Optimizing Postoperative Glucose Management in CABG Patients: Exploring Early Transition to Subcutaneous Insulin
Abstract
1. Introduction
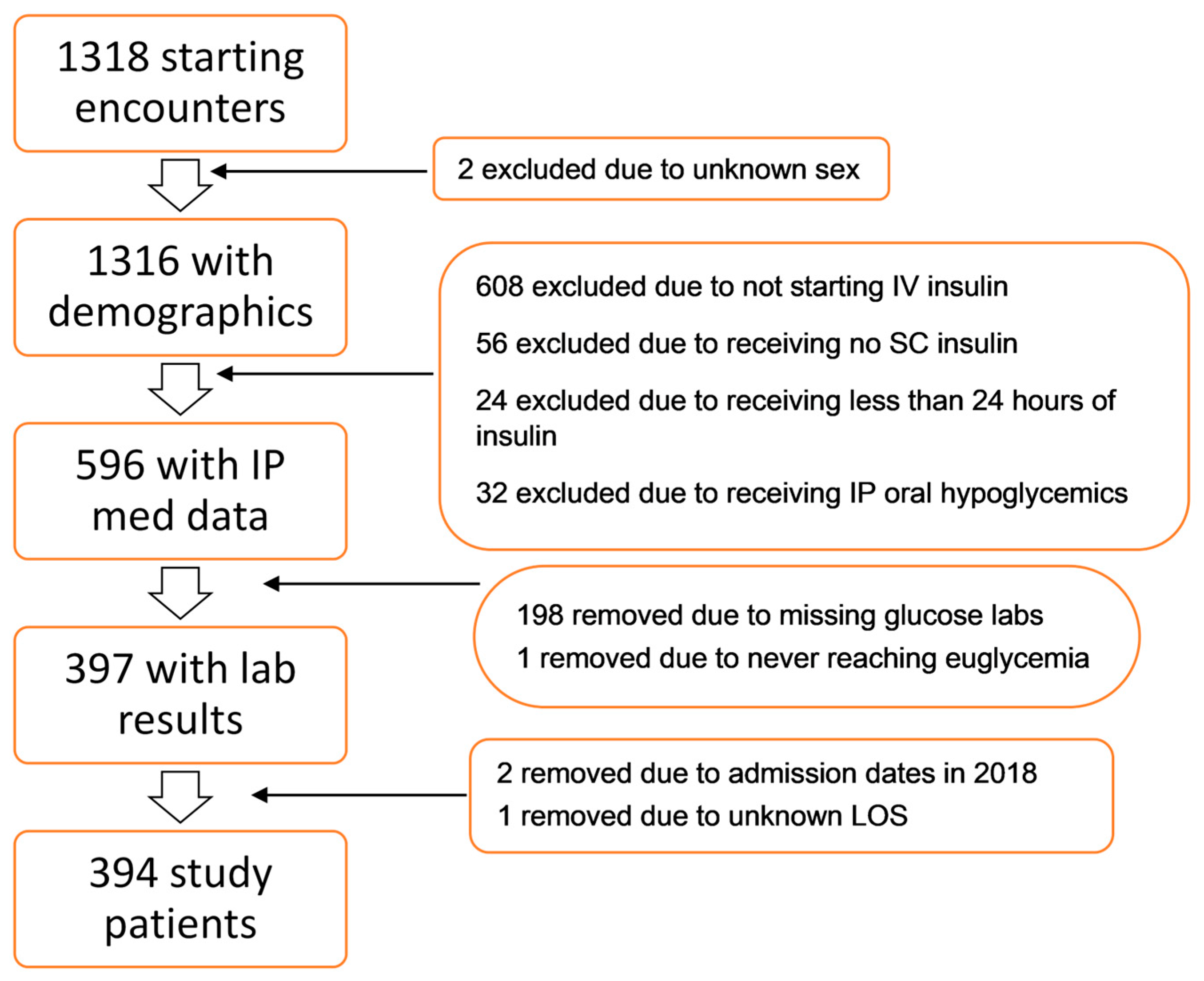
2. Materials and Methods
2.1. Population
2.2. Outcomes Studied
2.3. Statistical Analysis
2.4. Definitions
3. Results
3.1. Population Characteristics
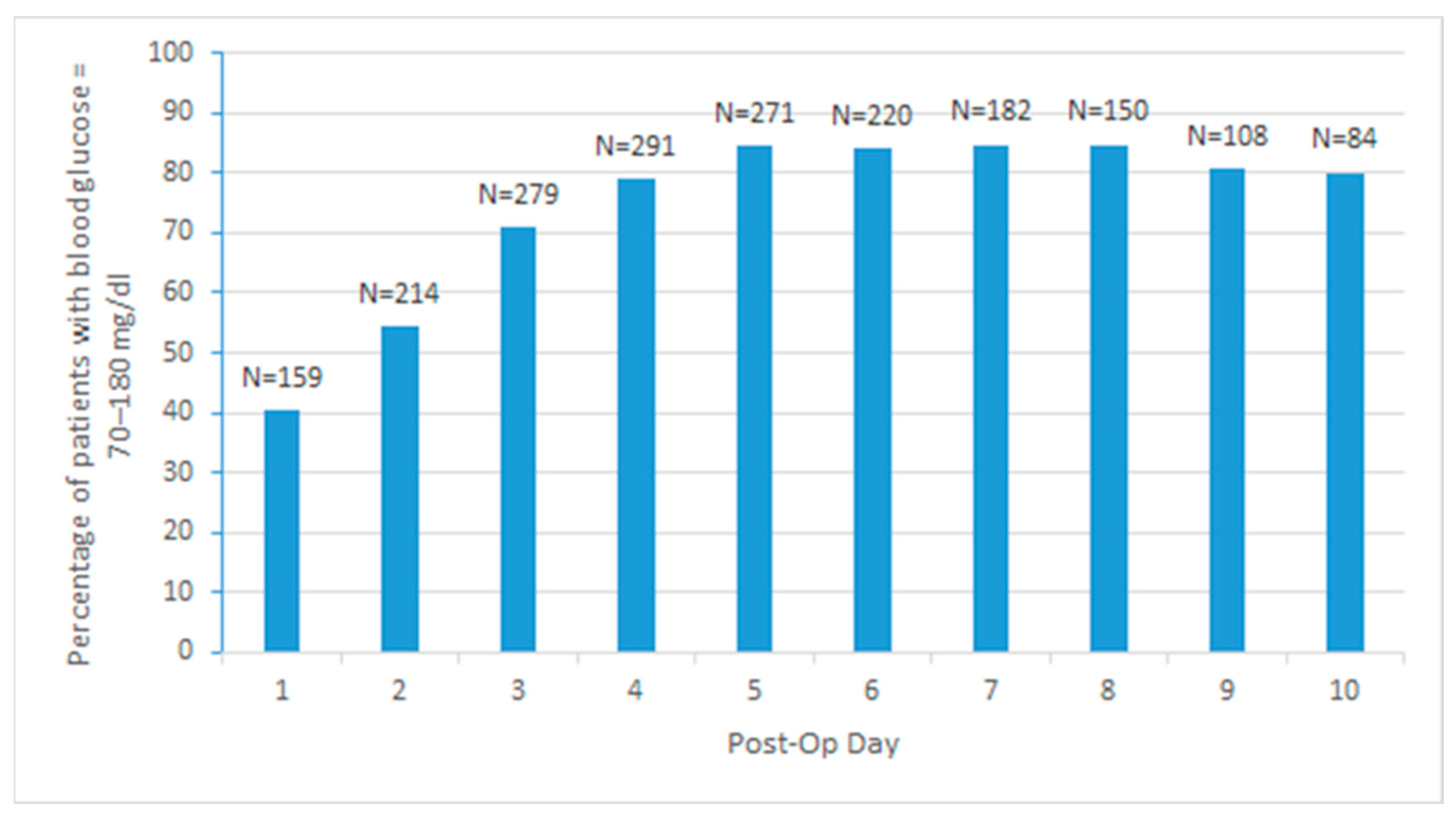
3.2. Glycemic Control
3.3. Transition from IV to SC Insulin During POD1 and the Effect on LOS and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CABG | Coronary artery bypass graft surgery |
| HbA1C | Hemoglobin A1C |
| ICU | Intensive care unit |
| IRB | Institutional Review Board |
| LOS | Length of stay |
| NPO | Nothing by mouth |
| POD1 | Postoperative day 1 |
| SC | Subcutaneous |
| STS | Society of Thoracic Surgeons |
References
- NICE-SUGAR Study Investigators; Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P. Intensive versus conventional glucose control in critically Ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.-L.; Puskas, J.D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Sabik, J.F.; Ainkaran, P.; Blackstone, E.H. Coronary artery bypass grafting in diabetics: A growing health care cost crisis. J. Thorac. Cardiovasc. Surg. 2015, 150, 304–312.e2. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.J.; Fayfman, M.; Umpierrez, G.E. Perioperative Management of Hyperglycemia and Diabetes in Cardiac Surgery Patients. Endocrinol. Metab. Clin. N. Am. 2018, 47, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Duggan, D.W.; Carlson, K.; Umpierrez, G.E. Perioperative Hyperglycemia Management: An Update: Erratum. Anesthesiology 2017, 126, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Ascione, R.; Rogers, C.; Rajakaruna, C.; Angelini, G. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation 2008, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Thiele, R.H.; Hucklenbruch, C.; Ma, J.Z.; Colquhoun, D.; Zuo, Z.; Nemergut, E.C.; Raphael, J. Admission hyperglycemia is associated with poor outcome after emergent coronary bypass grafting surgery. J. Crit. Care 2015, 30, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.L.; McDonnell, M.; Chipkin, S.R.; Furnary, A.P.; Engelman, R.M.; Sadhu, A.R.; Bridges, C.R.; Haan, C.K.; Svedjeholm, R.; Taegtmeyer, H.; et al. The society of thoracic surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann. Thorac. Surg. 2009, 87, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Kreider, K.E.; Lien, L.F. Transitioning safely from intravenous to subcutaneous insulin. Curr. Diabetes Rep. 2015, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.L.; Salm, T.V.; Engelman, R.; Orgill, D.; Gordon, S. Prevention and management of sternal wound infections. J. Thorac. Cardiovasc. Surg. 2016, 152, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.; Debaveye, Y.; Güiza, F.; Dubois, J.; De Bruyn, A.; Dauwe, D.; De Troy, E.; Casaer, M.P.; De Vlieger, G.; Haghedooren, R.; et al. Tight Blood-Glucose Control without Early Parenteral Nutrition in the ICU. N. Engl. J. Med. 2023, 389, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Furnary, A.P.; Gao, G.; Grunkemeier, G.L.; Wu, Y.; Zerr, K.J.; Bookin, S.O.; Floten, H.; Starr, A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2003, 125, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.L.; Chipkin, S.R.; Fitzgerald, C.A.; Bao, Y.; Cabral, H.; Apstein, C.S. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004, 109, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Peterson, E.D.; Albrecht, S.; Li, S.; Hirji, S.A.; Ferguson, T.; Smith, P.K.; Lopes, R.D. Glycemic control in patients undergoing coronary artery bypass graft surgery: Clinical features, predictors, and outcomes. J. Crit. Care 2017, 42, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.L.; McDonnell, M.M.; Chipkin, S.; Fitzgerald, C.; Bliss, C.; Cabral, H. Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Ann. Surg. 2011, 254, 458–464; discussion 463–464. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.Y.; Nuttall, G.A.; Abel, M.D.; Mullany, C.J.; Schaff, H.V.; O’Brien, P.C.; Johnson, M.G.; Williams, A.R.; Cutshall, S.M.; Mundy, L.M.; et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: A randomized trial. Ann. Intern. Med. 2007, 146, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.P.C.; Galas, F.R.B.G.; Hajjar, L.A.; Bello, C.N.; Piccioni, M.A., Jr. Intensive Perioperative Glucose Control Does Not Improve Outcomes of Patients Submitted to Open-Heart Surgery: A Randomized Controlled Trial. Clinics 2009, 64, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Schmeltz, L.R.; DeSantis, A.J.; Thiyagarajan, V.; Schmidt, K.; O’Shea-Mahler, E.; Johnson, D.; Henske, J.; McCarthy, P.M.; Gleason, T.G.; McGee, E.C.; et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care 2007, 30, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, A.; Struemph, K.; Behnen, E.; Schimmelpfennig, J. Pharmacy management of postoperative blood glucose in open heart surgery patients: Evaluation of an intravenous to subcutaneous insulin protocol. Hosp. Pharm. 2014, 49, 164–169. [Google Scholar] [CrossRef] [PubMed]
| Variable | Level | Total | Group 1 (Delayed Transition to SC Insulin) | Group 2 (Early Transition to SC * Insulin) | p-Value |
|---|---|---|---|---|---|
| N (%) | 394 (100.00) | 68 (17.26) | 326 (82.74) | ||
| Sex, N (%) | Male | 102 (25.89) | 18 (26.47) | 84 (25.77) | 0.88 |
| Female | 292 (74.11) | 50 (73.53) | 242 (74.23) | ||
| Race, N (%) | Non-White | 50 (12.69) | 9 (13.24) | 41 (12.58) | 0.88 |
| White | 344 (87.31) | 59 (86.76) | 285 (87.42) | ||
| Diabetes, N (%) | Yes | 175 (44.42) | 30 (44.12) | 145 (44.48) | 0.96 |
| No | 219 (55.58) | 38 (55.88) | 181 (55.52) | ||
| Age years, M (SD) | 67.34 (9.15) | 66.79 (9.34) | 67.45 (9.12) | 0.7 | |
| BMI kg/m2, M (SD) | 31.44 (5.97) | 32.25 (6.89) | 31.27 (5.75) | 0.32 | |
| Creatinine mg/dL, M (SD) | 1.18 (0.65) | 1.19 (0.50) | 1.18 (0.68) | 0.884 | |
| HbA1C ** %, M (SD) | 6.75 (1.59) | 6.94 (1.81) | 6.71 (1.54) | 0.45 |
| Indication for CABG | Total N of Cases | Transitioned by Postoperative Day 1. N (%) | Transitioned Later. N (%) |
|---|---|---|---|
