Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
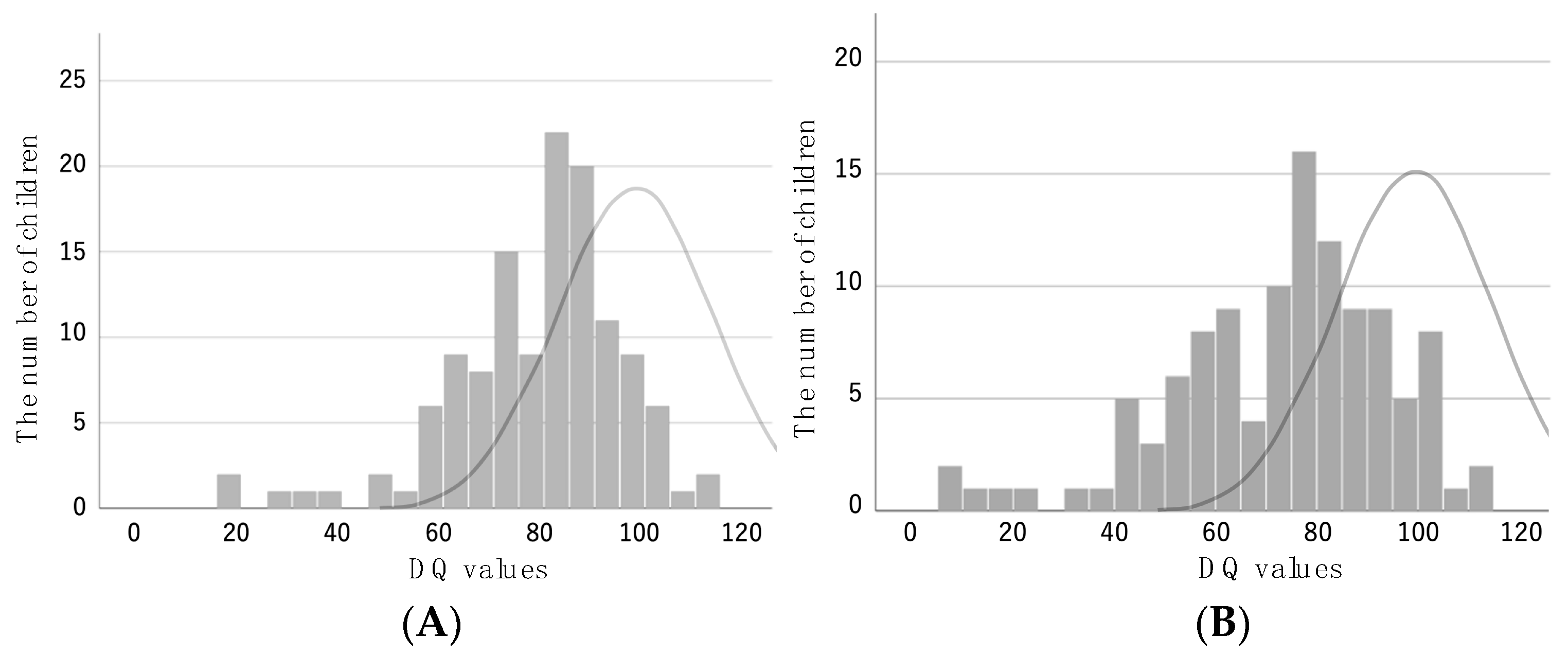
3.2. Developmental Test Results
3.3. Analysis of the Factors That Influenced the Developmental Test Results
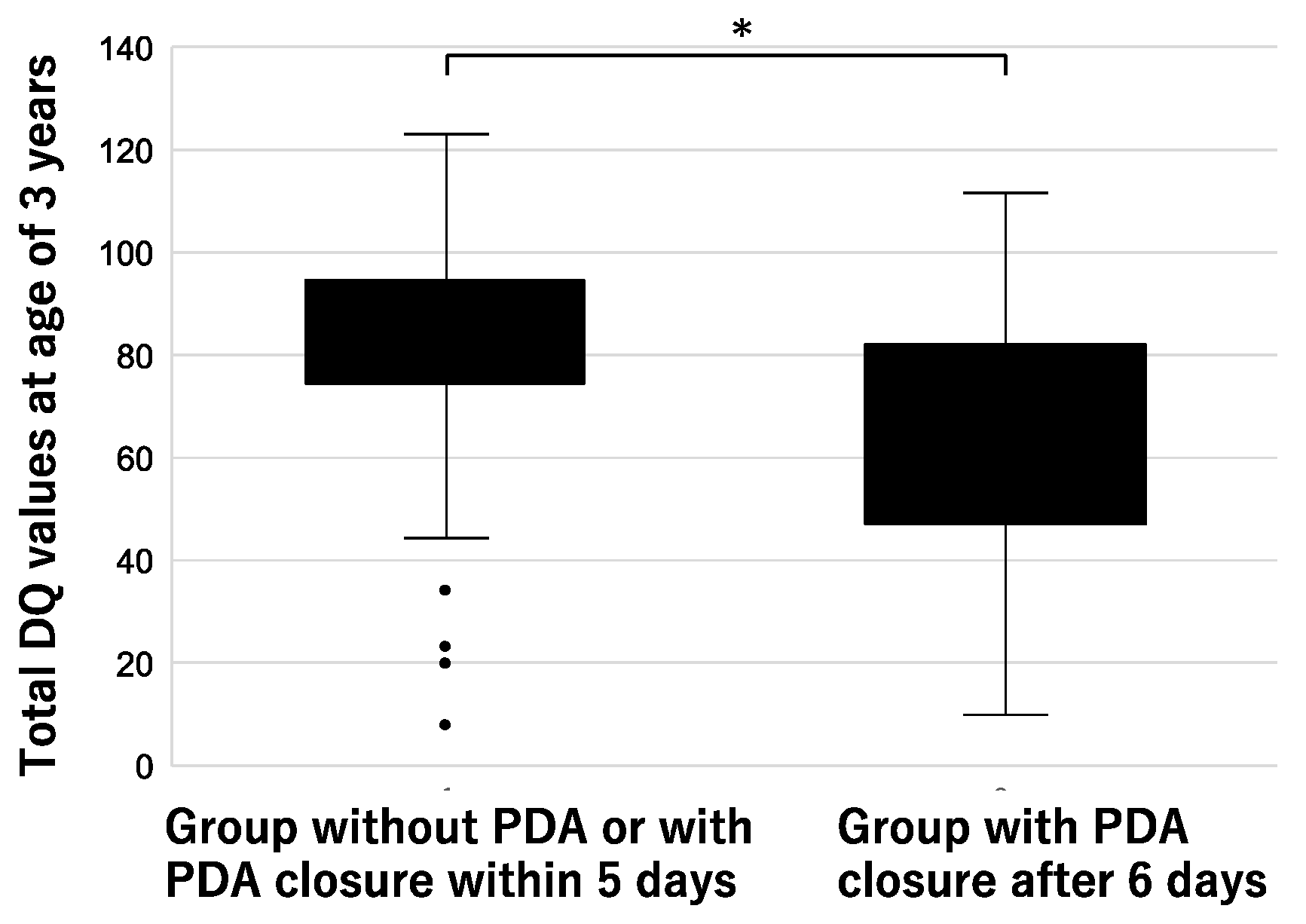
3.4. Comparison of Preterm Infants with and without PDA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aw, T.C.; Chan, B.; Singh, Y. Transport and Anaesthesia Consideration for Transcatheter Patent Ductus Arteriosus Closure in Premature Infants. J. Cardiovasc. Dev. Dis. 2023, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Hsu, R.; Lin, Y.-C.; Wong, T.-W.; Kan, C.-D.; Wang, J.-N. The Association of Patent Ductus Arteriosus with Inflammation: A Narrative Review of the Role of Inflammatory Biomarkers and Treatment Strategy in Premature Infants. Int. J. Mol. Sci. 2022, 23, 13877. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Weisz, D.E.; Reese, J.; Jain, A. Combination pharmacotherapy for patent ductus arteriosus: Rationale and evidence. Semin. Perinatol. 2023, 47, 151720. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Couto, J.; Murphy, G.M. Patent Ductus Arteriosus: Are Current Neonatal Treatment Options Better or Worse Than No Treatment at All? Semin. Perinatol. 2012, 36, 123–129. [Google Scholar] [CrossRef]
- Chock, V.Y.; Bhombal, S.; Variane, G.F.T.; Van Meurs, K.P.; Benitz, W.E. Ductus arteriosus and the preterm brain. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Dankhara, N.; Holla, I.; Ramarao, S.; Kalikkot Thekkeveedu, R. Bronchopulmonary Dysplasia: Pathogenesis and Pathophysiology. J. Clin. Med. 2023, 12, 4207. [Google Scholar] [CrossRef]
- Ghouse, F.; Idrobo Zapata, C.; Kasam Shiva, P.K.; Aguilar, A.; Siripragada, R.; Nair, N.; Vera, E.; Suresh, A. Closing the Gap: Investigation of Various Approaches in the Management of Patent Ductus Arteriosus. Cureus 2023, 15, e45009. [Google Scholar]
- Lembo, C.; El-Khuffash, A.; Fusch, C.; Iacobelli, S.; Lapillonne, A. Nutrition of the preterm infant with persistent ductus arteriosus: Existing evidence and practical implications. Pediatr. Res. 2023. [Google Scholar] [CrossRef]
- Rolland, A.; Shankar-Aguilera, S.; Diomandé, D.; Zupan-Simunek, V.; Boileau, P. Natural evolution of patent ductus arteriosus in the extremely preterm infant. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F55–F58. [Google Scholar] [CrossRef][Green Version]
