. JAHA 2021, 10, e020238. [Google Scholar] [CrossRef]
- Hanke, J.S.; Dogan, G.; Haverich, A.; Schmitto, J.D. First-in-man explantation of a HeartMate3 left ventricular assist device via customized plug. Eur. J. Cardiothorac. Surg. 2020, 57, 604–606. [Google Scholar] [CrossRef]
- Schmitto, J.D.; Rojas, S.V.; Hanke, J.S.; Avsar, M.; Haverich, A. Minimally invasive left ventricular assist device explantation after cardiac recovery: Surgical technical considerations. Artif. Organs 2014, 38, 507–510. [Google Scholar] [CrossRef]
- MacGowan, G.A.; Wrightson, N.; Robinson-Smith, N.; Woods, A.; Parry, G.; Gould, K.; Schueler, S. Myocardial recovery strategy with decommissioning for the HeartWare left ventricular assist device. ASAIO J. 2017, 63, 299–304. [Google Scholar] [CrossRef]
- Albulushi, A.; Goldsweig, A.M.; Stoller, D.; Delaney, J.W.; Um, J.; Lowes, B.; Zolty, R. Percutaneous deactivation of left ventricular assist devices. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 467–472. [Google Scholar] [CrossRef]
- Maybaum, S.; Mancini, D.; Xydas, S.; Starling, R.C.; Aaronson, K.; Pagani, F.D.; Miller, L.W.; Margulies, K.; McRee, S.; Frazier, O.H.; et al. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the LVAD Working Group. Circulation 2007, 115, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Weng, Y.; Siniawski, H.; Potapov, E.; Drews, T.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation 2008, 118, S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Weng, Y.; Siniawski, H.; Stepanenko, A.; Krabatsch, T.; Potapov, E.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: Criteria for weaning from ventricular assist devices. Eur. Heart J. 2011, 32, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Wever-Pinzon, J.; Selzman, C.H.; Wever-Pinzon, O.; Wever-Pinzon, O.; Catino, A.; Kfoury, A.G.; Diakos, N.A.; Reid, B.B.; McKellar, S.; Bonios, M.; et al. Impact of ischemic heart failure etiology on the incidence of cardiac recovery during mechanical unloading: A prospective study from the Utah Cardiac Recovery Program. J. Am. Coll. Cardiol. 2016, 68, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Saeed, O.; Murthy, S.; Bhatia, V.; Shin, J.J.; Wang, D.; Negassa, A.; Pullman, J.; Goldstein, D.J.; Maybaum, S. Combining neurohormonal blockade with continuous-flow left ventricular assist device support for myocardial recovery: A single-arm p rospective study. J. Heart Lung Transplant. 2013, 32, 305–312. [Google Scholar] [CrossRef]
- Liden, H.; Karason, K.; Bergh, C.; Nilsson, F.; Koul, B.; Wiklund, L. The feasibility of left ventricular mechanical support as a bridge to cardiac recovery. Eur. J. Heart Fail. 2007, 9, 525–530. [Google Scholar] [CrossRef]
- Lamarche, Y.; Kearns, M.; Josan, K.; Bashir, J.; Ignaszewski, A.; Kaan, A.; Kealy, J.; Moss, R.; Cheung, A. Successful weaning and explantation of the Heartmate II left ventricular assist device. Can. J. Cardiol. 2011, 27, 358–362. [Google Scholar] [CrossRef]
- Gorcsan, J.; Severyn, D.; Murali, S.; Kormos, R.L. Non-invasive assessment of myocardial recovery on chronic left ventricular assist device: Results associated with successful device removal. J. Heart Lung Transplant. 2003, 22, 1304–1313. [Google Scholar] [CrossRef]
- Khan, T.; Delgado, R.M.; Radovancevic, B.; Torre-Amione, G.; Abrams, J.; Miller, K.; Myers, T.; Okerberg, K.; Stetson, S.J.; Gregoric, I.; et al. Dobutamine stress echocardiography predicts myocardial improvement in patients supported by left ventricular assist devices (LVADs): Hemodynamic and histologic evidence of improvement before LVAD explantation. J. Heart Lung Transplant. 2003, 22, 137–146. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Starling, R.C.; Cooper, L.T.; Torre-Amione, G.; Wittstein, I.; Dec, G.W.; Markham, D.W.; Zucker, M.J.; Gorcsan, J., 3rd; McTiernan, C.; et al. Left ventricular assist device support and myocardial recovery in recent onset cardiomyopathy. J. Card. Fail. 2012, 18, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Drakos, S.; Terrovitis, J.; Anastasiou-Nana, M.; Nanas, J.N. Reverse remodeling during long-term mechanical unloading of the left ventricle. J. Mol. Cell. Cardiol. 2007, 43, 231–242. [Google Scholar] [CrossRef]
