Distinct Patterns of Smooth Muscle Phenotypic Modulation in Thoracic and Abdominal Aortic Aneurysms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Single-Cell RNA Sequencing Data Analysis
2.3. Human Thoracic and Abdominal Aortic Aneurysm Samples
2.4. Histology and Immunostaining
3. Results
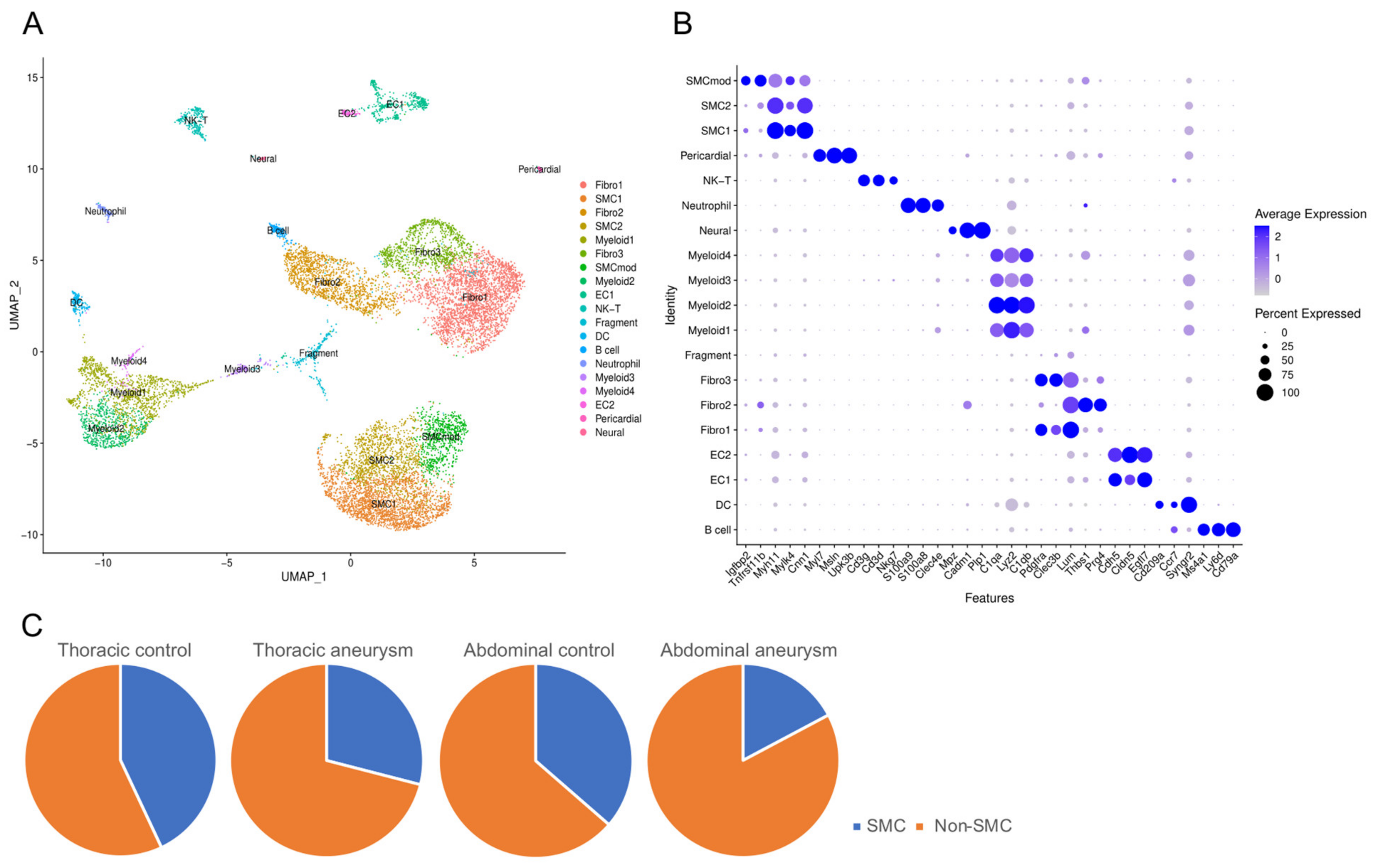
3.1. Single Cell Sequencing Meta-Analysis of Mouse Ascending and Abdominal Aortic Aneurysms
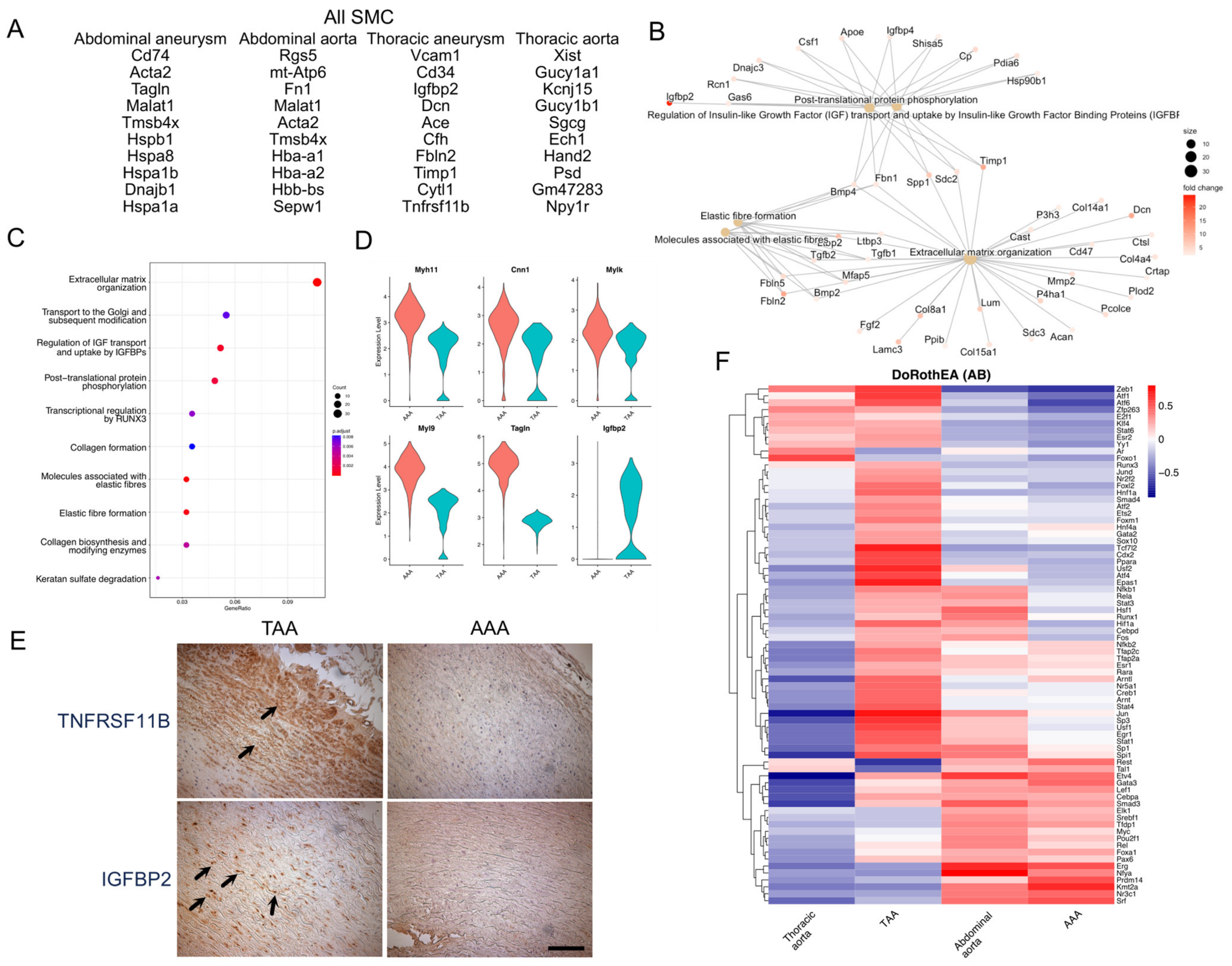
3.2. Phenotypically Modulated SMCs in Thoracic Aortic Aneurysms
3.3. Transcriptional Signatures of TAA and AAA SMCs
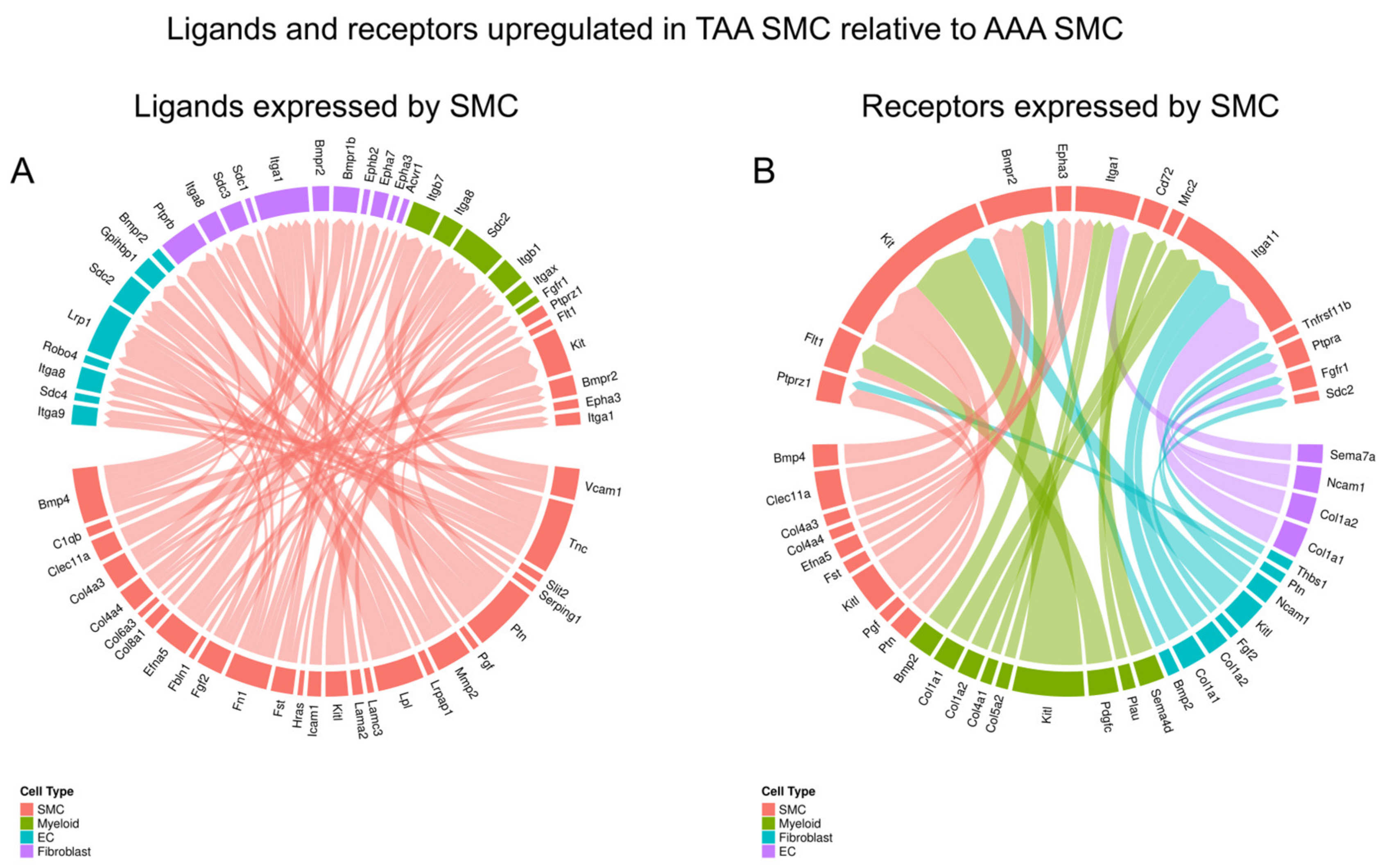
3.4. Cell–Cell Interaction Difference Between TAA and AAA SMCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lin, C.-J.; Lin, C.-Y.; Stitziel, N.O. Genetics of the extracellular matrix in aortic aneurysmal diseases. Matrix Biol. 2018, 71–72, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M. Thoracic and abdominal aortic aneurysms. Circulation 2005, 111, 816–828. [Google Scholar] [CrossRef]
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Absi, T.S.; Sundt, T.M., III; Tung, W.S.; Moon, M.; Lee, J.K.; Damiano, R.R.; Thompson, R.W. Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: Complementary DNA expression profiling in the molecular characterization of aortic disease. J. Thorac. Cardiovasc. Surg. 2003, 126, 344–357, discussion 357. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Chappell, J.; Raffort, J.; Lareyre, F.; Vandestienne, M.; Taylor, A.L.; Finigan, A.; Harrison, J.; Bennett, M.R.; Bruneval, P.; et al. Vascular Smooth Muscle Cell Plasticity and Autophagy in Dissecting Aortic Aneurysms. Arter. Thromb. Vasc. Biol. 2019, 39, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Qin, L.; Li, G.; Malagon-Lopez, J.; Wang, Z.; Bergaya, S.; Gujja, S.; Caulk, A.W.; Murtada, S.-I.; Zhang, X.; et al. Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell 2020, 26, 542–557.e11. [Google Scholar] [CrossRef]