- Isayama, T.; Mirea, L.; Mori, R.; Kusuda, S.; Fujimura, M.; Lee, S.K.; Shah, P.S. Patent ductus arteriosus management and outcomes in Japan and Canada: Comparison of proactive and selective approaches. Am. J. Perinatol. 2015, 32, 1087–1094. [Google Scholar] [CrossRef]
- Xiang, Y.; Jin, K.; Cai, Q.; Peng, Y.; Gan, Q. Clinical findings, diagnosis and therapy of patent ductus venosus in children: A case series. Cardiovasc. Diagn. Ther. 2022, 12, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Isayama, T.; Ye, X.Y.; Tokumasu, H.; Chiba, H.; Mitsuhashi, H.; Shahrook, S.; Kusuda, S.; Fujimura, M.; Toyoshima, K.; Mori, R. The effect of professional-led guideline workshops on clinical practice for the management of patent ductus arteriosus in preterm neonates in Japan: A controlled before-and-after study. Implement. Sci. 2015, 10, 67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benitz, W.E. Patent ductus arteriosus: To treat or not to treat? Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F80–F82. [Google Scholar] [CrossRef]
- Hamrick, S.E.; Hansmann, G. Patent ductus arteriosus of the preterm infant. Pediatrics 2010, 125, 1020–1030. [Google Scholar] [CrossRef]
- Noori, S. Patent ductus arteriosus in the preterm infant: To treat or not to treat? J. Perinatol. 2010, 30 (Suppl. S1), S31–S37. [Google Scholar] [CrossRef]
- May, L.A.; Masand, P.M.; Qureshi, A.M.; Jadhav, S.P. The ductus arteriosus: A review of embryology to intervention. Pediatr. Radiol. 2023, 53, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Lin, T.Y.; Liu, Y.C.; Yeh, J.L.; Hsu, J.H. Molecular Mechanisms Underlying Remodeling of Ductus Arteriosus: Looking beyond the Prostaglandin Pathway. Int. J. Mol. Sci. 2021, 22, 3238. [Google Scholar] [CrossRef] [PubMed]
- Janz-Robinson, E.M.; Badawi, N.; Walker, K.; Bajuk, B.; Abdel-Latif, M.E. Neurodevelopmental Outcomes of Premature Infants Treated for Patent Ductus Arteriosus: A Population-Based Cohort Study. J. Pediatr. 2015, 167, 1025–1032.e3. [Google Scholar] [CrossRef]
- Rheinlaender, C.; Helfenstein, D.; Pees, C.; Walch, E.; Czernik, C.; Obladen, M.; Koehne, P. Neurodevelopmental outcome after COX inhibitor treatment for patent ductus arteriosus. Early Hum. Dev. 2010, 86, 87–92. [Google Scholar] [CrossRef]
