Exploring QRS Area beyond Patient Selection in CRT—Can It Guide Left Ventricular Lead Placement?
Abstract
1. Introduction
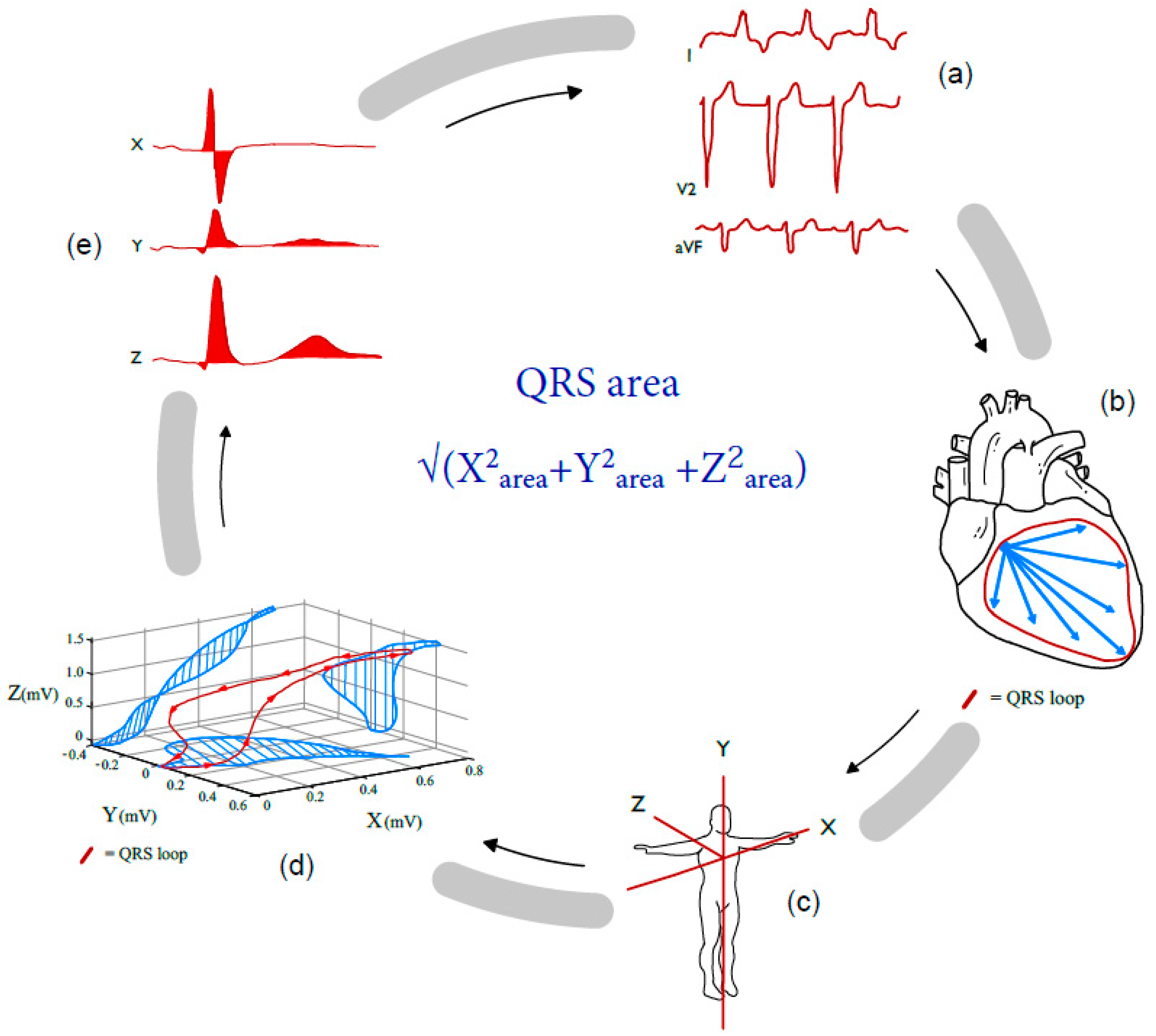
2. Twelve-Lead ECG-Derived Markers of Dyssynchrony; QRS Duration, Morphology and the Potential Role of Vectorcardiographic QRS Area
3. The Importance of LV Lead Position in Benefit from CRT
4. QRS Area in LVLP
5. QRS Area in Conduction System Pacing
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Weston, S.A.; Jacobsen, S.J.; Roger, V.L. Risk factors for heart failure: A population-based case-control study. Am. J. Med. 2009, 122, 1023–1028. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Dikdan, S.J.; Co, M.L.; Pavri, B.B. Dyssynchronous Heart Failure: A Clinical Review. Curr. Cardiol. Rep. 2022, 24, 1957–1972. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- van Stipdonk, A.M.W.; Ter Horst, I.; Kloosterman, M.; Engels, E.B.; Rienstra, M.; Crijns, H.; Vos, M.A.; van Gelder, I.C.; Prinzen, F.W.; Meine, M.; et al. QRS Area Is a Strong Determinant of Outcome in Cardiac Resynchronization Therapy. Circ. Arrhythmia Electrophysiol. 2018, 11, e006497. [Google Scholar] [CrossRef]
- Tokavanich, N.; Prasitlumkum, N.; Mongkonsritragoon, W.; Trongtorsak, A.; Cheungpasitporn, W.; Chokesuwattanaskul, R. QRS area as a predictor of cardiac resynchronization therapy response: A systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2022, 45, 393–400. [Google Scholar] [CrossRef]
- Mullens, W.; Grimm, R.A.; Verga, T.; Dresing, T.; Starling, R.C.; Wilkoff, B.L.; Tang, W.H. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J. Am. Coll. Cardiol. 2009, 53, 765–773. [Google Scholar] [CrossRef]
- Singh, J.P.; Klein, H.U.; Huang, D.T.; Reek, S.; Kuniss, M.; Quesada, A.; Barsheshet, A.; Cannom, D.; Goldenberg, I.; McNitt, S.; et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation 2011, 123, 1159–1166. [Google Scholar] [CrossRef]
- Bose, A.; Kandala, J.; Upadhyay, G.A.; Riedl, L.; Ahmado, I.; Padmanabhan, R.; Gewirtz, H.; Mulligan, L.J.; Singh, J.P. Impact of myocardial viability and left ventricular lead location on clinical outcome in cardiac resynchronization therapy recipients with ischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2014, 25, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.W.; Laksman, Z.; Gepstein, L. Making better scar: Emerging approaches for modifying mechanical and electrical properties following infarction and ablation. Prog. Biophys. Mol. Biol. 2016, 120, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.J.; Umar, F.; Panting, J.R.; Stegemann, B.; Leyva, F. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm 2016, 13, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.Z.; Virdee, M.S.; Palmer, C.R.; Pugh, P.J.; O’Halloran, D.; Elsik, M.; Read, P.A.; Begley, D.; Fynn, S.P.; Dutka, D.P. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: The TARGET study: A randomized, controlled trial. J. Am. Coll. Cardiol. 2012, 59, 1509–1518. [Google Scholar] [CrossRef]
- Arnold, A.D.; Shun-Shin, M.J.; Keene, D.; Howard, J.P.; Sohaib, S.M.A.; Wright, I.J.; Cole, G.D.; Qureshi, N.A.; Lefroy, D.C.; Koa-Wing, M.; et al. His Resynchronization Versus Biventricular Pacing in Patients With Heart Failure and Left Bundle Branch Block. J. Am. Coll. Cardiol. 2018, 72, 3112–3122. [Google Scholar] [CrossRef]
