Advanced Imaging Techniques for Atherosclerosis and Cardiovascular Calcification in Animal Models
Abstract
1. Introduction
2. Computed Tomography (CT)
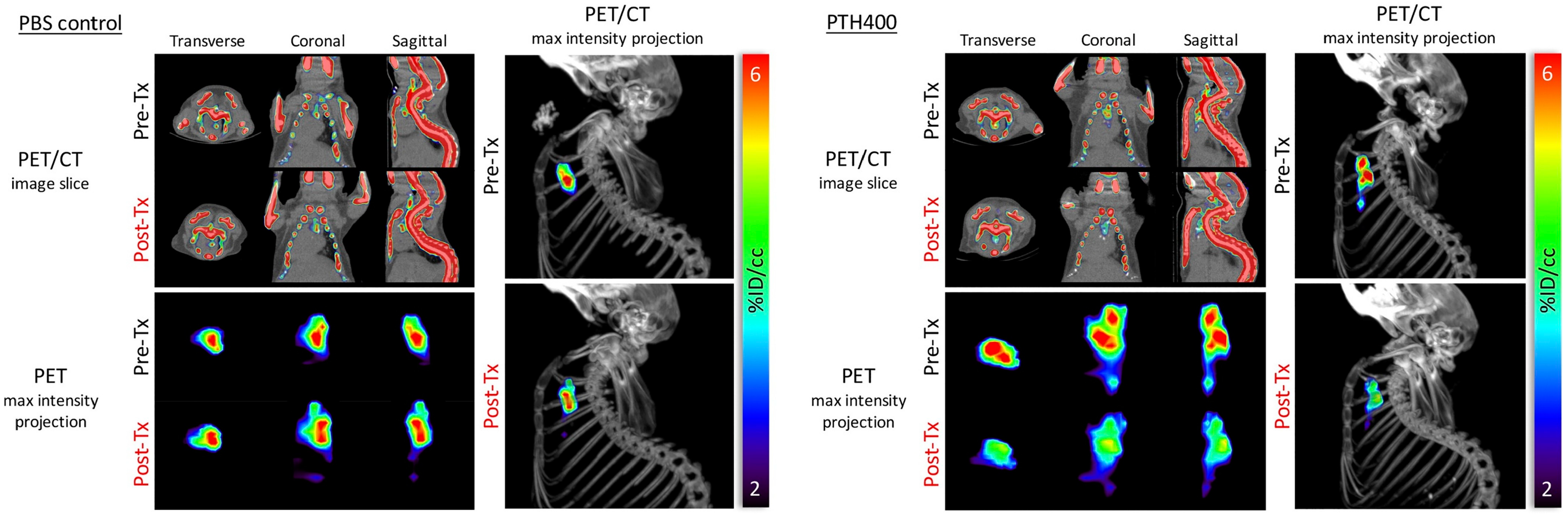
3. Positron Emission Tomography (PET)
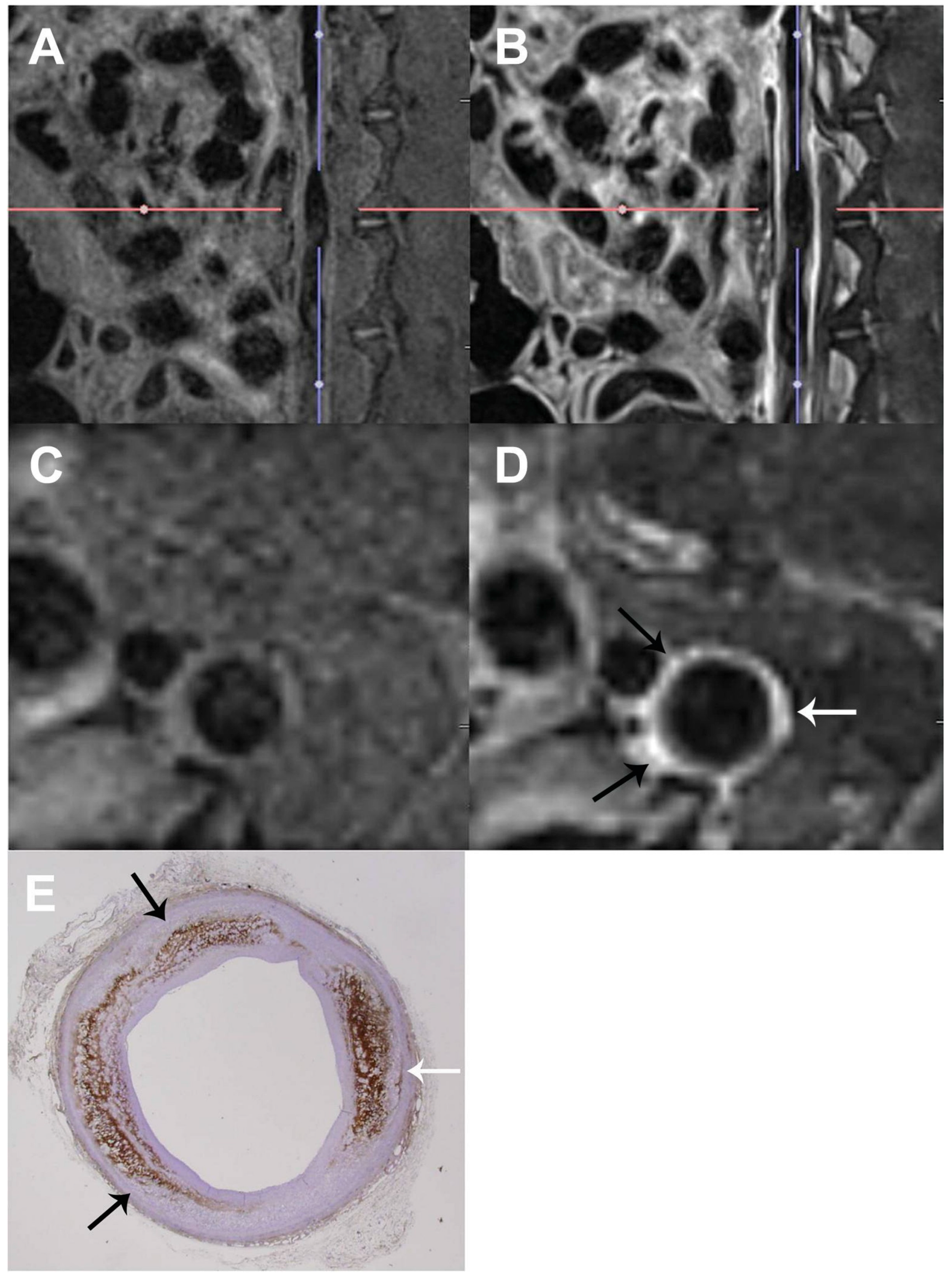
4. Magnetic Resonance Imaging (MRI)
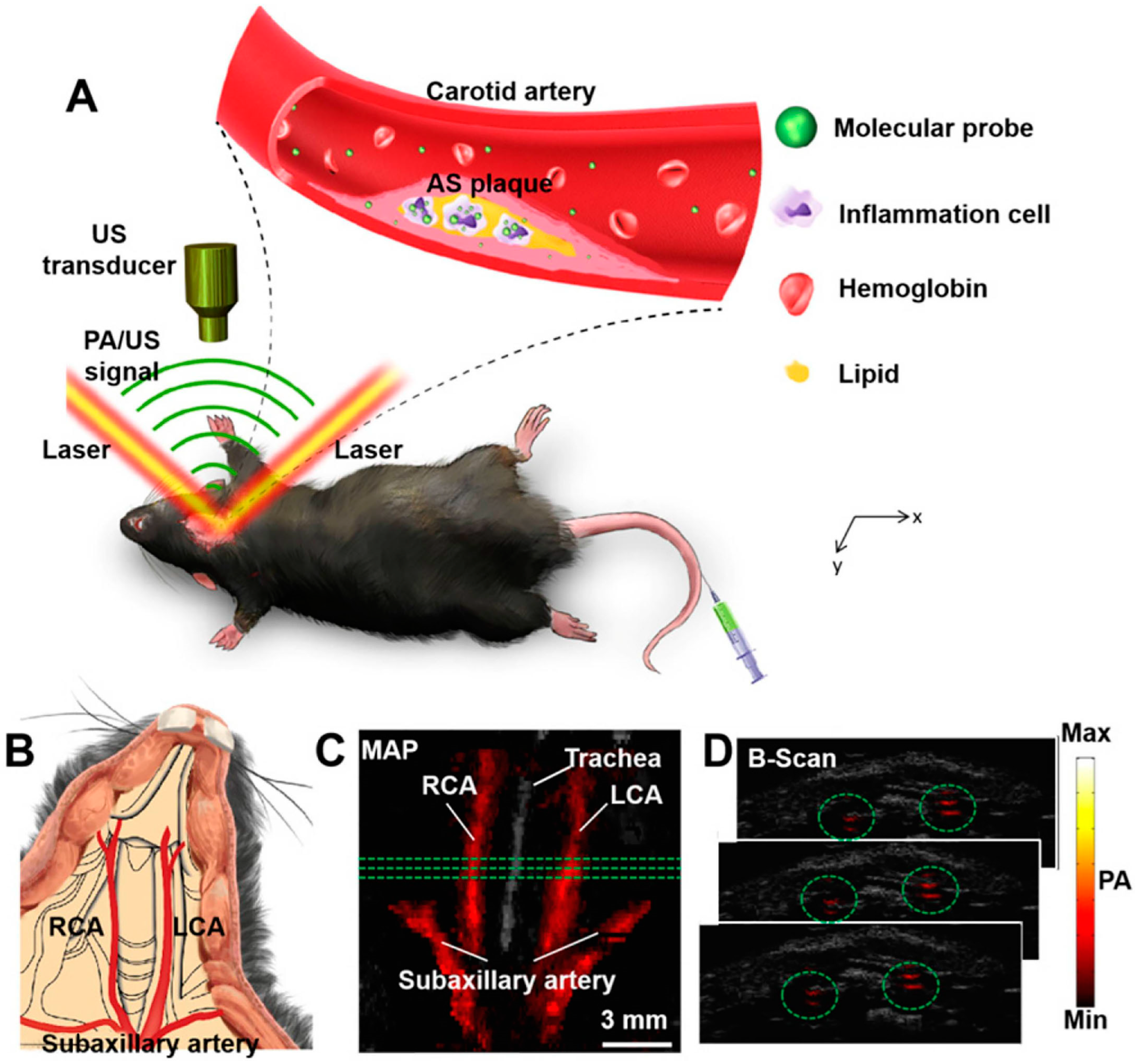
5. Photoacoustic Imaging (PAI)
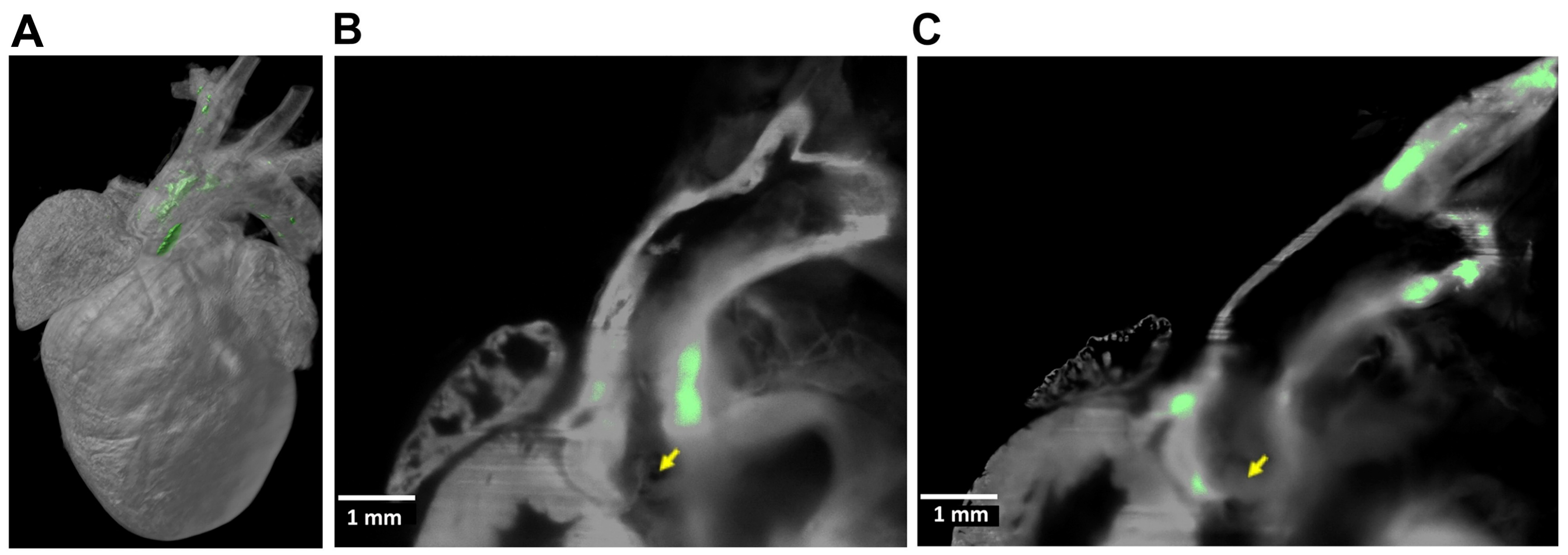
6. Light-Sheet Fluorescence Microscopy (LSFM)
7. Multimodal Approaches
8. Implications and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abedin, M.; Tintut, Y.; Demer, L.L. Vascular calcification: Mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St, H.C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Iacobini, C.; Fantauzzi, C.B.; Menini, S. The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Chow, L.A.; Hsu, J.J.; Perlowski, A.A.; Abedin, M.; Tobis, J.; Tintut, Y.; Mal, A.K.; Klug, W.S.; Demer, L.L. Mechanical stress analysis of a rigid inclusion in distensible material: A model of atherosclerotic calcification and plaque vulnerability. Am. J. Physiol. Circ. Physiol. 2009, 297, H802–H810. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Feng, G.; Fan, T.; Jiang, H.; Wang, Z. Advances in CT Techniques in Vascular Calcification. Front. Cardiovasc. Med. 2021, 8, 716822. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Advances in micro-CT imaging of small animals. Phys. Medica 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Borland, S.J.; Behnsen, J.; Ashton, N.; Francis, S.E.; Brennan, K.; Sherratt, M.J.; Withers, P.J.; Canfield, A.E. X-ray Micro-Computed Tomography: An Emerging Technology to Analyze Vascular Calcification in Animal Models. Int. J. Mol. Sci. 2020, 21, 4538. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Jaffer, F.A.; Gijsen, F.J.; van Soest, G.; Madden, S.P.; Courtney, B.K.; Fard, A.M.; Tenekecioglu, E.; Zeng, Y.; van der Steen, A.; et al. Hybrid intravascular imaging: Recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur. Heart J. 2017, 38, 400–412. [Google Scholar] [CrossRef]
- Dweck, M.R.; Aikawa, E.; Newby, D.E.; Tarkin, J.M.; Rudd, J.H.; Narula, J.; Fayad, Z.A. Noninvasive Molecular Imaging of Disease Activity in Atherosclerosis. Circ. Res. 2016, 119, 330–340. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Dweck, M.R.; Evans, N.R.; Takx, R.A.; Brown, A.J.; Tawakol, A.; Fayad, Z.A.; Rudd, J.H. Imaging Atherosclerosis. Circ. Res. 2016, 118, 750–769. [Google Scholar] [CrossRef]
- Brinjikji, W.; Huston, J.R.; Rabinstein, A.A.; Kim, G.M.; Lerman, A.; Lanzino, G. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Cate, T.T.; Powers, E.; Kim, C.; et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet 2019, 394, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.J.; Tintut, Y.; Demer, L.L. Murine models of atherosclerotic calcification. Curr. Drug Targets 2008, 9, 224–228. [Google Scholar] [CrossRef]