- Tauzin, L.; Joubert, C.; Noel, A.C.; Bouissou, A.; Moulies, M.E. Effect of persistent patent ductus arteriosus on mortality and morbidity in very low-birthweight infants. Acta Paediatr. 2012, 101, 419–423. [Google Scholar] [CrossRef]
- Wickremasinghe, A.C.; Rogers, E.E.; Piecuch, R.E.; Johnson, B.C.; Golden, S.; Moon-Grady, A.J.; Clyman, R.I. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J. Pediatr. 2012, 161, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Adrouche-Amrani, L.; Green, R.S.; Gluck, K.M.; Lin, J. Failure of a repeat course of cyclooxygenase inhibitor to close a PDA is a risk factor for developing chronic lung disease in ELBW infants. BMC Pediatr. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.C.; Kendrick, D.; Hagadorn, J.I.; Frantz, I.D., 3rd. Patent ductus arteriosus therapy: Impact on neonatal and 18-month outcome. Pediatrics 2009, 123, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Lee, J.Y.; Lee, J.H.; Kim, S.Y.; Sung, I.K.; Lee, J.Y. Impact of patient selection on outcomes of PDA in very low birth weight infants. Early Hum. Dev. 2013, 89, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Avsar, M.K.; Demir, T.; Celiksular, C.; Zeybek, C. Bedside PDA ligation in premature infants less than 28 weeks and 1000 grams. J. Cardiothorac. Surg. 2016, 11, 146. [Google Scholar] [CrossRef][Green Version]
- Chock, V.Y.; Goel, V.V.; Palma, J.P.; Luh, T.M.; Wang, N.A.; Gaskari, S.; Punn, R.; Silverman, N.H.; Benitz, W.E. Changing Management of the Patent Ductus Arteriosus: Effect on Neonatal Outcomes and Resource Utilization. Am. J. Perinatol. 2017, 34, 990–995. [Google Scholar]
- Cooke, R.W. Perinatal and postnatal factors in very preterm infants and subsequent cognitive and motor abilities. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F60–F63. [Google Scholar] [CrossRef]
- Lemmers, P.M.; Benders, M.J.; D’Ascenzo, R.; Zethof, J.; Alderliesten, T.; Kersbergen, K.J.; Isgum, I.; de Vries, L.S.; Groenendaal, F.; van Bel, F. Patent Ductus Arteriosus and Brain Volume. Pediatrics 2016, 137, e20153090. [Google Scholar] [CrossRef]
- Obst, S.; Herz, J.; Alejandre Alcazar, M.A.; Endesfelder, S.; Möbius, M.A.; Rüdiger, M.; Felderhoff-Müser, U.; Bendix, I. Perinatal Hyperoxia and Developmental Consequences on the Lung-Brain Axis. Oxid. Med. Cell. Longev. 2022, 2022, 5784146. [Google Scholar] [CrossRef]