| Valvular Heart Disease | 40 | 35 (87.5%) | 5 (12.5%) |
| Acute Coronary Syndrome (ACS) | 133 | 90 (67.7%) | 43 (32.3%) |
| Ischemic Heart Disease with Triple Vessel Involvement | 221 | 201 (91%) | 20 (9%) |
| Total | Normal BMI ** | Overweight | Obese | Severely Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | N D/C * | N | % | N | % | N | % | N | % | N | % |
| 1 | 0 | 159 | 40.36 | 22 | 61.11 | 66 | 43.42 | 63 | 37.06 | 8 | 22.22 |
| 2 | 0 | 214 | 54.31 | 23 | 63.89 | 90 | 59.21 | 90 | 52.94 | 11 | 30.56 |
| 3 | 0 | 279 | 70.81 | 28 | 77.78 | 114 | 75 | 121 | 71.18 | 16 | 44.44 |
| 4 | 25 | 291 | 78.86 | 26 | 78.79 | 111 | 79.29 | 132 | 81.99 | 22 | 62.86 |
| 5 | 73 | 271 | 84.42 | 22 | 78.57 | 104 | 85.95 | 120 | 86.33 | 25 | 75.76 |
| 6 | 132 | 220 | 83.97 | 18 | 75 | 83 | 86.46 | 95 | 84.82 | 24 | 80 |
| 7 | 179 | 182 | 84.65 | 14 | 73.68 | 68 | 86.08 | 80 | 86.96 | 20 | 80 |
| 8 | 217 | 150 | 84.75 | 12 | 75 | 57 | 86.36 | 63 | 87.5 | 18 | 78.26 |
| 9 | 260 | 108 | 80.6 | 10 | 71.43 | 46 | 83.64 | 40 | 83.33 | 12 | 70.59 |
| 10 | 289 | 84 | 80 | 6 | 60 | 38 | 84.44 | 35 | 87.5 | 5 | 50 |
| Day | Total Patients Discharged | Number of Group 1 Patients Who Were Discharged | Number of Group 2 Patients Who Were Discharged | Total Euglycemic Patients N (%) | Group 1 Euglycemic Patients N (%) | Group 2 Euglycemic Patients N (%) |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 159 (40.36) | 32 (47.06) | 127 (38.96) |
| 2 | 0 | 0 | 0 | 214 (54.31) | 39 (57.35) | 175 (53.68) |
| 3 | 0 | 0 | 0 | 279 (70.81) | 46 (67.65) | 233 (71.47) |
| 4 | 25 | 1 | 24 | 291 (78.86) | 52 (77.61) | 302 (79.14) |
| 5 | 73 | 6 | 67 | 271 (84.42) | 51 (82.26) | 220 (84.94) |
| 6 | 132 | 13 | 119 | 220 (83.97) | 45 (81.82) | 175 (84.54) |
| 7 | 179 | 21 | 158 | 182 (84.65) | 38 (80.85) | 144 (85.71) |
| 8 | 217 | 29 | 188 | 150 (84.75) | 30 (76.92) | 120 (86.96) |
| 9 | 260 | 38 | 222 | 108 (80.60) | 21 (70.00) | 87 (83.35) |
| 10 | 289 | 45 | 244 | 84 (80.00) | 17 (73.91) | 67 (81.71) |