- Molina, E.; Shah, P.; Kiernan, M.; Cornwell, W.K., 3rd; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. J. Heart Lung Transplant. 2021, 111, 778–792. [Google Scholar]
- Siméon, S.; Flécher, E.; Revest, M.; Niculescu, M.; Roussel, J.C.; Michel, M.; Leprince, P.; Tattevin, P. Left ventricular assist device-related infections: A multi centric study. Clin. Microbiol. Infect. 2017, 23, 748–751. [Google Scholar] [CrossRef]
- Almarzooq, Z.I.; Varshney, A.S.; Vaduganathan, M.; Pareek, M.; Stewart, G.C.; Estep, J.D.; Mehra, M.R. Expanding the scope of multimodality imaging in durable mechanical circulatory support. JACC Cardiovasc. Imaging 2020, 13, 1069–1081. [Google Scholar] [CrossRef]
- Immohr, M.B.; Boeken, U.; Mueller, F.; Prashovikj, E.; Morshuis, M.; Böttger, C.; Aubin, H.; Gummert, J.; Akhyari, P.; Lichtenberg, A.; et al. Complications of left ventricular assist devices causing high urgency status on waiting list: Impact on outcome after heart transplantation. ESC Heart Fail. J. 2021, 8, 1253–1262. [Google Scholar] [CrossRef]


| All the following criteria must be present despite optimal guideline-directed treatment: |
|---|
| 1. Severe and persistent symptoms of heart failure [NYHA class III (advanced) or IV]. |
| 2. Severe cardiac dysfunction defined by a reduced LVEF ≤30%, isolated RV failure (e.g., ARVC) or non-operable severe valve abnormalities or congenital abnormalities, or persistently high (or increasing) BNP or NT-proBNP values and data of severe diastolic dysfunction or LV structural abnormalities according to the ESC definition of HFpEF and HFmrEF. |
| 3. Episodes of pulmonary or systemic congestion requiring high-dose intravenous diuretics (or diuretic combinations) or episodes of low output requiring inotropes or vasoactive drugs or malignant arrhythmias causing >1 unplannedvisit or hospitalization in the last 12 months. |
| 4. Severe impairment of exercise capacity with Inability to exercise or low 6MWD (<300 m) or pVO2 (<12–14 mL/kg/min), estimated to be of cardiac origin. |
| In addition to the above, extra-cardiac organ dysfunction due to heart failure (e.g., cardiac cachexia, liver, or kidney dysfunction) or type 2 pulmonary hypertension may be present but are not required. |
| I: Inotropes (iv) |
|---|
| N: NYHA IIIb-IV or persistently elevated natriuretic peptides |
| E: End-organ dysfunction |
| E: Ejection fraction ≤35% |
| D: Defibrillator shocks |
| H: Hospitalizations >1 in prior 12 months |
| E: Edema despite escalating diuretics |
| L: Low blood pressure ≤90 mmHg, high heart rate |
| P: Prognostic medication progressive intolerance/down-titration of guideline-directed medical therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulos, M.; Bonios, M.J.; Dimopoulos, S.; Leontiadis, E.; Gouziouta, A.; Kogerakis, N.; Koliopoulou, A.; Elaiopoulos, D.; Vlahodimitris, I.; Chronaki, M.; et al. Advanced Heart Failure: Therapeutic Options and Challenges in the Evolving Field of Left Ventricular Assist Devices. J. Cardiovasc. Dev. Dis. 2024, 11, 61. https://doi.org/10.3390/jcdd11020061
Antonopoulos M, Bonios MJ, Dimopoulos S, Leontiadis E, Gouziouta A, Kogerakis N, Koliopoulou A, Elaiopoulos D, Vlahodimitris I, Chronaki M, et al. Advanced Heart Failure: Therapeutic Options and Challenges in the Evolving Field of Left Ventricular Assist Devices. Journal of Cardiovascular Development and Disease. 2024; 11(2):61. https://doi.org/10.3390/jcdd11020061
Chicago/Turabian StyleAntonopoulos, Michael, Michael J. Bonios, Stavros Dimopoulos, Evangelos Leontiadis, Aggeliki Gouziouta, Nektarios Kogerakis, Antigone Koliopoulou, Dimitris Elaiopoulos, Ioannis Vlahodimitris, Maria Chronaki, and et al. 2024. "Advanced Heart Failure: Therapeutic Options and Challenges in the Evolving Field of Left Ventricular Assist Devices" Journal of Cardiovascular Development and Disease 11, no. 2: 61. https://doi.org/10.3390/jcdd11020061
APA StyleAntonopoulos, M., Bonios, M. J., Dimopoulos, S., Leontiadis, E., Gouziouta, A., Kogerakis, N., Koliopoulou, A., Elaiopoulos, D., Vlahodimitris, I., Chronaki, M., Chamogeorgakis, T., Drakos, S. G., & Adamopoulos, S. (2024). Advanced Heart Failure: Therapeutic Options and Challenges in the Evolving Field of Left Ventricular Assist Devices. Journal of Cardiovascular Development and Disease, 11(2), 61. https://doi.org/10.3390/jcdd11020061