- Rademaker, K.J.; de Vries, W.B. Long-term effects of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Semin. Fetal Neonatal Med. 2009, 14, 171–177. [Google Scholar] [CrossRef]
- Robles, I.; Eidsness, M.A.; Travis, K.E.; Feldman, H.M.; Dubner, S.E. Effects of postnatal glucocorticoids on brain structure in preterm infants, a scoping review. Neurosci. Biobehav. Rev. 2023, 145, 105034. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Hensley, G.; Roy, L.; Brown, S.; Ramaciotti, C.; Rosenfeld, C.R. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 2006, 117, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Olowoyeye, A.; Nnamdi-Nwosu, O.; Manalastas, M.; Okwundu, C. A Network Meta-Analysis of Intravenous Versus Oral Acetaminophen for Patent Ductus Arteriosus. Pediatr. Cardiol. 2023, 44, 748–756. [Google Scholar] [CrossRef]
- Yokoyama, U.; Minamisawa, S.; Katayama, A.; Tang, T.; Suzuki, S.; Iwatsubo, K.; Iwasaki, S.; Kurotani, R.; Okumura, S.; Sato, M.; et al. Differential regulation of vascular tone and remodeling via stimulation of type 2 and type 6 adenylyl cyclases in the ductus arteriosus. Circ. Res. 2010, 106, 1882–1892. [Google Scholar] [CrossRef]
- Kabra, N.S.; Schmidt, B.; Roberts, R.S.; Doyle, L.W.; Papile, L.; Fanaroff, A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: Results from the Trial of Indomethacin Prophylaxis in Preterms. J. Pediatr. 2007, 150, 229–234.e1. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.I.; Chang, Y.S.; Ahn, S.Y.; Jo, H.S.; Yang, M.; Park, W.S. Conservative Non-intervention Approach for Hemodynamically Significant Patent Ductus Arteriosus in Extremely Preterm Infants. Front. Pediatr. 2020, 8, 605134. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.I.; Choi, S.Y.; Park, J.H.; Lee, M.S.; Yoo, H.S.; Ahn, S.Y.; Chang, Y.S.; Park, W.S. The timing of surgical ligation for patent ductus arteriosus is associated with neonatal morbidity in extremely preterm infants born at 23–25 weeks of gestation. J. Korean Med. Sci. 2014, 29, 581–586. [Google Scholar] [CrossRef]
- Linnane, N.; Kenny, D.P.; Hijazi, Z.M. Congenital heart disease: Addressing the need for novel lower-risk percutaneous interventional strategies. Expert Rev. Cardiovasc. Ther. 2023, 21, 329–336. [Google Scholar] [CrossRef]
- Mitra, S.; de Boode, W.P.; Weisz, D.E.; Shah, P.S. Interventions for patent ductus arteriosus (PDA) in preterm infants: An overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2023, 4, Cd013588. [Google Scholar]
