The Impact of Frailty on Outcomes of Proximal Aortic Aneurysm Surgery: A Nationwide Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Population and Definitions
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes
3.3. Multivariable Analysis in Frail Patients
3.4. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Q.-L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty syndrome: Implications and challenges for health care policy. Risk Manag. Healthc. Policy 2019, 12, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Shinall, M.C., Jr.; Youk, A.; Massarweh, N.N.; Shireman, P.K.; Arya, S.; George, E.L.; Hall, D.E. Association of preoperative frailty and operative stress with mortality after elective vs. emergency surgery. JAMA Netw. Open 2020, 3, e2010358. [Google Scholar] [CrossRef]
- Oakland, K.; Nadler, R.; Cresswell, L.; Jackson, D.; Coughlin, P.A. Systematic review and meta-analysis of the association between frailty and outcome in surgical patients. Ann. R. Coll. Surg. Engl. 2016, 98, 80–85. [Google Scholar] [CrossRef]
- Sepehri, A.; Beggs, T.; Hassan, A.; Rigatto, C.; Shaw-Daigle, C.; Tangri, N.; Arora, R.C. The impact of frailty on outcomes after cardiac surgery: A systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, 3110–3117. [Google Scholar] [CrossRef]
- Lee, D.H.; Buth, K.J.; Martin, B.-J.; Yip, A.M.; Hirsch, G.M. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010, 121, 973–978. [Google Scholar] [CrossRef]
- Iyengar, A.; Goel, N.; Kelly, J.J.; Han, J.; Brown, C.R.; Khurshan, F.; Chen, Z.; Desai, N. Effects of frailty on outcomes and 30-day readmissions after surgical mitral valve replacement. Ann. Thorac. Surg. 2020, 109, 1120–1126. [Google Scholar] [CrossRef]
- Bagnall, N.M.; Faiz, O.; Darzi, A.; Athanasiou, T. What is the utility of preoperative frailty assessment for risk stratification in cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 398–402. [Google Scholar] [CrossRef]
- Neuman, H.B.; Weiss, J.M.; Leverson, G.; O’Connor, E.S.; Greenblatt, D.Y.; Loconte, N.K.; Greenberg, C.C.; Smith, M.A. Predictors of short-term postoperative survival after elective colectomy in colon cancer patients ≥ 80 years of age. Ann. Surg. Oncol. 2013, 20, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Bryson, G.L.; van Walraven, C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: A population-based cohort study. JAMA Surg. 2016, 151, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.T.; Tu, J.V.; Dupuis, J.-Y.; Bader Eddeen, A.; Sun, L.Y. Association of frailty and long-term survival in patients undergoing coronary artery bypass grafting. J. Am. Heart Assoc. 2018, 7, e009882. [Google Scholar] [CrossRef] [PubMed]