- Rickard, J.; Jackson, K.; Gold, M.; Biffi, M.; Ziacchi, M.; Silverstein, J.; Ramza, B.; Metzl, M.; Grubman, E.; Abben, R.; et al. Electrocardiogram Belt guidance for left ventricular lead placement and biventricular pacing optimization. Heart Rhythm 2023, 20, 537–544. [Google Scholar] [CrossRef]
- Molhoek, S.G.; Bax, J.J.; Boersma, E.; Erven, L.V.; Bootsma, M.; Steendijk, P.; Van Der Wall, E.E.; Schalij, M.J. QRS duration and shortening to predict clinical response to cardiac resynchronization therapy in patients with end-stage heart failure. Pacing Clin. Electrophysiol. 2004, 27, 308–313. [Google Scholar] [CrossRef]
- Okafor, O.; Zegard, A.; van Dam, P.; Stegemann, B.; Qiu, T.; Marshall, H.; Leyva, F. Changes in QRS Area and QRS Duration After Cardiac Resynchronization Therapy Predict Cardiac Mortality, Heart Failure Hospitalizations, and Ventricular Arrhythmias. J. Am. Heart Assoc. 2019, 8, e013539. [Google Scholar] [CrossRef]
- van Stipdonk, A.M.W.; Vanbelle, S.; Ter Horst, I.A.H.; Luermans, J.G.; Meine, M.; Maass, A.H.; Auricchio, A.; Prinzen, F.W.; Vernooy, K. Large variability in clinical judgement and definitions of left bundle branch block to identify candidates for cardiac resynchronisation therapy. Int. J. Cardiol. 2019, 286, 61–65. [Google Scholar] [CrossRef]
- Brandtvig, T.O.; Marinko, S.; Farouq, M.; Brandt, J.; Mörtsell, D.; Wang, L.; Chaudhry, U.; Saba, S.; Borgquist, R. Association between left ventricular lead position and intrinsic QRS morphology with regard to clinical outcome in cardiac resynchronization therapy for heart failure. Ann. Noninvasive Electrocardiol. 2023, 28, e13065. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, R.; Marinko, S.; Platonov, P.G.; Wang, L.; Chaudhry, U.; Mortsell, G. Structured optimization of QRS duration reduction post-Cardiac Resynchronization Therapy is feasible and shorter QRS duration is associated with better clinical outcome. Europace 2022, 24 (Suppl. S1), euac053.503. [Google Scholar] [CrossRef]
- Bazoukis, G.; Naka, K.K.; Alsheikh-Ali, A.; Tse, G.; Letsas, K.P.; Korantzopoulos, P.; Liu, T.; Yeung, C.; Efremidis, M.; Tsioufis, K.; et al. Association of QRS narrowing with response to cardiac resynchronization therapy-a systematic review and meta-analysis of observational studies. Heart Fail Rev. 2020, 25, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.D.; Bode, M.F.; Gettes, L.; Jensen, B.C.; Mounsey, J.P.; Chung, E.H. Prominent R wave in ECG lead V1 predicts improvement of left ventricular ejection fraction after cardiac resynchronization therapy in patients with or without left bundle branch block. Heart Rhythm 2015, 12, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.B.; Alshehri, S.; van Deursen, C.J.; Wecke, L.; Bergfeldt, L.; Vernooy, K.; Prinzen, F.W. The synthesized vectorcardiogram resembles the measured vectorcardiogram in patients with dyssynchronous heart failure. J. Electrocardiol. 2015, 48, 586–592. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, C.J.; Vernooy, K.; Dudink, E.; Bergfeldt, L.; Crijns, H.J.; Prinzen, F.W.; Wecke, L. Vectorcardiographic QRS area as a novel predictor of response to cardiac resynchronization therapy. J. Electrocardiol. 2015, 48, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.M.; Blaauw, Y.; Dinh, T.; Pison, L.; Crijns, H.J.; Prinzen, F.W.; Vernooy, K. Left ventricular lead placement in the latest activated region guided by coronary venous electroanatomic mapping. Europace 2015, 17, 84–93. [Google Scholar] [CrossRef]