- Shamsuzzaman, S.; Deaton, R.A.; Salamon, A.; Doviak, H.; Serbulea, V.; Milosek, V.M.; Evans, M.A.; Karnewar, S.; Saibaba, S.; Alencar, G.F.; et al. Novel Mouse Model of Myocardial Infarction, Plaque Rupture, and Stroke Shows Improved Survival With Myeloperoxidase Inhibition. Circulation 2024, 150, 687–705. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC-Cardiovasc. Imag. 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Self, T.S.; Ginn-Hedman, A.M.; Kaulfus, C.N.; Newell-Fugate, A.E.; Weeks, B.R.; Heaps, C.L. Iodine-enhanced micro-computed tomography of atherosclerotic plaque morphology complements conventional histology. Atherosclerosis 2020, 313, 43–49. [Google Scholar] [CrossRef]
- Faight, E.; Verdelis, K.; Ahearn, J.M.; Shields, K.J. 3D MicroCT spatial and temporal characterization of thoracic aorta perivascular adipose tissue and plaque volumes in the ApoE-/- mouse model. Adipocyte 2018, 7, 156–165. [Google Scholar] [CrossRef]
- Armstrong, Z.B.; Boughner, D.R.; Drangova, M.; Rogers, K.A. Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc. Res. 2011, 90, 165–170. [Google Scholar] [CrossRef]
- Choi, B.G.; Vilahur, G.; Zafar, M.U.; Cardoso, L.; Yadegar, D.; Ibanez, B.; Tunstead, J.; Viles-Gonzalez, J.F.; Schaffler, M.B.; Fuster, V.; et al. Selective estrogen receptor modulation influences atherosclerotic plaque composition in a rabbit menopause model. Atherosclerosis 2008, 201, 76–84. [Google Scholar] [CrossRef]
- Panetta, D.; Pelosi, G.; Viglione, F.; Kusmic, C.; Terreni, M.; Belcari, N.; Guerra, A.D.; Athanasiou, L.; Exarchos, T.; Fotiadis, D.I.; et al. Quantitative micro-CT based coronary artery profiling using interactive local thresholding and cylindrical coordinates. Technol. Health Care 2015, 23, 557–570. [Google Scholar] [CrossRef]
- Sriranjan, R.S.; Tarkin, J.M.; Evans, N.R.; Le, E.P.V.; Chowdhury, M.M.; Rudd, J. Atherosclerosis imaging using PET: Insights and applications. Brit J. Pharmacol. 2021, 178, 2186–2203. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, D.; Chen, X.; Liang, Y.; Guo, F.; Wu, C.; Jia, L.; Hou, Z.; Li, W.; He, Z.; et al. (18)F-ASEM Imaging for Evaluating Atherosclerotic Plaques Linked to α7-Nicotinic Acetylcholine Receptor. Front. Bioeng. Biotechnol. 2021, 9, 684221. [Google Scholar] [CrossRef]
- Wang, D.; Yao, Y.; Wang, S.; Zhang, H.; He, Z.X. The Availability of the α7-Nicotinic Acetylcholine Receptor in Early Identification of Vulnerable Atherosclerotic Plaques: A Study Using a Novel (18)F-Label Radioligand PET. Front. Bioeng. Biotechnol. 2021, 9, 640037. [Google Scholar] [CrossRef] [PubMed]
- Horti, A.G.; Gao, Y.; Kuwabara, H.; Wang, Y.; Abazyan, S.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Sahibzada, N.; Holt, D.P.; et al. 18F-ASEM, a radiolabeled antagonist for imaging the α7-nicotinic acetylcholine receptor with PET. J. Nucl. Med. 2014, 55, 672–677. [Google Scholar] [CrossRef]
- Jurtz, V.I.; Skovbjerg, G.; Salinas, C.G.; Roostalu, U.; Pedersen, L.; Hecksher-Sorensen, J.; Rolin, B.; Nyberg, M.; van de Bunt, M.; Ingvorsen, C. Deep learning reveals 3D atherosclerotic plaque distribution and composition. Sci. Rep. 2020, 10, 21523. [Google Scholar] [CrossRef]
- Jarrett, B.R.; Correa, C.; Ma, K.L.; Louie, A.Y. In vivo mapping of vascular inflammation using multimodal imaging. PLoS ONE 2010, 5, e13254. [Google Scholar] [CrossRef]