- Sathanandam, S.K.; Gutfinger, D.; O’Brien, L.; Forbes, T.J.; Gillespie, M.J.; Berman, D.P.; Armstrong, A.K.; Shahanavaz, S.; Jones, T.K.; Morray, B.H.; et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter. Cardiovasc. Interv. 2020, 96, 1266–1276. [Google Scholar] [CrossRef]
- Dimas, V.V.; Takao, C.; Ing, F.F.; Mattamal, R.; Nugent, A.W.; Grifka, R.G.; Mullins, C.E.; Justino, H. Outcomes of Transcatheter Occlusion of Patent Ductus Arteriosus in Infants Weighing ≤6 kg. JACC Cardiovasc. Interv. 2010, 3, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Cheatham, S.L.; Deyo, G.M.; Leopold, S.; Ball, M.K.; Smith, C.V.; Garg, V.; Holzer, R.J.; Cheatham, J.P.; Berman, D.P. Percutaneous Patent Ductus Arteriosus (PDA) Closure in Very Preterm Infants: Feasibility and Complications. J. Am. Heart Assoc. 2016, 5, e002923. [Google Scholar] [CrossRef]
- Georgiev, S.; Tanase, D.; Eicken, A.; Peters, J.; Hörer, J.; Ewert, P. Transvenous, Echocardiographically Guided Closure of Persistent Ductus Arteriosus in 11 Premature Infants: A Pilot Study. JACC Cardiovasc. Interv. 2021, 14, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Pouldar, T.M.; Wong, R.; Almeida-Jones, M.; Zahn, E.; Lubin, L. Bedside Transcatheter Patent Ductus Arteriosus Device Occlusion in an Extremely Low Birth Weight Neonate: A Novel Approach in a High-Risk Population. Case Rep. Anesthesiol. 2021, 2021, 4716997. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Balduf, K.; Chilakala, S.; Washington, K.; Allen, K.; Knott-Craig, C.; Rush Waller, B.; Philip, R. Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter. Cardiovasc. Interv. 2019, 93, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Whiting, S.; Cunningham, J.; Zurakowski, D.; Apalodimas, L.; Waller, B.R.; Philip, R.; Qureshi, A.M. Practice variation in the management of patent ductus arteriosus in extremely low birth weight infants in the United States: Survey results among cardiologists and neonatologists. Congenit. Heart Dis. 2019, 14, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.M.; Madison, M.; Lorant, D.; Hoyer, M.; Alexy, R. Comparison of ‘post-patent ductus arteriosus ligation syndrome’ in premature infants after surgical ligation vs. percutaneous closure. J. Perinatol. 2020, 40, 324–329. [Google Scholar] [CrossRef]
