Nicotine Potentially Alters Endothelial Inflammation and Cell Adhesion via LGALS9
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
2.2. Plate Coating
2.3. Experimental Conditions
2.4. RNA Isolation and Complementary DNA Synthesis
2.5. Library Preparation and Sequencing
2.6. Sequencing Data Analysis
2.7. RT-qPCR
2.8. In Silico Gene Interaction Analysis
2.9. Statistics
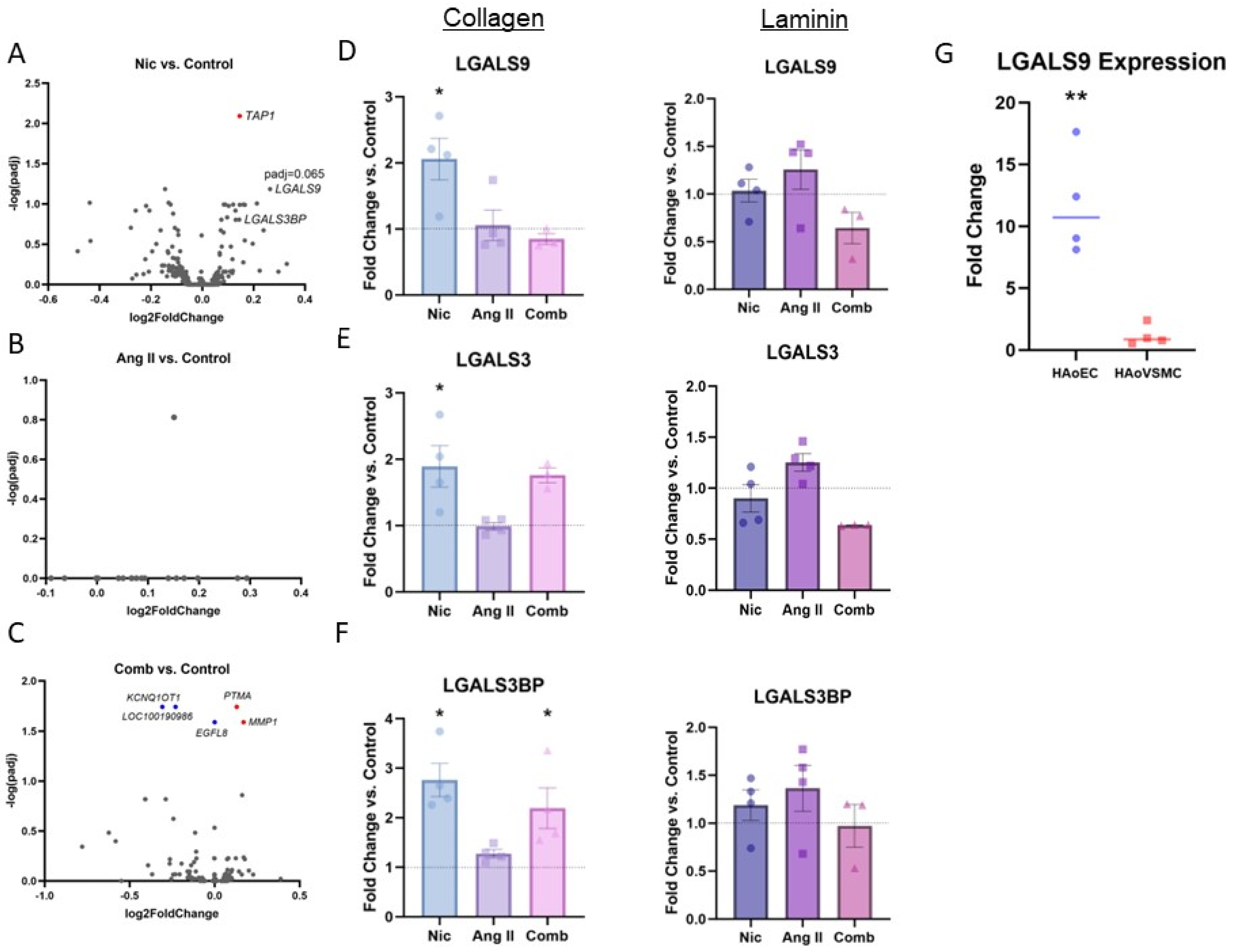
3. Results
3.1. RNA-Sequencing Analysis
3.2. RT-qPCR
3.3. In Silico Gene Interacton Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial Function and Dysfunction: Testing and Clinical Relevance: Testing and Clinical Relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of Endothelial Activation and Dysfunction in Cardiovascular Diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, E.; Stranz, A.; Yang, H.; Hsieh, M.; Se, C.; Zhou, T. Endothelial Dysfunction in the Pathogenesis of Abdominal Aortic Aneurysm. Biomolecules 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Seta, F.; Cohen, R.A. The Endothelium: Paracrine Mediator of Aortic Dissection. Circulation 2014, 129, 2629–2632. [Google Scholar] [CrossRef]
- Whitehead, A.K.; Erwin, A.P.; Yue, X. Nicotine and Vascular Dysfunction. Acta Physiol. 2021, 231, e13631. [Google Scholar] [CrossRef]
- Braß, S.M.; Wagenhäuser, M.U.; Simon, F.; Schelzig, H.; Mulorz, J. Elektrische Zigaretten—Stand der Forschung aus gefäßmedizinischer Sicht. Gefasschirurgie 2023, 28, 140–144. [Google Scholar] [CrossRef]
- Wagenhäuser, M.U.; Schellinger, I.N.; Yoshino, T.; Toyama, K.; Kayama, Y.; Deng, A.; Guenther, S.P.; Petzold, A.; Mulorz, J.; Mulorz, P.; et al. Chronic Nicotine Exposure Induces Murine Aortic Remodeling and Stiffness Segmentation-Implications for Abdominal Aortic Aneurysm Susceptibility. Front. Physiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Kobeissi, E.; Hibino, M.; Pan, H.; Aune, D. Blood Pressure, Hypertension and the Risk of Abdominal Aortic Aneurysms: A Systematic Review and Meta-Analysis of Cohort Studies. Eur. J. Epidemiol. 