- Abrams, C.; Roy, L.; Weiner, J.P. Development and Evaluation of the Johns Hopkins University Risk Adjustment Models for Medicare+Choice Plan Payment; Johns Hopkins University: Baltimore, MD, USA, 2003. [Google Scholar]
- Williams, J.B.; Peterson, E.D.; Zhao, Y.; O’Brien, S.M.; Andersen, N.D.; Miller, D.C.; Chen, E.P.; Hughes, G.C. Contemporary results for proximal aortic replacement in North America. J. Am. Coll. Cardiol. 2012, 60, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl, E.S.; Immer, F.F.; Stalder, M.; Englberger, L.; Eckstein, F.S.; Schmidli, J.; Carrel, T.P. Technical advances improved outcome in patients undergoing surgery of the ascending aorta and/or aortic arch: Ten years experience. Eur. J. Cardiothorac. Surg. 2008, 34, 595–599. [Google Scholar] [CrossRef]
- Ganapathi, A.M.; Englum, B.R.; Hanna, J.M.; Schechter, M.A.; Gaca, J.G.; Hurwitz, L.M.; Hughes, G.C. Frailty and risk in proximal aortic surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 186–191.e1. [Google Scholar] [CrossRef]
- HCUP-US NIS Overview. Available online: https://hcup-us.ahrq.gov/nisoverview.jsp (accessed on 20 December 2023).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Eckart, A.; Hauser, S.I.; Haubitz, S.; Struja, T.; Kutz, A.; Koch, D.; Neeser, O.; Meier, M.A.; Mueller, B.; Schuetz, P. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: Results of a prospective, observational study. BMJ Open 2019, 9, e026923. [Google Scholar] [CrossRef]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Khera, R.; Angraal, S.; Couch, T.; Welsh, J.W.; Nallamothu, B.K.; Girotra, S.; Chan, P.S.; Krumholz, H.M. Adherence to methodological standards in research using the National Inpatient Sample. JAMA 2017, 318, 2011–2018. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; McBurnie, M.A.; Newman, A.; Tracy, R.P.; Kop, W.J.; Hirsch, C.H.; Gottdiener, J.; Fried, L.P. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2002, 162, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Langmann, G.A.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Inflammatory markers and frailty in long-term care residents. J. Am. Geriatr. Soc. 2017, 65, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Dodson, J.A.; Hochman, J.S.; Roe, M.T.; Chen, A.Y.; Chaudhry, S.I.; Katz, S.; Zhong, H.; Radford, M.J.; Udell, J.A.; Bagai, A.; et al. The association of frailty with in-hospital bleeding among older adults with acute myocardial infarction: Insights from the ACTION Registry. JACC Cardiovasc. Interv. 2018, 11, 2287–2296. [Google Scholar] [CrossRef]
- Li, C.; Xu, L.; Guan, C.; Zhao, L.; Luo, C.; Zhou, B.; Zhang, X.; Wang, J.; Zhao, J.; Huang, J.; et al. Malnutrition screening and acute kidney injury in hospitalised patients: A retrospective study over a 5-year period from China. Br. J. Nutr. 2020, 123, 337–346. [Google Scholar] [CrossRef]