- Pedroza, A.J.; Tashima, Y.; Shad, R.; Cheng, P.; Wirka, R.; Churovich, S.; Nakamura, K.; Yokoyama, N.; Cui, J.Z.; Iosef, C.; et al. Single-Cell Transcriptomic Profiling of Vascular Smooth Muscle Cell Phenotype Modulation in Marfan Syndrome Aortic Aneurysm. Arter. Thromb. Vasc. Biol. 2020, 40, 2195–2211. [Google Scholar] [CrossRef]
- Zhao, G.; Lu, H.; Chang, Z.; Zhao, Y.; Zhu, T.; Chang, L.; Guo, Y.; Garcia-Barrio, M.T.; Chen, Y.E.; Zhang, J. Single-cell RNA sequencing reveals the cellular heterogeneity of aneurysmal infrarenal abdominal aorta. Cardiovasc. Res. 2021, 117, 1402–1416. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, T.; Stranz, A.; DeRoo, E.; Liu, B. Single-Cell RNA Sequencing Reveals Heterogeneity of Vascular Cells in Early Stage Murine Abdominal Aortic Aneurysm—Brief Report. Arter. Thromb. Vasc. Biol. 2021, 41, 1158–1166. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Holland, C.H.; Ibrahim, M.M.; Turei, D.; Saez-Rodriguez, J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 2019, 29, 1363–1375. [Google Scholar] [CrossRef]
- Raredon, M.S.B.; Yang, J.; Garritano, J.; Wang, M.; Kushnir, D.; Schupp, J.C.; Adams, T.S.; Greaney, A.M.; Leiby, K.L.; Kaminski, N.; et al. Computation and visualization of cell–cell signaling topologies in single-cell systems data using Connectome. Sci. Rep. 2022, 12, 4187. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.H.; Tanevski, J.; Perales-Paton, J.; Gleixner, J.; Kumar, M.P.; Mereu, E.; Joughin, B.A.; Stegle, O.; Lauffenburger, D.A.; Heyn, H.; et al. Robustness and applicability of transcription factor and pathway analysis tools on single-cell RNA-seq data. Genome Biol. 2020, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Du, W.; Ren, L.; Hamblin, M.H.; Becker, R.C.; Chen, Y.E.; Fan, Y. Vascular Smooth Muscle Cells in Aortic Aneurysm: From Genetics to Mechanisms. J. Am. Heart Assoc. 