- Postnov, A.A.; D’Haese, P.C.; Neven, E.; De Clerck, N.M.; Persy, V.P. Possibilities and limits of X-ray microtomography for in vivo and ex vivo detection of vascular calcifications. Int. J. Cardiovasc. Imaging 2009, 25, 615–624. [Google Scholar] [CrossRef]
- Hsu, J.J.; Lu, J.; Umar, S.; Lee, J.T.; Kulkarni, R.P.; Ding, Y.; Chang, C.C.; Hsiai, T.K.; Hokugo, A.; Gkouveris, I.; et al. Effects of teriparatide on morphology of aortic calcification in aged hyperlipidemic mice. Am. J. Physiol. Circ. Physiol. 2018, 314, H1203–H1213. [Google Scholar] [CrossRef]
- Lloyd, D.J.; Helmering, J.; Kaufman, S.A.; Turk, J.; Silva, M.; Vasquez, S.; Weinstein, D.; Johnston, B.; Hale, C.; Veniant, M.M. A volumetric method for quantifying atherosclerosis in mice by using microCT: Comparison to en face. PLoS ONE 2011, 6, e18800. [Google Scholar] [CrossRef]
- Yang, D.-Y.; Zhu, Y.; Kong, J.-Q.; Gong, X.-J.; Xie, Z.-H.; Mei, W.-Y.; Luo, C.-F.; Du, Z.-M.; Zhuang, X.-D.; Liao, X.-X. “Light in and Sound Out”: Review of Photoacoustic Imaging in Cardiovascular Medicine. IEEE Access 2019, 7, 38890–38901. [Google Scholar] [CrossRef]
- Stadelmann, V.A.; Boyd, G.; Guillot, M.; Bienvenu, J.G.; Glaus, C.; Varela, A. Automatic Quantification of Atherosclerosis in Contrast-Enhanced MicroCT Scans of Mouse Aortas Ex Vivo. Int. J. Biomed. Imaging 2021, 2021, 4998786. [Google Scholar] [CrossRef] [PubMed]
- Kampschulte, M.; Langheinirch, A.C.; Sender, J.; Litzlbauer, H.D.; Althohn, U.; Schwab, J.D.; Alejandre-Lafont, E.; Martels, G.; Krombach, G.A. Nano-Computed Tomography: Technique and Applications. Rofo-Fortschr. Rontg. 2016, 188, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, J.; Neyt, S.; Debie, P.; Descamps, B.; Devoogdt, N.; Cleeren, F.; Bormans, G.; Broisat, A.; Caveliers, V.; Xavier, C.; et al. Improved Detection of Molecular Markers of Atherosclerotic Plaques Using Sub-Millimeter PET Imaging. Molecules 2020, 25, 1838. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, S. Atherosclerosis in Animals is a Separate Type of Atherosclerosis that has Nothing to do with the Two Types of Atherosclerosis in Humans. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Wu, M.; van der Steen, A.F.; Regar, E.; van Soest, G.; Wu, M.; van der Steen, A.F.; Regar, E.; van Soest, G. Emerging Technology Update Intravascular Photoacoustic Imaging of Vulnerable Atherosclerotic Plaque. Interv. Cardiol. 2016, 11, 120–123. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, Y.; He, Y.; Shu, C.; Chen, D.; Zhang, J.; Chen, J.; Liu, C.; Sheng, Z.; Liu, H.; et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics 2020, 10, 4694–4704. [Google Scholar] [CrossRef]
- McCabe, J.J.; Evans, N.R.; Gorey, S.; Bhakta, S.; Rudd, J.; Kelly, P.J. Imaging Carotid Plaque Inflammation Using Positron Emission Tomography: Emerging Role in Clinical Stroke Care, Research Applications, and Future Directions. Cells 2023, 12, 2073. [Google Scholar] [CrossRef]
- Lee, Y.T.; Laxton, V.; Lin, H.Y.; Chan, Y.; Fitzgerald-Smith, S.; To, T.; Yan, B.P.; Liu, T.; Tse, G. Animal models of atherosclerosis. Biomed. Rep. 2017, 6, 259–266. [Google Scholar] [CrossRef]
- Chen, H.; Wu, T.; Kerwin, W.S.; Yuan, C. Atherosclerotic plaque inflammation quantification using dynamic contrast-enhanced (DCE) MRI. Quant. Imag. Med. Surg. 2013, 3, 298–301. [Google Scholar] [CrossRef]