- Maass, A.H.; Vernooy, K.; Wijers, S.C.; van ’t Sant, J.; Cramer, M.J.; Meine, M.; Allaart, C.P.; De Lange, F.J.; Prinzen, F.W.; Gerritse, B.; et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: Results from the Markers and Response to CRT (MARC) study. Europace 2018, 20, e1–e10. [Google Scholar] [CrossRef]
- Leyva, F.; Foley, P.W.; Chalil, S.; Ratib, K.; Smith, R.E.; Prinzen, F.; Auricchio, A. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2011, 13, 29. [Google Scholar] [CrossRef]
- Butter, C.; Auricchio, A.; Stellbrink, C.; Fleck, E.; Ding, J.; Yu, Y.; Huvelle, E.; Spinelli, J. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001, 104, 3026–3029. [Google Scholar] [CrossRef]
- Singh, J.P.; Fan, D.; Heist, E.K.; Alabiad, C.R.; Taub, C.; Reddy, V.; Mansour, M.; Picard, M.H.; Ruskin, J.N.; Mela, T. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm 2006, 3, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Zareba, W.; McNitt, S.; Singh, J.; Hall, W.J.; Polonsky, S.; Goldenberg, I.; Huang, D.T.; Merkely, B.; Wang, P.J.; et al. Left ventricular lead location and the risk of ventricular arrhythmias in the MADIT-CRT trial. Eur. Heart J. 2013, 34, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ypenburg, C.; Schalij, M.J.; Bleeker, G.B.; Steendijk, P.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.; van der Wall, E.E.; Bax, J.J. Extent of viability to predict response to cardiac resynchronization therapy in ischemic heart failure patients. J. Nucl. Med. 2006, 47, 1565–1570. [Google Scholar] [PubMed]
- Xu, Y.Z.; Cha, Y.M.; Feng, D.; Powell, B.D.; Wiste, H.J.; Hua, W.; Chareonthaitawee, P. Impact of myocardial scarring on outcomes of cardiac resynchronization therapy: Extent or location? J. Nucl. Med. 2012, 53, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bisson, A.; Pucheux, J.; Andre, C.; Bernard, A.; Pierre, B.; Babuty, D.; Fauchier, L.; Clementy, N. Localization of Left Ventricular Lead Electrodes in Relation to Myocardial Scar in Patients Undergoing Cardiac Resynchronization Therapy. J. Am. Heart Assoc. 2018, 7, e009502. [Google Scholar] [CrossRef] [PubMed]
- Huntjens, P.R.; Walmsley, J.; Ploux, S.; Bordachar, P.; Prinzen, F.W.; Delhaas, T.; Lumens, J. Influence of left ventricular lead position relative to scar location on response to cardiac resynchronization therapy: A model study. Europace 2014, 16 (Suppl. S4), iv62–iv68. [Google Scholar] [CrossRef]
- Stephansen, C.; Sommer, A.; Kronborg, M.B.; Jensen, J.M.; Nørgaard, B.L.; Gerdes, C.; Kristensen, J.; Jensen, H.K.; Fyenbo, D.B.; Bouchelouche, K.; et al. Electrically vs. imaging-guided left ventricular lead placement in cardiac resynchronization therapy: A randomized controlled trial. Europace 2019, 21, 1369–1377. [Google Scholar] [CrossRef]