2021, 10, e023601. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Moehle, C.W.; Pei, H.; Walton, S.P.; Yang, Z.; Kron, I.L.; Lau, C.L.; Owens, G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009, 138, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Mecham, R.P. Tissue-specific smooth muscle cell subtypes identified by transcriptional profiling. Int. J. Biochem. Cell Biol. 2021, 139, 106055. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Dawson, A.; Vasquez, H.G.; Ageedi, W.; Zhang, C.; Luo, W.; Chen, R.; Li, Y.; Kim, S.; et al. Single-Cell Transcriptome Analysis Reveals Dynamic Cell Populations and Differential Gene Expression Patterns in Control and Aneurysmal Human Aortic Tissue. Circulation 2020, 142, 1374–1388. [Google Scholar] [CrossRef]
- Thijssen, C.G.; Dekker, S.; Bons, L.R.; Geenen, L.W.; Gökalp, A.L.; Takkenberg, J.J.; Mokhles, M.M.; Bekkers, J.A.; Boersma, E.; Bouwens, E.; et al. Novel biomarkers associated with thoracic aortic disease. Int. J. Cardiol. 2023, 378, 115–122. [Google Scholar] [CrossRef]
- Saddic, L.; Orosco, A.; Guo, D.; Milewicz, D.M.; Troxlair, D.; Heide, R.V.; Herrington, D.; Wang, Y.; Azizzadeh, A.; Parker, S.J. Proteomic analysis of descending thoracic aorta identifies unique and universal signatures of aneurysm and dissection. JVS Vasc. Sci. 2022, 3, 85–181. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Staiculescu, M.C.; Cocciolone, A.J.; Procknow, J.D.; Kim, J.; Wagenseil, J.E. Comparative gene array analyses of severe elastic fiber defects in late embryonic and newborn mouse aorta. Physiol. Genom. 2018, 50, 988–1001. [Google Scholar] [CrossRef]