- Ludvigsen, T.P.; Pedersen, S.F.; Vegge, A.; Ripa, R.S.; Johannesen, H.H.; Hansen, A.E.; Lofgren, J.; Schumacher-Petersen, C.; Kirk, R.K.; Pedersen, H.D.; et al. (18)F-FDG PET/MR-imaging in a Gottingen Minipig model of atherosclerosis: Correlations with histology and quantitative gene expression. Atherosclerosis 2019, 285, 55–63. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, D.; Diyabalanage, H.; Ramsay, I.A.; Rotile, N.J.; Mauskapf, A.; Choi, J.K.; Witzel, T.; Humblet, V.; Jaffer, F.A.; Brownell, A.L.; et al. Imaging High-Risk Atherothrombosis Using a Novel Fibrin-Binding Positron Emission Tomography Probe. Stroke 2022, 53, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Burtea, C.; Laurent, S.; Murariu, O.; Rattat, D.; Toubeau, G.; Verbruggen, A.; Vansthertem, D.; Elst, L.V.; Muller, R.N. Molecular imaging of α v β3 integrin expression in atherosclerotic plaques with a mimetic of RGD peptide grafted to Gd-DTPA. Cardiovasc. Res. 2008, 78, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Leahy, M.; Wijns, W.; Kolios, M.; Zafar, J.; Johnson, N.; Sharif, F. Photoacoustic cardiovascular imaging: A new technique for imaging of atherosclerosis and vulnerable plaque detection. Biomed. Phys. Eng. Express 2018, 4, 32002. [Google Scholar] [CrossRef]
- Ferraro, B.; Giustetto, P.; Schengel, O.; Weckbach, L.T.; Maegdefessel, L.; Soehnlein, O. Longitudinal In Vivo Monitoring of Atheroprogression in Hypercholesterolemic Mice Using Photoacoustic Imaging. Thromb. Haemost. 2023, 123, 545–554. [Google Scholar] [CrossRef]
- Cai, K.; Caruthers, S.D.; Huang, W.; Williams, T.A.; Zhang, H.; Wickline, S.A.; Lanza, G.M.; Winter, P.M. MR molecular imaging of aortic angiogenesis. JACC-Cardiovasc. Imaging 2010, 3, 824–832. [Google Scholar] [CrossRef]
- Calcagno, C.; Lairez, O.; Hawkins, J.; Kerr, S.W.; Dugas, M.S.; Simpson, T.; Epskamp, J.; Robson, P.M.; Eldib, M.; Bander, I.; et al. Combined PET/DCE-MRI in a Rabbit Model of Atherosclerosis: Integrated Quantification of Plaque Inflammation, Permeability, and Burden During Treatment with a Leukotriene A4 Hydrolase Inhibitor. JACC-Cardiovasc. Imaging 2018, 11, 291–301. [Google Scholar] [CrossRef]
- Calcagno, C.; Mani, V.; Ramachandran, S.; Fayad, Z.A. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis 2010, 13, 87–99. [Google Scholar] [CrossRef]
- Olarte, O.E.; Andilla, J.; Gualda, E.J.; Loza-Alvarez, P. Light-sheet microscopy: A tutorial. Adv. Opt. Photonics 2018, 10, 111–179. [Google Scholar] [CrossRef]
- Chang, C.C.; Chu, A.; Meyer, S.; Ding, Y.; Sun, M.M.; Abiri, P.; Baek, K.I.; Gudapati, V.; Ding, X.; Guihard, P.; et al. Three-dimensional Imaging Coupled with Topological Quantification Uncovers Retinal Vascular Plexuses Undergoing Obliteration. Theranostics 2021, 11, 1162–1175. [Google Scholar] [CrossRef]
- Xian, J.Z.; Lu, M.; Fong, F.; Qiao, R.; Patel, N.R.; Abeydeera, D.; Iriana, S.; Demer, L.L.; Tintut, Y. Statin Effects on Vascular Calcification: Microarchitectural Changes in Aortic Calcium Deposits in Aged Hyperlipidemic Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e185–e192. [Google Scholar] [CrossRef]