| Outcome | Total | Group 1 (Delayed Transition to SC Insulin) | Group 2 (Early Transition to SC Insulin) | p-Value |
|---|---|---|---|---|
| LOS days, M (SD) | 8.47 (5.78) | 10.43 (7.19) | 8.06 (5.36) | <0.001 |
| ICU LOS days, M (SD) | 7.65 (5.14) | 9.74 (6.75) | 7.22 (4.64) | <0.001 |
| Day1 Avg Glucose mg/dL, M (SD) | 164.06 (13.69) | 163.82 (19.55) | 164.11 (12.16) | 0.9 |
| Postop Avg Glucose mg/dL, M (SD) | 158.19 (15.20) | 155.28 (15.33) | 158.80 (15.13) | 0.19 |
| Maintenance of Euglycemia, N (%) | 98.54 (25.01) | 69.5 (17.65) | 29 (7.36) | <0.05 |
| Mortality, N (%) | 5 (1.27) | 1 (1.47) | 4 (1.23) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzghoul, H.; Weimer, J.; Antigua, A.; Maule, G.; Ismail, M.F.; Althunibat, W.; Reddy, R.; Khan, A.A.; Sher, N.; Meadows, R.; et al. Optimizing Postoperative Glucose Management in CABG Patients: Exploring Early Transition to Subcutaneous Insulin. J. Cardiovasc. Dev. Dis. 2024, 11, 348. https://doi.org/10.3390/jcdd11110348
Alzghoul H, Weimer J, Antigua A, Maule G, Ismail MF, Althunibat W, Reddy R, Khan AA, Sher N, Meadows R, et al. Optimizing Postoperative Glucose Management in CABG Patients: Exploring Early Transition to Subcutaneous Insulin. Journal of Cardiovascular Development and Disease. 2024; 11(11):348. https://doi.org/10.3390/jcdd11110348
Chicago/Turabian StyleAlzghoul, Hamza, Joel Weimer, Abigail Antigua, Geran Maule, Mohamed F. Ismail, Ward Althunibat, Raju Reddy, Abdul Ahad Khan, Nehan Sher, Robyn Meadows, and et al. 2024. "Optimizing Postoperative Glucose Management in CABG Patients: Exploring Early Transition to Subcutaneous Insulin" Journal of Cardiovascular Development and Disease 11, no. 11: 348. https://doi.org/10.3390/jcdd11110348
APA StyleAlzghoul, H., Weimer, J., Antigua, A., Maule, G., Ismail, M. F., Althunibat, W., Reddy, R., Khan, A. A., Sher, N., Meadows, R., & Khan, A. (2024). Optimizing Postoperative Glucose Management in CABG Patients: Exploring Early Transition to Subcutaneous Insulin. Journal of Cardiovascular Development and Disease, 11(11), 348. https://doi.org/10.3390/jcdd11110348