- Tkatch, R.; Musich, S.; MacLeod, S.; Alsgaard, K.; Hawkins, K.; Yeh, C.S. Population Health Management for Older Adults: Review of Interventions for Promoting Successful Aging Across the Health Continuum. Gerontol. Geriatr. Med. 2016, 2, 2333721416667877. [Google Scholar] [CrossRef]
- Wanamaker, K.M.; Hirji, S.A.; Del Val, F.R.; Yammine, M.; Lee, J.; McGurk, S.; Shekar, P.; Kaneko, T. Proximal aortic surgery in the elderly population: Is advanced age a contraindication for surgery? J. Thorac. Cardiovasc. Surg. 2019, 157, 53–63. [Google Scholar] [CrossRef]
- Anselmi, A.; Abbate, A.; Girola, F.; Nasso, G.; Biondi-Zoccai, G.G.L.; Possati, G.; Gaudino, M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: A review of evidence. Eur. J. Cardiothorac. Surg. 2004, 25, 304–311. [Google Scholar] [CrossRef]
- Park, C.B.; Suri, R.M.; Burkhart, H.M.; Greason, K.L.; Dearani, J.A.; Schaff, H.V.; Sundt, T.M. Identifying patients at particular risk of injury during repeat sternotomy: Analysis of 2555 cardiac reoperations. J. Thorac. Cardiovasc. Surg. 2010, 140, 1028–1035. [Google Scholar] [CrossRef]
- Chikwe, J.; Toyoda, N.; Anyanwu, A.C.; Itagaki, S.; Egorova, N.N.; Boateng, P.; El-Eshmawi, A.; Adams, D.H. Relation of Mitral Valve Surgery Volume to Repair Rate, Durability, and Survival. J. Am. Coll. Cardiol. 2017. [CrossRef]
- Shuhaiber, J.; Isaacs, A.J.; Sedrakyan, A. The Effect of Center Volume on In-Hospital Mortality After Aortic and Mitral Valve Surgical Procedures: A Population-Based Study. Ann. Thorac. Surg. 2015, 100, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Xirasagar, S.; Tsao, N.-W.; Hwang, Y.-T.; Kuo, N.-W.; Lee, H.-C. Volume-outcome relationships in coronary artery bypass graft surgery patients: 5-year major cardiovascular event outcomes. J. Thorac. Cardiovasc. Surg. 2008, 135, 923–930. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mori, M.; Shioda, K.; Wang, X.; Mangi, A.A.; Yun, J.J.; Darr, U.; Elefteriades, J.A.; Geirsson, A. Perioperative Risk Profiles and Volume-Outcome Relationships in Proximal Thoracic Aortic Surgery. Ann. Thorac. Surg. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.N.; Walston, J.D.; Brummel, N.E.; Deiner, S.; Brown, C.H., 4th; Kennedy, M.; Hurria, A. Frailty for Surgeons: Review of a National Institute on Aging Conference on Frailty for Specialists. J. Am. Coll. Surg. 2015, 221, 1083–1092. [Google Scholar] [CrossRef]
| Non-Frail (n = 5340) | Frail (n = 405) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Mean Age, years (SD) | 61.8 (0.4) | 68.7 (1.3) | <0.001 * |
| Female [N (%)] | 1640 (30.7%) | 165 (40.7%) | <0.05 * |
| Comorbidities [N (%)] | |||
| Atrial Fibrillation | 1295 (24.3%) | 115 (28.4%) | 0.40 |
| Coronary Artery Disease | 235 (4.4%) | 50 (12.3%) | <0.001 * |
| Dyslipidemia | 2430 (45.5%) | 180 (44.4%) | 0.85 |
