Computed Tomography Angiography-Derived Scores for Prediction of Chronic Total Occlusion Percutaneous Coronary Intervention Using the Hybrid Algorithm
Abstract
:1. Introduction
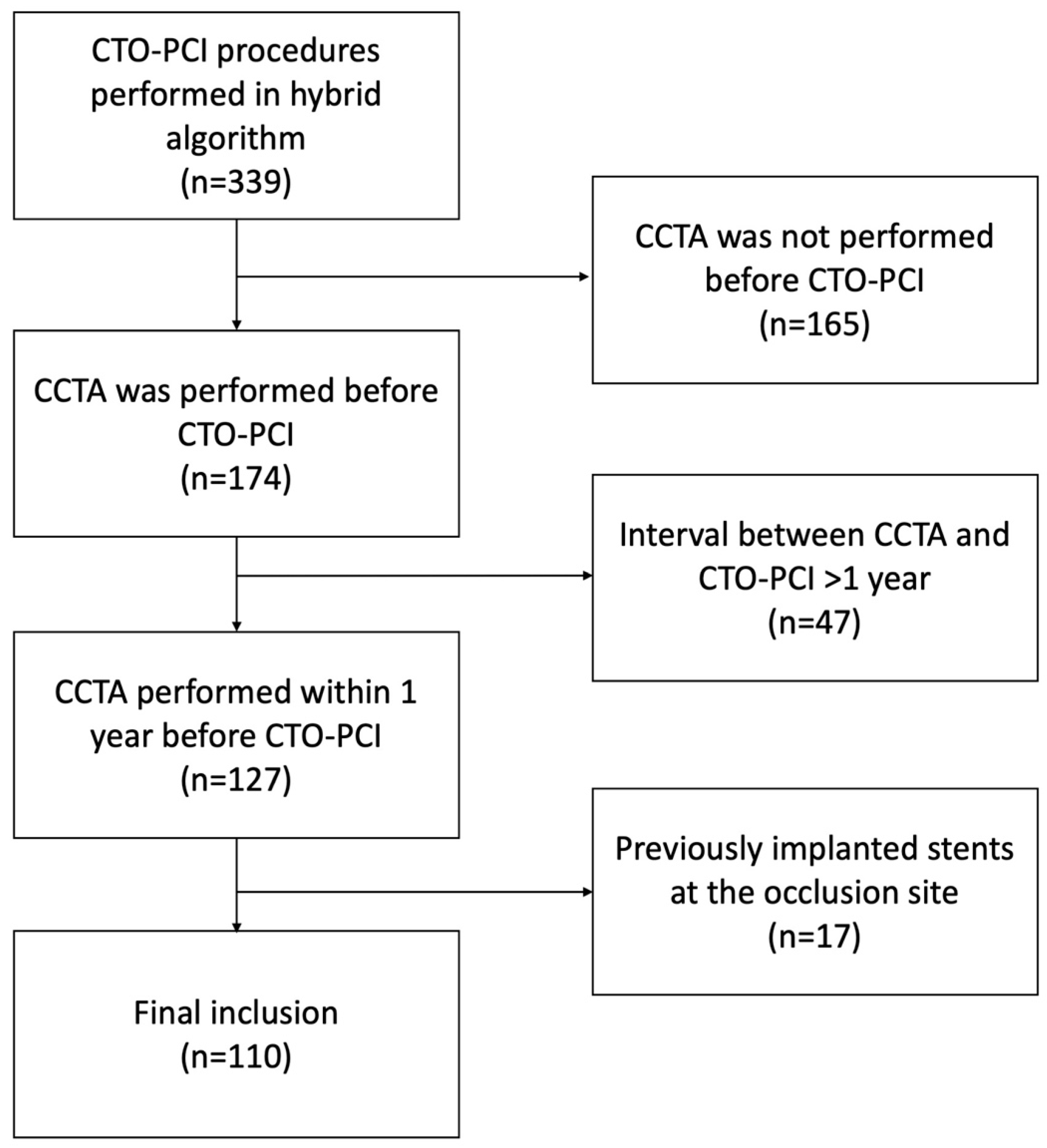
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Endpoints
2.3. CCTA Protocol, Image Reconstruction, and Analysis
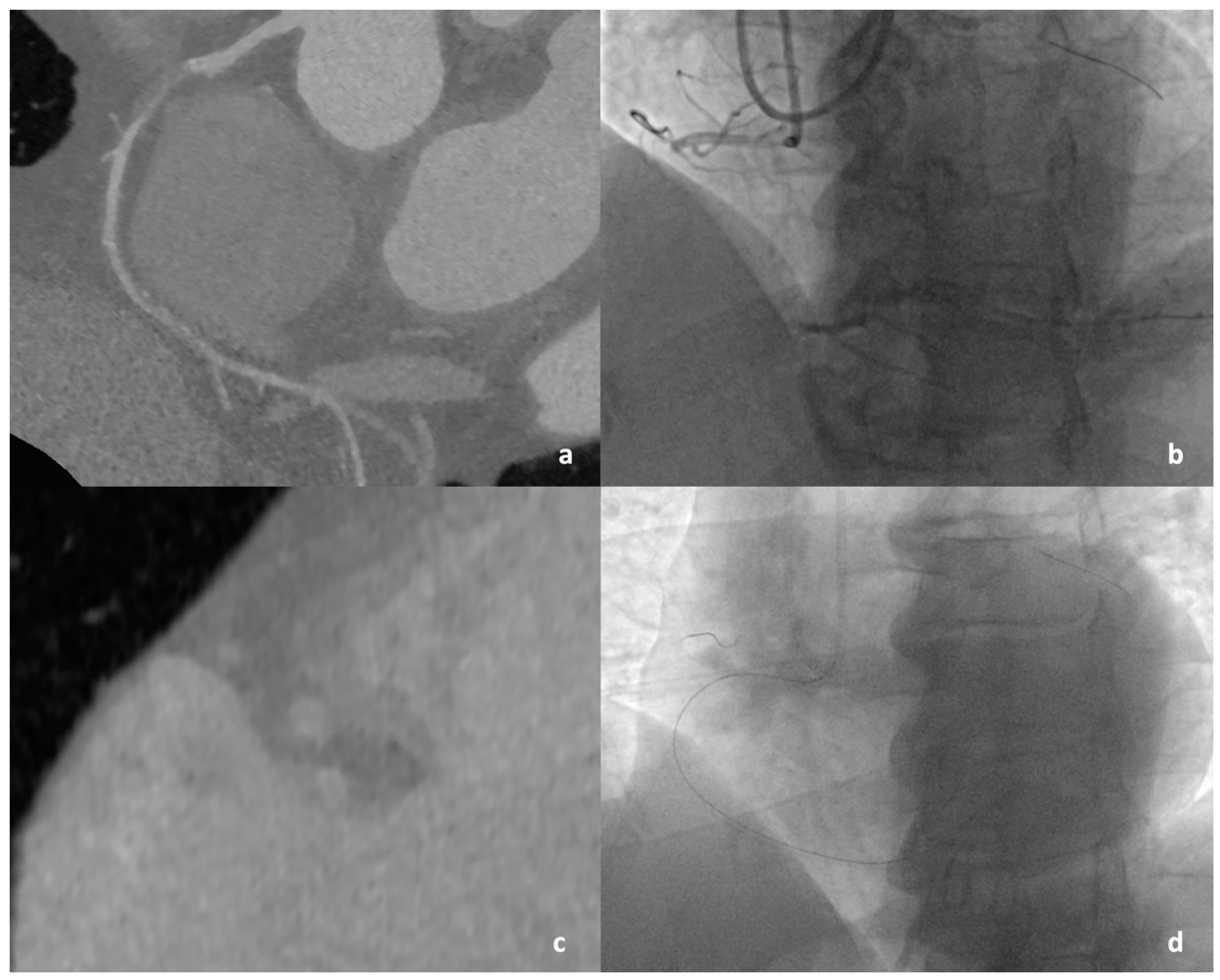
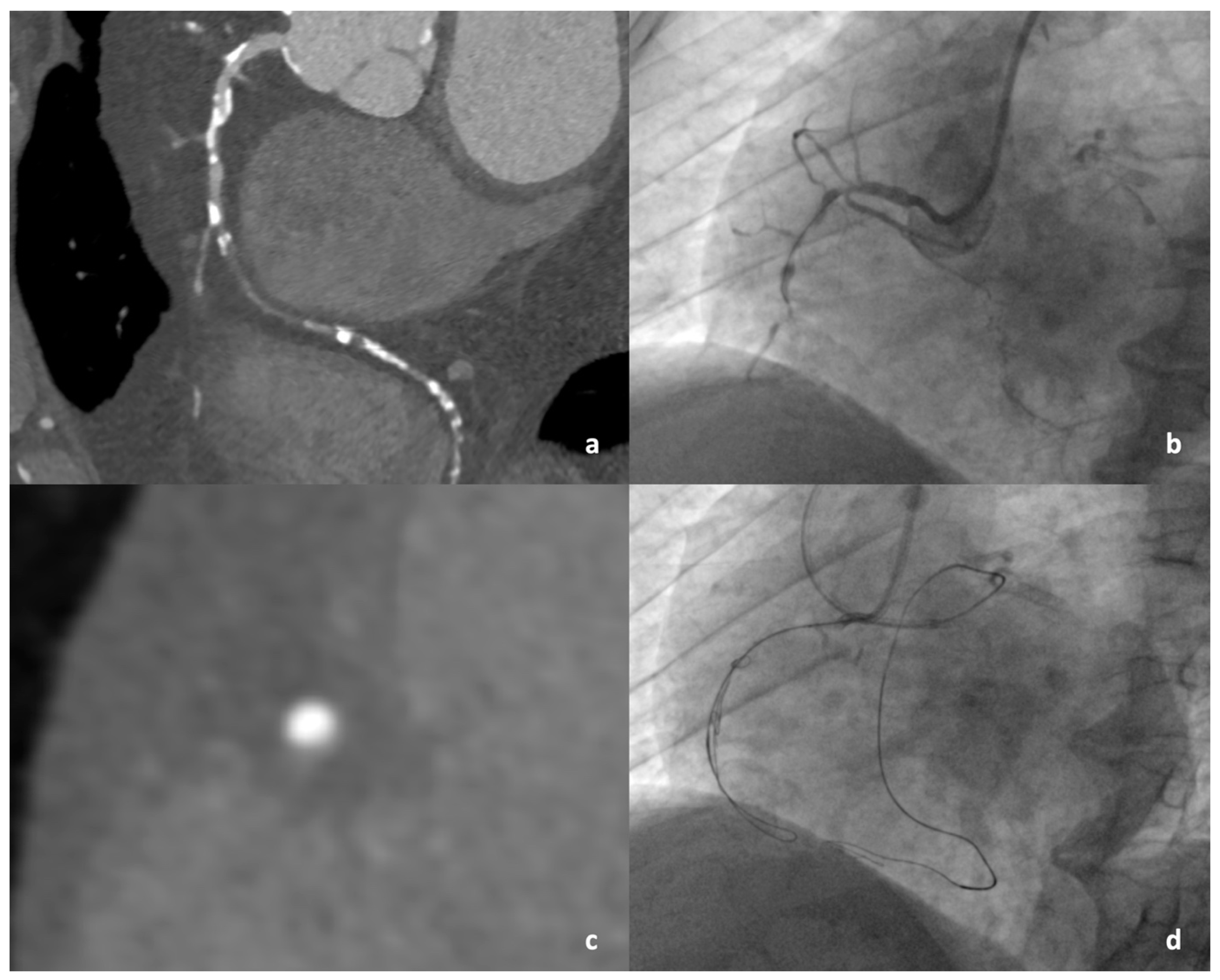
2.4. Coronary Angiography and CTO PCI
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Angiographic and Procedural Characteristics
3.3. Computed Tomographic Characteristics
3.4. Diagnostic Accuracy of CCTA-Derived Scores
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.P.; Driessen, R.S.; Stuijfzand, W.J.; Raijmakers, P.G.; Danad, I.; Dens, J.; Spratt, J.C.; Hanratty, C.G.; Walsh, S.J.; Boellaard, R.; et al. Recovery of myocardial perfusion after percutaneous coronary intervention of chronic total occlusions is comparable to hemodynamically significant non-occlusive lesions. Catheter. Cardiovasc. Interv. 2019, 93, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Cockburn, J.; Clayton, T.C.; Ludman, P.; Cotton, J.; Spratt, J.; Redwood, S.; de Belder, M.; de Belder, A.; Hill, J.; et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: Analysis from the U.K. Central Cardiac Audit Database. J. Am. Coll. Cardiol. 2014, 64, 235–243, Erratum in J. Am. Coll. Cardiol. 2014, 64, 1538. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.J.; Hanratty, C.G.; McEntegart, M.; Strange, J.W.; Rigger, J.; Henriksen, P.A.; Smith, E.J.; Wilson, S.J.; Hill, J.M.; Mehmedbegovic, Z.; et al. Intravascular Healing Is Not Affected by Approaches in Contemporary CTO PCI: The CONSISTENT CTO Study. JACC Cardiovasc. Interv. 2020, 13, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Habara, M.; Tsuchikane, E.; Shimizu, K.; Kashima, Y.; Shimoji, K.; Nakamura, S.; Niizeki, T.; Tsutsumi, T.; Ito, Y.; Kawasaki, T.; et al. Japanese multicenter registry evaluating the antegrade dissection reentry with cardiac computerized tomography for chronic coronary total occlusion. Cardiovasc. Interv. Ther. 2022, 37, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, J.; Tajti, P.; Rangan, B.V.; Halligan, S.C.; Allen, R.H.; Nicholson, W.J.; Harvey, J.E.; Spaedy, A.J.; Jaffer, F.A.; Grantham, J.A.; et al. Randomized Comparison of a CrossBoss First Versus Standard Wire Escalation Strategy for Crossing Coronary Chronic Total Occlusions: The CrossBoss First Trial. JACC Cardiovasc. Interv. 2018, 11, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Brilakis, E.S.; Grantham, J.A.; Rinfret, S.; Wyman, R.M.; Burke, M.N.; Karmpaliotis, D.; Lembo, N.; Pershad, A.; Kandzari, D.E.; Buller, C.E.; et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 2012, 5, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: The J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef]
- Maeremans, J.; Walsh, S.; Knaapen, P.; Spratt, J.C.; Avran, A.; Hanratty, C.G.; Faurie, B.; Agostoni, P.; Bressollette, E.; Kayaert, P.; et al. The Hybrid Algorithm for Treating Chronic Total Occlusions in Europe: The RECHARGE Registry. J. Am. Coll. Cardiol. 2016, 68, 1958–1970. [Google Scholar] [CrossRef]
- Opolski, M.P.; Achenbach, S.; Schuhbäck, A.; Rolf, A.; Möllmann, H.; Nef, H.; Rixe, J.; Renker, M.; Witkowski, A.; Kepka, C.; et al. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion: Insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc. Interv. 2015, 8, 257–267. [Google Scholar] [CrossRef]
- Yu, C.-W.; Lee, H.-J.; Suh, J.; Lee, N.-H.; Park, S.-M.; Park, T.K.; Yang, J.H.; Bin Song, Y.; Hahn, J.-Y.; Choi, S.H.; et al. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion: Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ. Cardiovasc. Imaging 2017, 10, e005800. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Otsuji, S.; Hasegawa, K.; Arita, T.; Takiuchi, S.; Fujii, K.; Yabuki, M.; Ibuki, M.; Nagayama, S.; Ishibuchi, K.; et al. Accuracy of J-CTO Score Derived from Computed Tomography Versus Angiography to Predict Successful Percutaneous Coronary Intervention. JACC Cardiovasc. Imaging 2018, 11 Pt 1, 209–217. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Tesche, C.; Schoepf, U.J.; Pannell, J.T.; He, Y.; Huang, R.; Chen, Y.; Li, J.; Song, X. CT Angiography-Derived RECHARGE Score Predicts Successful Percutaneous Coronary Intervention in Patients with Chronic Total Occlusion. Korean J. Radiol. 2021, 22, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhou, J.; Zhang, W.; Zhou, Y.; Du, L.; Tian, F.; Guo, J.; Chen, L.; Cao, F.; Chen, Y. Comparison of CT-RECTOR and J-CTO scores to predict chronic total occlusion difficulty for percutaneous coronary intervention. Int. J. Cardiol. 2017, 235, 169–175. [Google Scholar] [CrossRef]
- Galassi, A.R.; Werner, G.S.; Boukhris, M.; Azzalini, L.; Mashayekhi, K.; Carlino, M.; Avran, A.; Konstantinidis, N.V.; Grancini, L.; Bryniarski, L.; et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2019, 15, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, N.; Zhang, J.; Li, M.; Lu, Z.; Wei, M.; Lu, B.; Zhang, Y. Procedural success of CTO recanalization: Comparison of the J-CTO score determined by coronary CT angiography to invasive angiography. J. Cardiovasc. Comput. Tomogr. 2015, 9, 578–584. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Yamawaki, M.; Mori, S.; Tsutsumi, M.; Makino, K.; Chisiki, T.; Shirai, S.; Mizusawa, M.; Kobayashi, N.; Ito, Y. Predictive performance of J-Calc-CTO score for guidewire crossing through chronic total occlusion lesions within 30 minutes: J-CTO score modified by computed tomography coronary angiography. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 100, 560–567. [Google Scholar] [CrossRef]
- Rolf, A.; Werner, G.S.; Schuhbäck, A.; Rixe, J.; Möllmann, H.; Nef, H.M.; Gundermann, C.; Liebetrau, C.; Krombach, G.A.; Hamm, C.W.; et al. Preprocedural coronary CT angiography significantly improves success rates of PCI for chronic total occlusion. Int. J. Cardiovasc. Imaging 2013, 29, 1819–1827. [Google Scholar] [CrossRef]
- Opolski, M.P.; Zysk, A.; Wolny, R.; Debski, A.; Witkowski, A. Coronary CTA co-registration for guiding antegrade dissection re-entry in chronic total occlusion percutaneous coronary intervention. J. Cardiovasc. Comput. Tomogr. 2022, 16, e14–e16. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, B.K.; Cho, I.; Kim, H.Y.; Rha, S.W.; Lee, S.H.; Park, S.M.; Kim, Y.H.; Chang, H.J.; Ahn, C.M.; et al. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention: A Randomized Trial. JACC. Cardiovasc. Imaging 2021, 14, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P.; Nap, A.; Knaapen, P. A computed tomography algorithm for crossing coronary chronic total occlusions: Riding on the wave of the proximal cap and distal vessel segment. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2021, 29, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P.; Kwiecinski, J.; Oleksiak, A.; Kruk, M.; Debski, A.; Knaapen, P.; Schumacher, S.P.; Zysk, A.; Witkowski, A.; Kepka, C. Feasibility of computed tomography perfusion in patients with chronic total occlusion undergoing percutaneous coronary intervention. J. Cardiovasc. Comput. Tomogr. 2022, 16, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Oleksiak, A.; Kruk, M.; Zysk, A.; Debski, A.; Knaapen, P.; Schumacher, S.P.; Barbero, U.; Witkowski, A.; Kepka, C.; et al. Computed tomography perfusion and angiography in patients with chronic total occlusion undergoing percutaneous coronary intervention. Atherosclerosis 2023, 381, 117174. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]

| Total (n = 110) | Time-Efficient GW Crossing (n = 59) | No Time-Efficient GW Crossing (n = 51) | p-Value | |
|---|---|---|---|---|
| Age | 67.0 (60.3–72.0) | 67.0 (56.9–72.0) | 67.0 (62.0–69.6) | 0.196 |
| Male | 88 (80.0%) | 46 (78.0%) | 42 (82.4%) | 0.637 |
| BMI | 28.82 (26.76, 31.59) | 28.93 (26.44, 31.82) | 28.73 (27.31, 31.16) | 0.767 |
| Family history of CAD | 13 (11.8%) | 8 (13.6%) | 5 (9.8%) | 0.572 |
| Current smoker | 27 (24.5%) | 15 (25.4%) | 12 (23.5%) | 1.000 |
| Hypertension | 91 (82.7%) | 51 (86.4%) | 40 (78.4%) | 0.317 |
| Dyslipidemia | 92 (83.6%) | 52 (88.1%) | 40 (78.4%) | 0.202 |
| Diabetes mellitus | 34 (30.9%) | 15 (25.4%) | 19 (37.3%) | 0.217 |
| Renal disease | 16 (14.5%) | 8 (13.6%) | 8 (15.7%) | 0.792 |
| Creatinine | 1.0 (0.9–1.1) | 0.9 (0.9–1.0) | 1.0 (0.8–1.1) | 0.367 |
| eGFR | 89.8 (71.9–114.5) | 93.6 (77.2–112.6) | 88.0 (69.1–114.9) | 0.642 |