- Daniel, R.-S.; Schmidt, G.-K.; Nakanishi, H.; Smayra, K.; Mascara, M.-N.; Vankayalapati, D.-K.; Matar, R.-H.; Than, C.-A.; Shiakos, G.; Tzanavaros, I. Transcatheter Closure vs. Surgical Ligation in Preterm Infants with Patent Ductus Arteriosus: A Systematic Review and Meta-Analysis. Congenit. Heart Dis. 2023, 18, 245–265. [Google Scholar] [CrossRef]
- Regan, W.; Benbrik, N.; Sharma, S.-R.; Auriau, J.; Bouvaist, H.; Bautista-Rodriguez, C.; Sirico, D.; Aw, T.-C.; di Salvo, G.; Foldvari, S.; et al. Improved ventilation in premature babies after transcatheter versus surgical closure of patent ductus arteriosus. Int. J. Cardiol. 2020, 311, 22–27. [Google Scholar] [CrossRef]
- Francescato, G.; Doni, D.; Annoni, G.; Capolupo, I.; Ciarmoli, E.; Corsini, I.; Gatelli, I.F.; Salvadori, S.; Testa, A.; Butera, G. Transcatheter closure in preterm infants with patent ductus arteriosus: Feasibility, results, hemodynamic monitoring and future prospectives. Ital. J. Pediatr. 2023, 49, 147. [Google Scholar] [CrossRef]
- Goto, T. Concerns Remain Regarding the Association of Sitting Time and Physical Activity with Cancer Survivorship. JAMA Oncol. 2022, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Goto, T. Kit Use May Not Be Key to Improved Prognosis. J. Thorac. Oncol. 2023, 18, e79–e80. [Google Scholar] [CrossRef] [PubMed]
| PDA Present | PDA Absent | p Value | |
|---|---|---|---|
| Number of patients | 114 | 155 | |
| Male/female | 47/67 | 77/78 | 0.17 |
| Birth weight (g) | 899 (376–1496) | 1232 (496–1802) | <0.01 |
| SD value of term birth weight | −3.28 (−15.3–0.31) | −2.56 (−17.5–1.56) | <0.05 |
| Enteral nutrition (day) | 17.7 (8–60) | 9.8 (1–48) | <0.05 |
| Chronic lung disease (%) | 70 (61.4) | 25 (16.1) | <0.01 |
| Intraventricular hemorrhage (%) | 13 (11.4) | 1 (0.65) | <0.01 |
| Periventricular leukomalacia (%) | 5 (4.4) | 1 (0.65) | 0.10 |
| Sepsis (%) | 10 (8.8) | 9 (5.8) | 0.49 |
| Idiopathic gastrointestinal perforation (%) | 1 (0.9) | 1 (0.65) | 0.62 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Pearson’s Correlation Coefficient | p Value | t Value | p Value | |
| Birth weight | 0.220 | 0.013 | 0.826 | 0.410 |
| PDA closure date | −0.241 | 0.006 | −2.451 | 0.016 |
| SD value of term birth weight | 0.347 | <0.001 | 2.480 | 0.015 |
| Chronic lung disease | −0.235 | 0.080 | 2.371 | 0.019 |