2019, 34, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Otaki, Y.; Kobeissi, E.; Pan, H.; Hibino, H.; Taddese, H.; Majeed, A.; Verma, S.; Konta, T.; Yamagata, K.; et al. Blood Pressure, Hypertension, and the Risk of Aortic Dissection Incidence and Mortality: Results from the J-SCH Study, the UK Biobank Study, and a Meta-Analysis of Cohort Studies. Circulation 2022, 145, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Metsärinne, K.; Tikkanen, I. Role of Angiotensin II in Blood Pressure Regulation and in the Pathophysiology of Cardiovascular Disorders. J. Hum. Hypertens. 1995, 9 (Suppl. 5), S19–S24. [Google Scholar] [PubMed]
- Catt, K.J.; Cran, E.; Zimmet, P.Z.; Best, J.B.; Cain, M.D.; Coghlan, J.P. Angiotensin II Blood-Levels in Human Hypertension. Lancet 1971, 1, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, L.; Li, Z.; Xiao, D.; Zhang, Y.; Zhang, H.; Mokembo, J.N.; Monayo, S.M.; Jha, N.K.; Kopylov, P.; et al. Endothelial to Mesenchymal Transition Contributes to Nicotine-Induced Atherosclerosis. Theranostics 2020, 10, 5276–5289. [Google Scholar] [CrossRef]
- Mulorz, J.; Ibing, W.; Cappallo, M.; Braß, S.M.; Takeuchi, K.; Raaz, U.; Schellinger, I.N.; Krott, K.J.; Schelzig, H.; Aubin, H.; et al. Ethanol Enhances Endothelial Rigidity by Targeting VE-Cadherin-Implications for Acute Aortic Dissection. J. Clin. Med. 2023, 12, 4967. [Google Scholar] [CrossRef]
- Miyao, M.; Cicalese, S.; Kawai, T.; Cooper, H.A.; Boyer, M.J.; Elliott, K.J.; Forrester, S.J.; Kuroda, R.; Rizzo, V.; Hashimoto, T.; et al. Involvement of Senescence and Mitochondrial Fission in Endothelial Cell Pro-Inflammatory Phenotype Induced by Angiotensin II. Int. J. Mol. Sci. 2020, 21, 3112. [Google Scholar] [CrossRef]
- Nassar, L.R.; Barber, G.P.; Benet-Pagès, A.; Casper, J.; Clawson, H.; Diekhans, M.; Fischer, C.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, B.T.; et al. The UCSC Genome Browser Database: 2023 Update. Nucleic Acids Res. 2023, 51, D1188–D1195. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA Prediction Server: Biological Network Integration for Gene Prioritization and Predicting Gene Function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.C.O. Venny 2.1.0. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 15 July 2023).