- Souilhol, C.; Ayllon, B.T.; Li, X.; Diagbouga, M.R.; Zhou, Z.; Canham, L.; Roddie, H.; Pirri, D.; Chambers, E.V.; Dunning, M.J.; et al. JAG1-NOTCH4 mechanosensing drives atherosclerosis. Sci. Adv. 2022, 8, eabo7958. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Ng, T.S.C.; House, A.; Tang, T.; Zheng, L.; Tu, C.; Peake, J.; Espiritu, I.E.; Ma, K.; Pinkerton, K.; et al. Contrast-Enhanced, Molecular Imaging of Vascular Inflammation in the Mouse Model by Simultaneous PET/MRI. BioRxiv 2019. [Google Scholar] [CrossRef]
- Varasteh, Z.; De Rose, F.; Mohanta, S.; Li, Y.; Zhang, X.; Miritsch, B.; Scafetta, G.; Yin, C.; Sager, H.B.; Glasl, S.; et al. Imaging atherosclerotic plaques by targeting Galectin-3 and activated macrophages using ((89)Zr)-DFO- Galectin3-F(ab’)(2) mAb. Theranostics 2021, 11, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Xu, Y.; Sahebkar, A.; Xu, S. CD36 in Atherosclerosis: Pathophysiological Mechanisms and Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 59. [Google Scholar] [CrossRef]
- Choromanska, B.; Mysliwiec, P.; Choromanska, K.; Dadan, J.; Chabowski, A. The role of CD36 receptor in the pathogenesis of atherosclerosis. Adv. Clin. Exp. Med. 2017, 26, 717–722. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, J.; Langenbacher, A.D.; Baek, K.I.; Lee, J.; Chang, C.C.; Hsu, J.J.; Kulkarni, R.P.; Belperio, J.; Shi, W.; et al. Multiscale light-sheet for rapid imaging of cardiopulmonary system. JCI Insight 2018, 3, e121396. [Google Scholar] [CrossRef]
- Kopecky, C.; Pandzic, E.; Parmar, A.; Szajer, J.; Lee, V.; Dupuy, A.; Arthur, A.; Fok, S.; Whan, R.; Ryder, W.J.; et al. Translocator protein localises to CD11b(+) macrophages in atherosclerosis. Atherosclerosis 2019, 284, 153–159. [Google Scholar] [CrossRef]
- Baek, K.I.; Ding, Y.; Chang, C.C.; Chang, M.; Packard, R.R.S.; Hsu, J.J.; Fei, P.; Hsiai, T.K. Advanced microscopy to elucidate cardiovascular injury and regeneration: 4D light-sheet imaging. Prog. Biophys. Mol. Biol. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Ding, Y.; Lee, J.; Hsu, J.J.; Chang, C.C.; Baek, K.I.; Ranjbarvaziri, S.; Ardehali, R.; Packard, R.; Hsiai, T.K. Light-Sheet Imaging to Elucidate Cardiovascular Injury and Repair. Curr. Cardiol. Rep. 2018, 20, 35. [Google Scholar] [CrossRef]
- Lee, J.; Vedula, V.; Baek, K.I.; Chen, J.; Hsu, J.J.; Ding, Y.; Chang, C.C.; Kang, H.; Small, A.; Fei, P.; et al. Spatial and temporal variations in hemodynamic forces initiate cardiac trabeculation. JCI Insight 2018, 3, e96672. [Google Scholar] [CrossRef]
- Becher, T.; Riascos-Bernal, D.F.; Kramer, D.J.; Almonte, V.M.; Chi, J.; Tong, T.; Oliveira-Paula, G.H.; Koleilat, I.; Chen, W.; Cohen, P.; et al. Three-Dimensional Imaging Provides Detailed Atherosclerotic Plaque Morphology and Reveals Angiogenesis After Carotid Artery Ligation. Circ. Res. 2020, 126, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Riascos-Bernal, D.F.; Chi, J.; Cohen, P.; Sibinga, N. Three-Dimensional Visualization of Atherosclerotic Vessels by Tissue Clearing and Light-Sheet Fluorescence Microscopy. Methods Mol. Biol. 2022, 2419, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Cartaya, A.E.; Maiocchi, S.; Buglak, N.E.; Torzone, S.; Messinger, G.; Bahnson, E.S.M. Application of Machine Learning and Virtual Reality for Volumetric Analysis of Arterial Lesions. BioRxiv 2023. [Google Scholar] [CrossRef]