- Tsuruda, T.; Yamashita, A.; Otsu, M.; Koide, M.; Nakamichi, Y.; Sekita-Hatakeyama, Y.; Hatakeyama, K.; Funamoto, T.; Chosa, E.; Asada, Y.; et al. Angiotensin II Induces Aortic Rupture and Dissection in Osteoprotegerin-Deficient Mice. J. Am. Heart Assoc. 2022, 11, e025336. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Mecham, R.P. The Vascular Smooth Muscle Cell: Molecular and Biological Responses to the Extracellular Matrix; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Kumar, M.S.; Owens, G.K. Combinatorial control of smooth muscle–specific gene expression. Arter. Thromb. Vasc. Biol. 2003, 23, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; Lu, H.; Hornsby, W.E.; Crone, B.; Wang, G.T.; Guo, D.-C.; Sendamarai, A.K.; Devineni, P.; Lin, M.; Zhou, W.; et al. Regulatory variants in TCF7L2 are associated with thoracic aortic aneurysm. Am. J. Hum. Genet. 2021, 108, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Tromp, G.; Kuivaniemi, H.; Gretarsdottir, S.; Baas, A.F.; Giusti, B.; Strauss, E.; Hof, F.N.V.; Webb, T.R.; Erdman, R.; et al. Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ. Res. 2017, 120, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Davis, E.C.; Chapman, S.L.; Budatha, M.; Marmorstein, L.Y.; Word, R.A.; Yanagisawa, H. Fibulin-4 deficiency results in ascending aortic aneurysms: A potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 2010, 106, 583–592. [Google Scholar] [CrossRef]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef]
- Lin, C.J.; Keating, C.; Roth, R.; Zayed, M.; Lin, C.Y.; Mecham, R.P. Distinct patterns of smooth muscle phenotypic modulation in thoracic and abdominal aortic aneurysm. J. Am. Coll. Cardiol. 2023, 81 (Suppl. S8), 2021. [Google Scholar] [CrossRef]

| Tissue | TAA | AAA | AAA |
|---|---|---|---|
| Study | Pedroza [7] | Zhao [8] | Yang [9] |
| GEO accession | GSE153534 | GSE152583 | GSE164678 |
| Model | Fbn1C1041G/+ | Elastase 14 days | CaCl2 4 days |
| Control age | 24 weeks | 10 weeks | 12 weeks |
| Gender | Mixed in control, male in aneurysm | Male | Male |
| Isolation method | Enzymatic | Enzymatic | Enzymatic |