| PAD | 29 (26.4%) | 13 (22.0%) | 16 (31.4%) | 0.286 |
| TIA/stroke | 9 (8.2%) | 6 (10.2%) | 3 (5.9%) | 0.500 |
| Heart failure | 24 (21.8%) | 10 (16.9%) | 14 (27.5%) | 0.248 |
| LVEF ≤ 40 | 16 (14.5%) | 9 (15.3%) | 7 (13.7%) | 1.000 |
| COPD | 9 (8.2%) | 5 (8.5%) | 4 (7.8%) | 1.000 |
| CCS | 2.0 (0.0–3.0) | 2.0 (0.5–3.0) | 2.0 (0.0–3.0) | 0.862 |
| NYHA | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.213 |
| NYHA ≥ II | 34 (30.9%) | 16 (27.1%) | 18 (35.3%) | 0.411 |
| CCS ≥ 2 | 74 (67.3%) | 40 (67.8%) | 34 (66.7%) | 1.000 |
| Prior MI | 53 (48.2%) | 25 (42.4%) | 28 (54.9%) | 0.251 |
| Prior CABG | 22 (20.0%) | 8 (13.6%) | 14 (27.5%) | 0.094 |
| Prior PCI | 66 (60.0%) | 35 (59.3%) | 31 (60.8%) | 1.000 |
| Unknown or >12 months’ duration of CTO | 98 (89.1%) | 50 (84.7%) | 48 (94.1%) | 0.137 |
| Reattempt at CTO | 24 (21.8%) | 11 (18.6%) | 13 (25.5%) | 0.489 |
| Grafted CTO vessel | 19 (17.3%) | 8 (13.6%) | 11 (21.6%) | 0.317 |
| Total (n = 110) | Time-Efficient GW Crossing (n = 59) | No Time-Efficient GW Crossing (n = 51) | p-Value | |
|---|---|---|---|---|
| CTO in RCA | 53 (48.2%) | 26 (44.1%) | 27 (52.9%) | 0.444 |
| CTO in LAD | 44 (40.0%) | 24 (40.7%) | 20 (39.2%) | 1.000 |
| CTO in CX | 13 (11.8%) | 9 (15.3%) | 4 (7.8%) | 0.255 |
| Successful GW crossing at any time | 100 (90.9%) | 59 (100.0%) | 41 (80.4%) | <0.001 |
| Restoration of TIMI 3 flow | 98 (89.1%) | 59 (100.0%) | 39 (76.5%) | <0.001 |
| Any AW | 106 (96.4%) | 59 (100.0%) | 47 (92.2%) | 0.043 |
| Any ADR | 26 (23.6%) | 1 (1.7%) | 25 (49.0%) | <0.001 |
| Any RW | 32 (29.1%) | 1 (1.7%) | 31 (60.8%) | <0.001 |
| Any RDR | 9 (8.18%) | 0 (0.00%) | 9 (17.65%) | 0.001 |
| Any DART | 29 (26.4%) | 1 (1.7%) | 28 (54.9%) | <0.001 |
| Retrograde approach | 34 (30.9%) | 1 (1.7%) | 33 (64.7%) | <0.001 |
| Any non-AW strategy | 40 (36.4%) | 2 (3.4%) | 38 (74.5%) | <0.001 |
| No. strategies applied | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 2.0 (1.0–3.0) | <0.001 |
| Successful AW | 71 (64.5%) | 57 (96.6%) | 14 (27.5%) | <0.001 |
| Successful ADR | 8 (7.3%) | 1 (1.7%) | 7 (13.7%) | 0.024 |
| Successful RW | 12 (10.9%) | 1 (1.7%) | 11 (21.6%) | 0.001 |