| Periventricular leukomalacia | −0.292 | 0.001 | 3.431 | 0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Pearson’s Correlation Coefficient | p Value | t Value | p Value | |
| Birth weight | 0.218 | 0.020 | 0.661 | 0.510 |
| PDA closure date | −0.234 | 0.012 | −2.034 | 0.044 |
| SD value of term birth weight | 0.399 | <0.001 | 4.285 | <0.001 |
| Chronic lung disease | −0.206 | 0.028 | 1.026 | 0.307 |
| Periventricular leukomalacia | −0.163 | 0.083 | 1.175 | 0.242 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Dependent Variables | Pearson’s Correlation Coefficient | p Value | t Value | p Value |
| Posture/motor | −0.312 | 0.001 | −3.444 | 0.001 |
| Cognition/adaptation | −0.208 | 0.027 | −0.651 | 0.517 |
| Language/sociality | −0.132 | 0.163 | −0.158 | 0.874 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Pearson’s Correlation Coefficient | p Value | t Value | p Value | |
| Birth weight | 0.375 | <0.001 | 2.163 | 0.031 |
| PDA exposure time | −0.273 | <0.001 | −2.866 | 0.004 |
| SD value of term birth weight | 0.277 | <0.001 | 2.562 | 0.011 |
| Chronic lung disease | 0.355 | <0.001 | 2.131 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, N.; Goto, T.; Katsumata, N.; Murakami, Y.; Shinohara, T.; Maebayashi, Y.; Sakakibara, A.; Saito, C.; Hasebe, Y.; Hoshiai, M.; et al. Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome. J. Cardiovasc. Dev. Dis. 2024, 11, 26. https://doi.org/10.3390/jcdd11010026
Kikuchi N, Goto T, Katsumata N, Murakami Y, Shinohara T, Maebayashi Y, Sakakibara A, Saito C, Hasebe Y, Hoshiai M, et al. Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome. Journal of Cardiovascular Development and Disease. 2024; 11(1):26. https://doi.org/10.3390/jcdd11010026
Chicago/Turabian StyleKikuchi, Natsumi, Taichiro Goto, Nobuyuki Katsumata, Yasushi Murakami, Tamao Shinohara, Yuki Maebayashi, Aiko Sakakibara, Chisato Saito, Yohei Hasebe, Minako Hoshiai, and et al. 2024. "Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome" Journal of Cardiovascular Development and Disease 11, no. 1: 26. https://doi.org/10.3390/jcdd11010026
APA StyleKikuchi, N., Goto, T., Katsumata, N., Murakami, Y., Shinohara, T., Maebayashi, Y., Sakakibara, A., Saito, C., Hasebe, Y., Hoshiai, M., Nemoto, A., & Naito, A. (2024). Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome. Journal of Cardiovascular Development and Disease, 11(1), 26. https://doi.org/10.3390/jcdd11010026