| Prior Myocardial Infarction | 240 (4.5%) | 40 (9.9%) | 0.03 * |
| Congestive Heart Failure | 1255 (23.5%) | 100 (24.7%) | 0.80 |
| Chronic Pulmonary Disease | 1105 (20.7%) | 125 (30.9%) | 0.04 * |
| Diabetes | 235 (4.4%) | 40 (9.9%) | 0.03 * |
| Hypertension | 3635 (68.1%) | 250 (61.7%) | 0.24 |
| Bicuspid Aortic Valve | 1285 (24.1%) | 45 (11.1%) | <0.01 * |
| Chronic Kidney Disease | 605 (11.3%) | 90 (22.2%) | <0.01 * |
| Prior CABG | 90 (1.7%) | 10 (2.5%) | 0.60 |
| Number of Charlson Comorbidities [N (%)] | |||
| One | 2360 (44.2%) | 75 (18.5%) | <0.001 * |
| Two | 1745 (32.7%) | 120 (29.6%) | |
| Three or more | 1235 (23.1%) | 210 (51.9%) | |
| Frailty-Defining Conditions [N (%)] | |||
| Malnutrition | 0 (0%) | 95 (23.5%) | |
| Dementia | 0 (0%) | 260 (64.2%) | |
| Impaired Vision | 0 (0%) | 0 (0%) | |
| Sacral Ulcer | 0 (0%) | 20 (4.9%) | |
| Urine Incontinence | 0 (0%) | 0 (0%) | |
| Fecal Incontinence | 0 (0%) | 0 (0%) | |
| Weight Loss | 0 (0%) | 10 (2.5%) | |
| Lacking Social Support | 0 (0%) | 20 (4.9%) | |
| Difficulty Walking | 0 (0%) | 25 (6.2%) | |
| Mechanical Fall | 0 (0%) | 10 (2.5%) | |
| Admission Characteristics | |||
| Admission on Weekend | 145 (2.7%) | 35 (8.6%) | <0.01 * |
| Elective Admission | 4425 (83.2%) | 275 (67.9%) | <0.01 * |
| Transfer Status | |||
| Not Transferred | 5060 (94.9%) | 360 (88.9%) | 0.07 |
| Transferred From a Different Acute Care Hospital | 230 (4.3%) | 35 (8.6%) | |
| Transferred From Another Type of Health Facility | 40 (0.8%) | 10 (2.5%) | |
| Non-Frail (n = 5340) | Frail (n = 405) | p-Value | |
|---|---|---|---|
| In-Hospital Outcomes | |||
| MACE [N (%)] | 3265 (61.1%) | 355 (87.7%) | <0.01 * |
| Acute Kidney Injury [N (%)] | 825 (15.4%) | 135 (33.3%) | <0.01 * |
| Complete Heart Block [N (%)] | 330 (6.2%) | 45 (11.1%) | 0.07 |
| Major Bleed [N (%)] | 2985 (55.9%) | 315 (77.8%) | <0.01 * |
| Stroke [N (%)] | 135 (2.5%) | 35 (8.6%) | <0.01 * |
| Death [N (%)] | 130 (2.4%) | 20 (4.9%) | 0.17 |
| Pacemaker Insertion [N (%)] | 130 (2.4%) | 30 (7.4%) | <0.01 * |
| Non-home Discharge [N (%)] | 2895 (54.3%) | 300 (74.1%) | <0.01 * |
| LOS (Mean Days, SE) | 8.3 (0.2) | 17.0 (1.7) | <0.01 * |
| Cost (Mean USD $, SE) | 228,573 (8855) | 419,515 (46,771) | <0.01 * |
| Hospital Factors | |||
| Bed Size [N (%)] | 0.78 | ||
| Small | 405 (7.6%) | 30 (7.4%) | |
| Medium | 835 (15.6%) | 75 (18.5%) | |
| Large | 4100 (76.8%) | 300 (74.1%) | |
| Ownership of Hospital [N (%)] | 0.77 | ||
| Government, Nonfederal | 410 (7.7%) | 30 (7.4%) | |
| Private, Not-for-Profit | 4630 (86.7%) | 345 (85.2%) | |
| Private, Invest Own | 300 (5.6%) | 30 (7.4%) | |
| Teaching Status of Hospital [N (%)] | -- | ||
| Rural | 65 (1.2%) | 0 (0%) | |
| Urban Non-Teaching | 395 (7.4%) | 10 (2.5%) | |
| Urban Teaching | 4880 (91.4%) | 395 (97.5%) | |