| Successful RDR | 9 (8.2%) | 0 (0.0%) | 9 (17.6%) | 0.001 |
| Change from AW to any non-AW strategy (min) | 27.0 (19.0–52.0) | 14.5 (10.2–18.8) | 30.0 (19.0–53.0) | 0.248 |
| Time of successful GW crossing (min) | 20.0 (8.0–83.2) | 9.0 (5.5–15.5) | 110.0 (67.0–142.0) | <0.001 |
| Duration of procedure (min) | 142.5 (92.2–209.5) | 97.0 (76.5–137.0) | 211.0 (162.5–233.5) | <0.001 |
| Fluoroscopy time (min) | 43.5 (27.4–73.4) | 29.0 (19.4–42.2) | 74.0 (55.8–94.7) | <0.001 |
| Radiation dose (mGy) | 1506.5 (794.0–2594.5) | 917.0 (501.9–1624.5) | 2531.0 (1436.0–3254.0) | <0.001 |
| Contrast volume (mL) | 200.0 (132.5–200.0) | 150.0 (100.0–200.0) | 200.0 (200.0–250.0) | <0.001 |
| Blunt proximal cap | 39 (35.5%) | 10 (16.9%) | 29 (56.9%) | <0.001 |
| Calcification | 17 (15.5%) | 4 (6.8%) | 13 (25.5%) | 0.008 |
| Tortuosity (°) | 25.0 (15.0–38.3) | 22.4 (14.1–32.5) | 30.0 (15.8–53.5) | 0.005 |
| Tortuosity > 45° | 23 (20.9%) | 6 (10.2%) | 17 (33.3%) | 0.004 |
| Occlusion length (mm) | 11.3 (7.0–22.7) | 9.0 (6.4–12.9) | 21.0 (11.2–27.3) | <0.001 |
| Length ≥ 20 mm | 34 (30.9%) | 7 (11.9%) | 27 (52.9%) | <0.001 |
| J-CTOCA Score | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 2.0 (1.0–3.0) | <0.001 |
| Total (n = 110) | Time-Efficient GW Crossing (n = 59) | No Time-Efficient GW Crossing (n = 51) | p-Value | |
|---|---|---|---|---|
| Blunt cap | 40 (36.4%) | 11 (18.6%) | 29 (56.9%) | <0.001 |
| Proximal adjacent SB | 57 (51.8%) | 25 (42.4%) | 32 (62.7%) | 0.037 |
| Occlusion length | 15.6 (9.3–23.9) | 12.0 (8.6–17.0) | 22.6 (16.4–28.9) | <0.001 |
| Occlusion length ≥ 15 mm | 56 (50.9%) | 16 (27.1%) | 40 (78.4%) | <0.001 |
| Occlusion length ≥ 20 mm | 39 (35.5%) | 9 (15.3%) | 30 (58.8%) | <0.001 |
| Tortuosity (°) | 27.0 (17.2–40.0) | 24.0 (16.5–34.5) | 32.0 (19.5–52.0) | 0.001 |
| Tortuosity > 45° | 23 (20.9%) | 3 (5.1%) | 20 (39.2%) | <0.001 |
| Any calcification | 89 (80.9%) | 46 (78.0%) | 43 (84.3%) | 0.470 |
| Calcium in entry of CTO | 56 (50.9%) | 30 (50.8%) | 26 (51.0%) | 1.000 |
| Calcium in body of CTO | 59 (53.6%) | 26 (44.1%) | 33 (64.7%) | 0.036 |