| Platform | 10X Chromium | 10X Chromium | 10X Chromium |
| Number of cells (aneurysm/control) | 3765/2823 | 1400/1392 | 1216/2035 |
| Tissue | TAA | AAA |
|---|---|---|
| Demographics | 21-year-old male, Marfan syndrome 22-year-old male, Marfan syndrome 36-year-old male, Marfan syndrome 65-year-old male, nonsyndromic 46-year-old male, bicuspid aortic valve | 52-year-old male 72-year-old female 62-year-old male |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-J.; Keating, C.; Roth, R.; Caliskan, Y.; Nazzal, M.; Exil, V.; DiPaolo, R.; Verma, D.R.; Harjai, K.; Zayed, M.; et al. Distinct Patterns of Smooth Muscle Phenotypic Modulation in Thoracic and Abdominal Aortic Aneurysms. J. Cardiovasc. Dev. Dis. 2024, 11, 349. https://doi.org/10.3390/jcdd11110349
Lin C-J, Keating C, Roth R, Caliskan Y, Nazzal M, Exil V, DiPaolo R, Verma DR, Harjai K, Zayed M, et al. Distinct Patterns of Smooth Muscle Phenotypic Modulation in Thoracic and Abdominal Aortic Aneurysms. Journal of Cardiovascular Development and Disease. 2024; 11(11):349. https://doi.org/10.3390/jcdd11110349
Chicago/Turabian StyleLin, Chien-Jung, Campbell Keating, Robyn Roth, Yasar Caliskan, Mustafa Nazzal, Vernat Exil, Richard DiPaolo, Divya Ratan Verma, Kishore Harjai, Mohamed Zayed, and et al. 2024. "Distinct Patterns of Smooth Muscle Phenotypic Modulation in Thoracic and Abdominal Aortic Aneurysms" Journal of Cardiovascular Development and Disease 11, no. 11: 349. https://doi.org/10.3390/jcdd11110349
APA StyleLin, C.-J., Keating, C., Roth, R., Caliskan, Y., Nazzal, M., Exil, V., DiPaolo, R., Verma, D. R., Harjai, K., Zayed, M., Lin, C.-Y., Mecham, R. P., & Jain, A. K. (2024). Distinct Patterns of Smooth Muscle Phenotypic Modulation in Thoracic and Abdominal Aortic Aneurysms. Journal of Cardiovascular Development and Disease, 11(11), 349. https://doi.org/10.3390/jcdd11110349