| Region of Hospital [N (%)] | 0.82 | ||
| Northeast | 1210 (22.7%) | 100 (24.7%) | |
| Midwest | 1375 (25.7%) | 105 (25.9%) | |
| South | 1725 (32.3%) | 140 (34.6%) | |
| West | 1030 (19.3%) | 60 (14.8%) | |
| Outcome | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Death | 1.40 | 0.43 | 4.50 | 0.575 |
| Stroke | 1.61 | 0.11 | 23.75 | 0.727 |
| Acute Kidney Injury | 2.09 | 1.13 | 3.84 | 0.018 * |
| Pacemaker Insertion | 3.05 | 0.96 | 9.72 | 0.060 |
| Complete Heart Block | 1.55 | 0.65 | 3.71 | 0.325 |
| Major Bleeding | 2.63 | 1.47 | 4.67 | 0.001 * |
| MACE | 4.29 | 1.88 | 9.78 | 0.001 * |
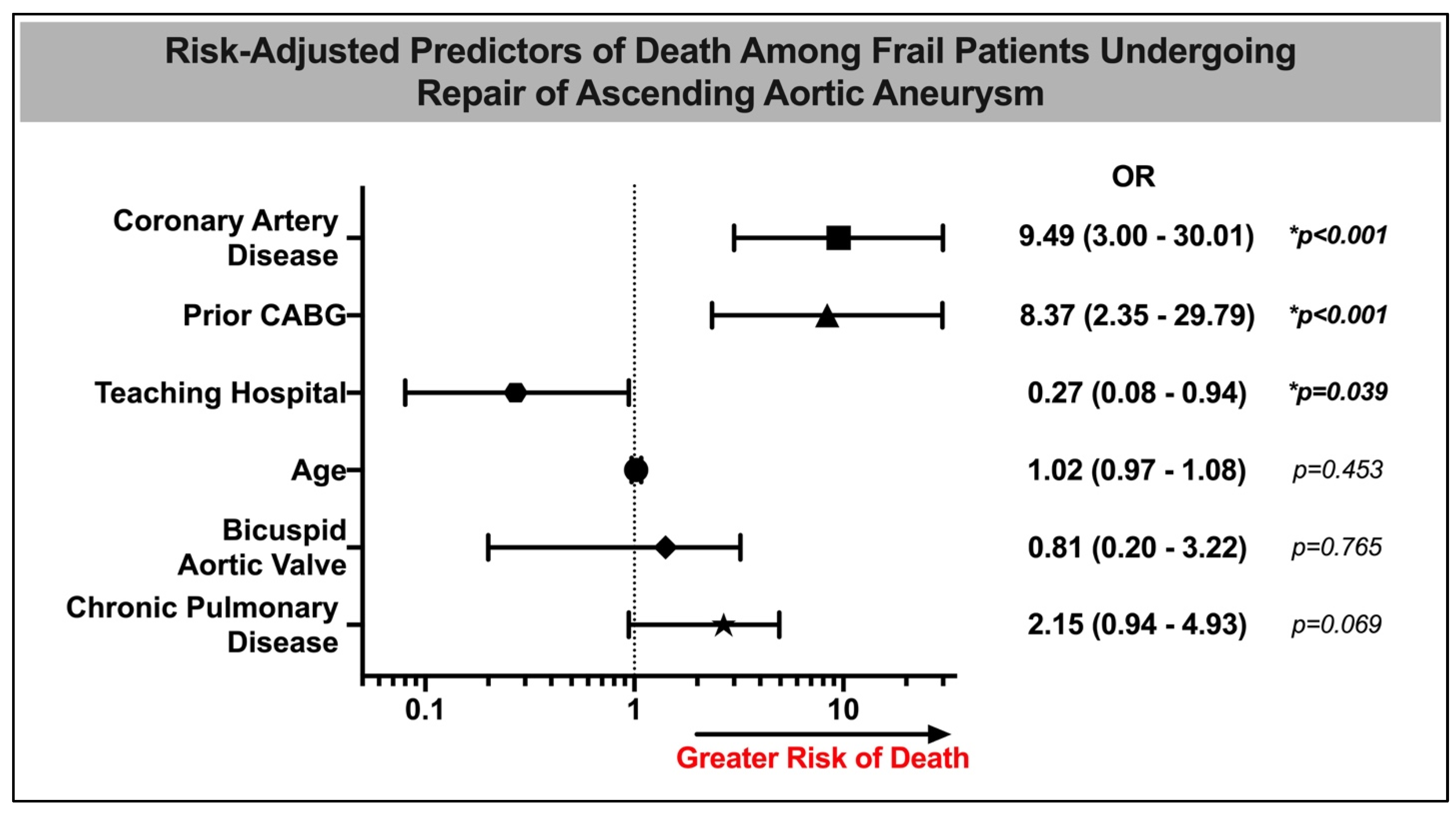
| Variable | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Age | 1.02 | 0.97 | 1.08 | 0.45 |
| Female | 0.47 | 0.18 | 1.26 | 0.13 |
| Dyslipidemia | 0.20 | 0.07 | 0.62 | 0.005 * |
| Bicuspid Aortic Valve | 0.81 | 0.20 | 3.22 | 0.75 |
| Hypertension | 0.60 | 0.18 | 2.04 | 0.41 |
| Chronic Pulmonary Disease | 2.15 | 0.94 | 4.93 | 0.07 |
| Chronic Kidney Disease | 1.95 | 0.43 | 8.77 | 0.38 |
| Coronary Artery Disease | 9.49 | 3.00 | 30.01 | <0.001 * |
| Atrial Fibrillation | 0.80 | 0.29 | 2.20 | 0.66 |
| Congestive Heart Failure | 2.15 | 0.96 | 4.85 | 0.064 |
| Teaching Hospital | 0.27 | 0.08 | 0.94 | 0.039 * |
| Prior CABG | 8.37 | 2.35 | 29.79 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Percy, E.D.; Faggion Vinholo, T.; Newell, P.; Singh, S.; Hirji, S.; Awtry, J.; Semco, R.; Chowdhury, M.; Reed, A.K.; Asokan, S.; et al. The Impact of Frailty on Outcomes of Proximal Aortic Aneurysm Surgery: A Nationwide Analysis. J. Cardiovasc. Dev. Dis. 2024, 11, 32. https://doi.org/10.3390/jcdd11010032
Percy ED, Faggion Vinholo T, Newell P, Singh S, Hirji S, Awtry J, Semco R, Chowdhury M, Reed AK, Asokan S, et al. The Impact of Frailty on Outcomes of Proximal Aortic Aneurysm Surgery: A Nationwide Analysis. Journal of Cardiovascular Development and Disease. 2024; 11(1):32. https://doi.org/10.3390/jcdd11010032
Chicago/Turabian StylePercy, Edward D., Thais Faggion Vinholo, Paige Newell, Supreet Singh, Sameer Hirji, Jake Awtry, Robert Semco, Muntasir Chowdhury, Alexander K. Reed, Sainath Asokan, and et al. 2024. "The Impact of Frailty on Outcomes of Proximal Aortic Aneurysm Surgery: A Nationwide Analysis" Journal of Cardiovascular Development and Disease 11, no. 1: 32. https://doi.org/10.3390/jcdd11010032
APA StylePercy, E. D., Faggion Vinholo, T., Newell, P., Singh, S., Hirji, S., Awtry, J., Semco, R., Chowdhury, M., Reed, A. K., Asokan, S., Malarczyk, A., Okoh, A., Harloff, M., Yazdchi, F., Kaneko, T., & Sabe, A. A. (2024). The Impact of Frailty on Outcomes of Proximal Aortic Aneurysm Surgery: A Nationwide Analysis. Journal of Cardiovascular Development and Disease, 11(1), 32. https://doi.org/10.3390/jcdd11010032