| Calcium in exit of CTO | 53 (48.2%) | 24 (40.7%) | 29 (56.9%) | 0.126 |
| Calcium ≥ 50% CSA | 45 (40.9%) | 16 (27.1%) | 29 (56.9%) | 0.002 |
| Calcium 100% CSA | 18 (16.4%) | 3 (5.1%) | 15 (29.4%) | 0.001 |
| Multiple occlusions | 16 (14.5%) | 3 (5.1%) | 13 (25.5%) | 0.003 |
| Diseased distal landing zone | 55 (50.0%) | 27 (45.8%) | 28 (54.9%) | 0.445 |
| Proximal reference lumen area | 6.6 (4.8–8.9) | 6.1 (4.7–8.6) | 7.3 (5.5–9.5) | 0.224 |
| Proximal reference maximal lumen diameter | 3.1 (2.7–3.7) | 3.0 (2.6–3.4) | 3.2 (2.8–3.7) | 0.088 |
| Proximal reference minimal lumen diameter | 2.7 (2.3–3.2) | 2.6 (2.2–3.1) | 2.8 (2.5–3.2) | 0.124 |
| Distal reference lumen area | 4.5 (3.3–5.9) | 4.5 (3.4–5.8) | 4.0 (3.2–6.0) | 0.952 |
| Distal reference maximal lumen diameter | 2.5 (2.1–3.0) | 2.7 (2.1–3.0) | 2.5 (2.1–3.0) | 0.754 |
| Distal reference minimal lumen diameter | 2.1 (1.9–2.6) | 2.1 (1.9–2.6) | 2.1 (1.8–2.5) | 0.673 |
| Maximal vessel area within occlusion site | 13.0 (8.8–18.0) | 13.0 (8.7–18.0) | 12.5 (9.3–18.0) | 0.408 |
| Remodeling index | 1.3 (1.0–1.7) | 1.3 (1.0–1.6) | 1.3 (0.9–1.8) | 0.962 |
| CCTA-derived CTO scores | ||||
| CT-RECTOR score | 2.0 (1.0–3.0) | 2.0 (1.0–2.0) | 3.0 (2.0–4.0) | <0.001 |
| KCCT score | 3.0 (2.0–5.0) | 2.0 (1.5–3.0) | 4.0 (3.0–6.0) | <0.001 |
| J-CTOCCTA score | 1.0 (0.0–3.0) | 1.0 (0.0–1.0) | 3.0 (1.0–3.0) | <0.001 |
| RECHARGECCTA score | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 3.0 (2.0–4.0) | <0.001 |
| AUC | AUC CI 95% | Criterion (Youden Index) | Sensitivity | Specificity | p (Area = 0.5) | |
|---|---|---|---|---|---|---|
| Successful time-efficient guidewire crossing | ||||||
| J-CTOCA | 0.784 | 0.696–0.857 | ≤1 | 88.14 | 62.75 | <0.001 |
| CT-RECTOR | 0.813 | 0.727–0.881 | ≤2 | 89.83 | 58.82 | <0.001 |
| KCCT | 0.839 | 0.757–0.902 | ≤3 | 84.75 | 70.59 | <0.001 |
| J-CTOCCTA | 0.815 | 0.73–0.883 | ≤1 | 79.66 | 70.59 | <0.001 |
| RECHARGECCTA | 0.817 | 0.731–0.884 | ≤2 | 89.83 | 62.75 | <0.001 |
| Final procedural success | ||||||
| J-CTOCA | 0.748 | 0.657–0.826 | ≤1 | 69.39 | 75 | 0.004 |
| CT-RECTOR | 0.824 | 0.739–0.89 | ≤2 | 72.45 | 75 | <0.001 |