- Hsu, J.J.; Fong, F.; Patel, R.; Qiao, R.; Lo, K.; Soundia, A.; Chang, C.C.; Le, V.; Tseng, C.H.; Demer, L.L.; et al. Changes in microarchitecture of atherosclerotic calcification assessed by (18)F-NaF PET and CT after a progressive exercise regimen in hyperlipidemic mice. J. Nucl. Cardiol. 2021, 28, 2207–2214. [Google Scholar] [CrossRef]
- Bartlett, B.; Ludewick, H.P.; Lee, S.; Verma, S.; Francis, R.J.; Dwivedi, G. Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque. Cells 2021, 10, 2573. [Google Scholar] [CrossRef]
- Meester, E.J.; Krenning, B.J.; de Swart, J.; Segbers, M.; Barrett, H.E.; Bernsen, M.R.; Van der Heiden, K.; de Jong, M. Perspectives on Small Animal Radionuclide Imaging; Considerations and Advances in Atherosclerosis. Front. Med. 2019, 6, 39. [Google Scholar] [CrossRef]
- Mayer, M.; Borja, A.J.; Hancin, E.C.; Auslander, T.; Revheim, M.E.; Moghbel, M.C.; Werner, T.J.; Alavi, A.; Rajapakse, C.S. Imaging Atherosclerosis by PET, With Emphasis on the Role of FDG and NaF as Potential Biomarkers for This Disorder. Front. Physiol. 2020, 11, 511391. [Google Scholar] [CrossRef]
- Creager, M.D.; Hohl, T.; Hutcheson, J.D.; Moss, A.J.; Schlotter, F.; Blaser, M.C.; Park, M.A.; Lee, L.H.; Singh, S.A.; Alcaide-Corral, C.J.; et al. (18)F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ.-Cardiovasc. Imaging 2019, 12, e007835. [Google Scholar] [CrossRef]
- Florea, A.; Sigl, J.P.; Morgenroth, A.; Vogg, A.; Sahnoun, S.; Winz, O.H.; Bucerius, J.; Schurgers, L.J.; Mottaghy, F.M. Sodium [(18)F]Fluoride PET Can Efficiently Monitor In Vivo Atherosclerotic Plaque Calcification Progression and Treatment. Cells 2021, 10, 275. [Google Scholar] [CrossRef]
- Davies, J.R.; Izquierdo-Garcia, D.; Rudd, J.H.; Figg, N.; Richards, H.K.; Bird, J.L.; Aigbirhio, F.I.; Davenport, A.P.; Weissberg, P.L.; Fryer, T.D.; et al. FDG-PET can distinguish inflamed from non-inflamed plaque in an animal model of atherosclerosis. Int. J. Cardiovasc. Imaging 2010, 26, 41–48. [Google Scholar] [CrossRef]
- Marzola, M.C.; Saboury, B.; Chondrogiannis, S.; Rampin, L.; Grassetto, G.; Ferretti, A.; Alavi, A.; Rubello, D. Role of FDG PET/CT in investigating the mechanisms underlying atherosclerotic plaque formation and evolution. Rev. Esp. Med. Nucl. Imagen Mol. 2013, 32, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Millon, A.; Canet-Soulas, E.; Boussel, L.; Fayad, Z.; Douek, P. Animal models of atherosclerosis and magnetic resonance imaging for monitoring plaque progression. Vascular 2014, 22, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Choi, Y.; Im, K.C. PET/MRI: Technical Challenges and Recent Advances. Nucl. Med. Mol. Imaging 2016, 50, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.R.; Tarkin, J.M.; Le, E.P.; Sriranjan, R.S.; Corovic, A.; Warburton, E.A.; Rudd, J.H. Integrated cardiovascular assessment of atherosclerosis using PET/MRI. Br. J. Radiol. 2020, 93, 20190921. [Google Scholar] [CrossRef]