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and Function of a Large Family of Animal Lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Thijssen, V.L. Galectins in Tumor Angiogenesis. Ann. Transl. Med. 2014, 2, 90. [Google Scholar] [CrossRef]
- Wada, J.; Kanwar, Y.S. Identification and Characterization of Galectin-9, a Novel Beta-Galactoside-Binding Mammalian Lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef]
- Manero-Rupérez, N.; Martínez-Bosch, N.; Barranco, L.E.; Visa, L.; Navarro, P. The Galectin Family as Molecular Targets: Hopes for Defeating Pancreatic Cancer. Cells 2020, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.J.; Krautter, F.; Blacksell, I.A.; Wright, R.D.; Austin-Williams, S.N.; Voisin, M.-B.; Hussain, M.T.; Law, H.L.; Niki, T.; Hirashima, M.; et al. Galectin-9 Mediates Neutrophil Capture and Adhesion in a CD44 and Β2 Integrin-Dependent Manner. FASEB J. 2022, 36, e22065. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin Expression in Cancer Diagnosis and Prognosis: A Systematic Review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Hulsmans, S.; Griffioen, A.W. The Galectin Profile of the Endothelium: Altered Expression and Localization in Activated and Tumor Endothelial Cells. Am. J. Pathol. 2008, 172, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Rabinovich, G.A. Galectins: Regulators of Acute and Chronic Inflammation: Galectins and Inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef]
- He, X.-W.; Li, W.-L.; Li, C.; Liu, P.; Shen, Y.-G.; Zhu, M.; Jin, X.-P. Serum Levels of Galectin-1, Galectin-3, and Galectin-9 Are Associated with Large Artery Atherosclerotic Stroke. Sci. Rep. 2017, 7, 40994. [Google Scholar] [CrossRef]
- Kremers, B.M.M.; Posma, J.N.; Heitmeier, S.; Glunz, J.; Ten Cate, H.; Pallares Robles, A.; Daemen, J.H.C.; Ten Cate-Hoek, A.J.; Mees, B.M.E.; Spronk, H.M.H. Discovery of Four Plasmatic Biomarkers Potentially Predicting Cardiovascular Outcome in Peripheral Artery Disease. Sci. Rep. 2022, 12, 18388. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, C.; Tang, H.; Zeng, Q.; Wang, X.; Zhu, Z.; Liu, Y.; Mao, X.; Zhong, Y. Serum Galectin-9 Levels Are Associated with Coronary Artery Disease in Chinese Individuals. Mediat. Inflamm. 2015, 2015, 457167. [Google Scholar] [CrossRef]
- Krautter, F.; Hussain, M.T.; Zhi, Z.; Lezama, D.R.; Manning, J.E.; Brown, E.; Marigliano, N.; Raucci, F.; Recio, C.; Chimen, M.; et al. Galectin-9: A Novel Promoter of Atherosclerosis Progression. Atherosclerosis 2022, 363, 57–68. [Google Scholar] [CrossRef]
- Yang, K.; Cui, S.; Wang, J.; Xu, T.; Du, H.; Yue, H.; Ye, H.; Guo, J.; Zhang, J.; Li, P.; et al. Early Progression of Abdominal Aortic Aneurysm Is Decelerated by Improved Endothelial Barrier Function via ALDH2-LIN28B-ELK3 Signaling. Adv. Sci. 2023, 10, e2302231. [Google Scholar] [CrossRef]
- Matsumoto, R.; Matsumoto, H.; Seki, M.; Hata, M.; Asano, Y.; Kanegasaki, S.; Stevens, R.L.; Hirashima, M. Human Ecalectin, a Variant of Human Galectin-9, Is a Novel Eosinophil Chemoattractant Produced by T Lymphocytes. J. Biol. Chem. 1998, 273, 16976–16984. [Google Scholar] [CrossRef] [PubMed]