| KCCT | 0.855 | 0.775–0.915 | ≤5 | 90.82 | 66.67 | <0.001 |
| J-CTOCCTA | 0.864 | 0.785–0.922 | ≤2 | 79.59 | 75 | <0.001 |
| RECHARGECCTA | 0.832 | 0.748–0.896 | ≤3 | 88.78 | 66.67 | <0.001 |
| Need for any non-AW strategy | ||||||
| J-CTOCA | 0.741 | 0.648–0.819 | >1 | 62.5 | 80 | <0.001 |
| CT-RECTOR | 0.777 | 0.688–0.851 | >2 | 60 | 82.86 | <0.001 |
| KCCT | 0.783 | 0.695–0.856 | >3 | 72.5 | 77.14 | <0.001 |
| J-CTOCCTA | 0.806 | 0.72–0.875 | >1 | 75 | 74.29 | <0.001 |
| RECHARGECCTA | 0.785 | 0.697–0.858 | >2 | 65 | 82.86 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyśk, A.; Wolny, R.; Kruk, M.; Kwieciński, J.; Dębski, A.; Barbero, U.; Kępka, C.; Demkow, M.; Witkowski, A.; Opolski, M.P. Computed Tomography Angiography-Derived Scores for Prediction of Chronic Total Occlusion Percutaneous Coronary Intervention Using the Hybrid Algorithm. J. Cardiovasc. Dev. Dis. 2024, 11, 3. https://doi.org/10.3390/jcdd11010003
Zyśk A, Wolny R, Kruk M, Kwieciński J, Dębski A, Barbero U, Kępka C, Demkow M, Witkowski A, Opolski MP. Computed Tomography Angiography-Derived Scores for Prediction of Chronic Total Occlusion Percutaneous Coronary Intervention Using the Hybrid Algorithm. Journal of Cardiovascular Development and Disease. 2024; 11(1):3. https://doi.org/10.3390/jcdd11010003
Chicago/Turabian StyleZyśk, Antoni, Rafał Wolny, Mariusz Kruk, Jacek Kwieciński, Artur Dębski, Umberto Barbero, Cezary Kępka, Marcin Demkow, Adam Witkowski, and Maksymilian P. Opolski. 2024. "Computed Tomography Angiography-Derived Scores for Prediction of Chronic Total Occlusion Percutaneous Coronary Intervention Using the Hybrid Algorithm" Journal of Cardiovascular Development and Disease 11, no. 1: 3. https://doi.org/10.3390/jcdd11010003
APA StyleZyśk, A., Wolny, R., Kruk, M., Kwieciński, J., Dębski, A., Barbero, U., Kępka, C., Demkow, M., Witkowski, A., & Opolski, M. P. (2024). Computed Tomography Angiography-Derived Scores for Prediction of Chronic Total Occlusion Percutaneous Coronary Intervention Using the Hybrid Algorithm. Journal of Cardiovascular Development and Disease, 11(1), 3. https://doi.org/10.3390/jcdd11010003