- Aizaz, M.; Moonen, R.; van der Pol, J.; Prieto, C.; Botnar, R.M.; Kooi, M.E. PET/MRI of atherosclerosis. Cardiovasc. Diagn. the 2020, 10, 1120–1139. [Google Scholar] [CrossRef]
- Calcagno, C. Dynamic Contrast Enhanced (Dce) Magnetic Resonance Imaging (Mri) of Atherosclerosis; New York University: New York, NY, USA, 2010. [Google Scholar]
- Calcagno, C.; Vucic, E.; Mani, V.; Goldschlager, G.; Fayad, Z.A. Reproducibility of black blood dynamic contrast-enhanced magnetic resonance imaging in aortic plaques of atherosclerotic rabbits. J. Magn. Reson. Imaging 2010, 32, 191–198. [Google Scholar] [CrossRef]
- Vucic, E.; Dickson, S.D.; Calcagno, C.; Rudd, J.H.; Moshier, E.; Hayashi, K.; Mounessa, J.S.; Roytman, M.; Moon, M.J.; Lin, J.; et al. Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC-Cardiovasc. Imag. 2011, 4, 1100–1109. [Google Scholar] [CrossRef]
- Andelovic, K.; Winter, P.; Jakob, P.M.; Bauer, W.R.; Herold, V.; Zernecke, A. Evaluation of Plaque Characteristics and Inflammation Using Magnetic Resonance Imaging. Biomedicines 2021, 9, 185. [Google Scholar] [CrossRef]
- Hur, J.; Park, J.; Kim, Y.J.; Lee, H.J.; Shim, H.S.; Choe, K.O.; Choi, B.W. Use of contrast enhancement and high-resolution 3D black-blood MRI to identify inflammation in atherosclerosis. JACC-Cardiovasc. Imaging 2010, 3, 1127–1135. [Google Scholar] [CrossRef]
- Segers, F.; Ruder, A.V.; Westra, M.M.; Lammers, T.; Dadfar, S.M.; Roemhild, K.; Lam, T.S.; Kooi, M.E.; Cleutjens, K.; Verheyen, F.K.; et al. Magnetic resonance imaging contrast-enhancement with superparamagnetic iron oxide nanoparticles amplifies macrophage foam cell apoptosis in human and murine atherosclerosis. Cardiovasc. Res. 2023, 118, 3346–3359. [Google Scholar] [CrossRef]
- de Vries, M.R.; Quax, P.H. Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr. Opin. Lipidol. 2016, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Parma, L.; Baganha, F.; Quax, P.; de Vries, M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kole, A.; Hui, J.; Zhang, Y.; Mai, J.; Alloosh, M.; Sturek, M.; Cheng, J.X. Fast assessment of lipid content in arteries in vivo by intravascular photoacoustic tomography. Sci. Rep. 2018, 8, 2400. [Google Scholar] [CrossRef] [PubMed]
- Iskander-Rizk, S.; Wu, M.; Springeling, G.; van Beusekom, H.; Mastik, F.; Hekkert, M.T.L.; Beurskens, R.; Hoogendoorn, A.; Hartman, E.; van der Steen, A.; et al. In vivo intravascular photoacoustic imaging of plaque lipid in coronary atherosclerosis. Eurointervention 2019, 15, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Sangha, G.S.; Goergen, C.J. Label-free photoacoustic and ultrasound imaging for murine atherosclerosis characterization. APL Bioeng. 2020, 4, 26102. [Google Scholar] [CrossRef]
- Lin, L.; Xie, Z.; Xu, M.; Wang, Y.; Li, S.; Yang, N.; Gong, X.; Liang, P.; Zhang, X.; Song, L.; et al. IVUS\IVPA hybrid intravascular molecular imaging of angiogenesis in atherosclerotic plaques via RGDfk peptide-targeted nanoprobes. Photoacoustics 2021, 22, 100262. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Z.; Zhong, Y.; Salazar, F.; Xu, C.; Ren, F.; Qu, L.; Wu, A.M.; Dai, H. In vivo NIR-II structured-illumination light-sheet microscopy. Proc. Natl. Acad. Sci. USA 2021, 118, e2023888118. [Google Scholar] [CrossRef]