- Krettek, A.; Sukhova, G.K.; Schönbeck, U.; Libby, P. Enhanced Expression of CD44 Variants in Human Atheroma and Abdominal Aortic Aneurysm: Possible Role for a Feedback Loop in Endothelial Cells. Am. J. Pathol. 2004, 165, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Jaskuła, K.; Sacharczuk, M.; Gaciong, Z.; Skiba, D.S. Cardiovascular Effects Mediated by HMMR and CD44. Mediat. Inflamm. 2021, 2021, 4977209. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Staudinger, C.; King, S.L.; Erickson, F.C.; Lau, L.S.; Bernasconi, A.; Luscinskas, F.W.; Perlyn, C.; Dimitroff, C.J. Galectin-9 Bridges Human B Cells to Vascular Endothelium While Programming Regulatory Pathways. J. Autoimmun. 2021, 117, 102575. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.P.; Nguyen, H.N.; Gomez-Rivas, E.; Jeong, Y.; Jonsson, A.H.; Chen, A.F.; Lange, J.K.; Dyer, G.S.; Blazar, P.; Earp, B.E.; et al. SLAMF7 Engagement Superactivates Macrophages in Acute and Chronic Inflammation. Sci. Immunol. 2022, 7, eabf2846. [Google Scholar] [CrossRef] [PubMed]
- van Wanrooij, E.J.A.; van Puijvelde, G.H.M.; de Vos, P.; Yagita, H.; van Berkel, T.J.C.; Kuiper, J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) Pathway Attenuates Atherogenesis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, X.; Shi, Y.; Ding, Y.; Li, X.; Xie, T.; Shi, Z.; Fu, W. Deficiency of ITGAM Attenuates Experimental Abdominal Aortic Aneurysm in Mice. J. Am. Heart Assoc. 2021, 10, e019900. [Google Scholar] [CrossRef]
- Friedrichs, J.; Torkko, J.M.; Helenius, J.; Teräväinen, T.P.; Füllekrug, J.; Muller, D.J.; Simons, K.; Manninen, A. Contributions of Galectin-3 and -9 to Epithelial Cell Adhesion Analyzed by Single Cell Force Spectroscopy. J. Biol. Chem. 2007, 282, 29375–29383. [Google Scholar] [CrossRef]
- Muramatsu, F.; Kidoya, H.; Naito, H.; Hayashi, Y.; Iba, T.; Takakura, N. Plakoglobin Maintains the Integrity of Vascular Endothelial Cell Junctions and Regulates VEGF-Induced Phosphorylation of VE-Cadherin. J. Biochem. 2017, 162, 55–62. [Google Scholar] [CrossRef]
- ’t Hart, D.C.; van der Vlag, J.; Nijenhuis, T. Laminar Flow Substantially Affects the Morphology and Functional Phenotype of Glomerular Endothelial Cells. PLoS ONE 2021, 16, e0251129. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braß, S.M.; Mazrekaj, A.; Mulorz, J.; Ibing, W.; Krott, K.-J.; Takeuchi, K.; Cappallo, M.; Liu, H.-H.; Elvers, M.; Schelzig, H.; et al. Nicotine Potentially Alters Endothelial Inflammation and Cell Adhesion via LGALS9. J. Cardiovasc. Dev. Dis. 2024, 11, 6. https://doi.org/10.3390/jcdd11010006
Braß SM, Mazrekaj A, Mulorz J, Ibing W, Krott K-J, Takeuchi K, Cappallo M, Liu H-H, Elvers M, Schelzig H, et al. Nicotine Potentially Alters Endothelial Inflammation and Cell Adhesion via LGALS9. Journal of Cardiovascular Development and Disease. 2024; 11(1):6. https://doi.org/10.3390/jcdd11010006
Chicago/Turabian StyleBraß, Sönke Maximilian, Agnesa Mazrekaj, Joscha Mulorz, Wiebke Ibing, Kim-Jürgen Krott, Kiku Takeuchi, Melanie Cappallo, Hsiang-Han Liu, Margitta Elvers, Hubert Schelzig, and et al. 2024. "Nicotine Potentially Alters Endothelial Inflammation and Cell Adhesion via LGALS9" Journal of Cardiovascular Development and Disease 11, no. 1: 6. https://doi.org/10.3390/jcdd11010006
APA StyleBraß, S. M., Mazrekaj, A., Mulorz, J., Ibing, W., Krott, K.-J., Takeuchi, K., Cappallo, M., Liu, H.-H., Elvers, M., Schelzig, H., & Wagenhäuser, M. U. (2024). Nicotine Potentially Alters Endothelial Inflammation and Cell Adhesion via LGALS9. Journal of Cardiovascular Development and Disease, 11(1), 6. https://doi.org/10.3390/jcdd11010006