- Hsu, J.J.; Vedula, V.; Baek, K.I.; Chen, C.; Chen, J.; Chou, M.I.; Lam, J.; Subhedar, S.; Wang, J.; Ding, Y.; et al. Contractile and hemodynamic forces coordinate Notch1b-mediated outflow tract valve formation. JCI Insight 2019, 5, e124460. [Google Scholar] [CrossRef]
- Ding, Y.; Abiri, A.; Abiri, P.; Li, S.; Chang, C.C.; Baek, K.I.; Hsu, J.J.; Sideris, E.; Li, Y.; Lee, J.; et al. Integrating light-sheet imaging with virtual reality to recapitulate developmental cardiac mechanics. JCI Insight 2017, 2, e97180. [Google Scholar] [CrossRef]
- Ding, Y.; Gudapati, V.; Lin, R.; Fei, Y.; Packard, R.; Song, S.; Chang, C.C.; Baek, K.I.; Wang, Z.; Roustaei, M.; et al. Saak Transform-Based Machine Learning for Light-Sheet Imaging of Cardiac Trabeculation. IEEE Trans. Bio-Med. Eng. 2021, 68, 225–235. [Google Scholar] [CrossRef]
- Reimann, C.; Brangsch, J.; Kaufmann, J.O.; Adams, L.C.; Onthank, D.C.; Thone-Reineke, C.; Robinson, S.P.; Hamm, B.; Botnar, R.M.; Makowski, M.R. Dual-probe molecular MRI for the in vivo characterization of atherosclerosis in a mouse model: Simultaneous assessment of plaque inflammation and extracellular-matrix remodeling. Sci. Rep. 2019, 9, 13827. [Google Scholar] [CrossRef]
- Ahmed, M.; Tegnebratt, T.; Tran, T.A.; Lu, L.; Damberg, P.; Gistera, A.; Tarnawski, L.; Bone, D.; Hedin, U.; Eriksson, P.; et al. Molecular Imaging of Inflammation in a Mouse Model of Atherosclerosis Using a Zirconium-89-Labeled Probe. Int. J. Nanomed. 2020, 15, 6137–6152. [Google Scholar] [CrossRef]

| Imaging Technique | Species | Advantages | Disadvantages |
|---|---|---|---|
| µCT | Mouse [15,16,17,18,19,20,21,22,23,24,25,26] Rat [27] Rabbit [23,28,29,30] Pig [31,32] |
|
|
| PET | Mouse [18,19,20,21,22,23,24,25,26,33,34,35,36] Rat [36] Rabbit [23,30,37,38,39] Pig [40] Baboon [41] |
|
|
| MRI | Mouse [34,35,36,42,43,44] Rat [36,45] Rabbit [30,39,46,47] Pig [40] |
|
|
| Photoacoustic Imaging (PAI) | Mouse [48,49,50] Rabbit [51,52] Pig [53] |
|
|
| Light-Sheet Fluorescence Microscopy (LSFM) | Zebrafish [54,55,56,57] Mouse [20,25,56,58,59,60,61,62,63] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Chang, C.-C.; Li, Q.; Tintut, Y.; Hsu, J.J. Advanced Imaging Techniques for Atherosclerosis and Cardiovascular Calcification in Animal Models. J. Cardiovasc. Dev. Dis. 2024, 11, 410. https://doi.org/10.3390/jcdd11120410
Ye L, Chang C-C, Li Q, Tintut Y, Hsu JJ. Advanced Imaging Techniques for Atherosclerosis and Cardiovascular Calcification in Animal Models. Journal of Cardiovascular Development and Disease. 2024; 11(12):410. https://doi.org/10.3390/jcdd11120410
Chicago/Turabian StyleYe, Lifang, Chih-Chiang Chang, Qian Li, Yin Tintut, and Jeffrey J. Hsu. 2024. "Advanced Imaging Techniques for Atherosclerosis and Cardiovascular Calcification in Animal Models" Journal of Cardiovascular Development and Disease 11, no. 12: 410. https://doi.org/10.3390/jcdd11120410
APA StyleYe, L., Chang, C.-C., Li, Q., Tintut, Y., & Hsu, J. J. (2024). Advanced Imaging Techniques for Atherosclerosis and Cardiovascular Calcification in Animal Models. Journal of Cardiovascular Development and Disease, 11(12), 410. https://doi.org/10.3390/jcdd11120410