- Nguyen, U.C.; Mafi-Rad, M.; Aben, J.P.; Smulders, M.W.; Engels, E.B.; van Stipdonk, A.M.; Luermans, J.G.; Bekkers, S.C.; Prinzen, F.W.; Vernooy, K. A novel approach for left ventricular lead placement in cardiac resynchronization therapy: Intraprocedural integration of coronary venous electroanatomic mapping with delayed enhancement cardiac magnetic resonance imaging. Heart Rhythm 2017, 14, 110–119. [Google Scholar] [CrossRef]
- Tseng, C.D.; Tseng, Y.Z.; Carson, W.; Lo, H.M.; Hsu, K.L.; Chiang, F.T.; Wu, T.L. Vectorcardiography in experimental myocardial infarction. Serial changes and correlation between QRS loop change and the infarction size. Jpn. Heart J. 1995, 36, 349–365. [Google Scholar] [CrossRef][Green Version]
- Nguyen, U.C.; Claridge, S.; Vernooy, K.; Engels, E.B.; Razavi, R.; Rinaldi, C.A.; Chen, Z.; Prinzen, F.W. Relationship between vectorcardiographic QRS(area), myocardial scar quantification, and response to cardiac resynchronization therapy. J. Electrocardiol. 2018, 51, 457–463. [Google Scholar] [CrossRef]
- Okafor, O.; Umar, F.; Zegard, A.; van Dam, P.; Walton, J.; Stegemann, B.; Marshall, H.; Leyva, F. Effect of QRS area reduction and myocardial scar on the hemodynamic response to cardiac resynchronization therapy. Heart Rhythm 2020, 17, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, M.A.; Zanon, F.; Salden, F.; van Stipdonk, A.; Marcantoni, L.; Engels, E.; Luermans, J.; Westra, S.; Prinzen, F.; Vernooy, K. Left Ventricular Lead Placement Guided by Reduction in QRS Area. J. Clin. Med. 2021, 10, 5935. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.B.; Thibault, B.; Mangual, J.; Badie, N.; McSpadden, L.C.; Calo, L.; Ritter, P.; Pappone, C.; Bode, K.; Varma, N.; et al. Dynamic atrioventricular delay programming improves ventricular electrical synchronization as evaluated by 3D vectorcardiography. J. Electrocardiol. 2020, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.B.; Mafi-Rad, M.; Hermans, B.J.M.; Aranda, A.; van Stipdonk, A.M.W.; Rienstra, M.; Scheerder, C.O.S.; Maass, A.H.; Prinzen, F.W.; Vernooy, K. Tailoring device settings in cardiac resynchronization therapy using electrograms from pacing electrodes. Europace 2018, 20, 1146–1153. [Google Scholar] [CrossRef]
- Liu, X.; Gu, M.; Niu, H.X.; Chen, X.; Cai, C.; Zhao, J.; Cai, M.; Zhou, X.; Gold, M.R.; Zhang, S.; et al. A Comparison of the Electrophysiological and Anatomic Characteristics of Pacing Different Branches of the Left Bundle Conduction System. Front. Cardiovasc. Med. 2021, 8, 781845. [Google Scholar] [CrossRef]
- Heckman, L.I.B.; Luermans, J.; Curila, K.; Van Stipdonk, A.M.W.; Westra, S.; Smisek, R.; Prinzen, F.W.; Vernooy, K. Comparing Ventricular Synchrony in Left Bundle Branch and Left Ventricular Septal Pacing in Pacemaker Patients. J. Clin. Med. 2021, 10, 822. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eerenberg, F.; Luermans, J.; Lumens, J.; Nguyên, U.C.; Vernooy, K.; van Stipdonk, A. Exploring QRS Area beyond Patient Selection in CRT—Can It Guide Left Ventricular Lead Placement? J. Cardiovasc. Dev. Dis. 2024, 11, 18. https://doi.org/10.3390/jcdd11010018
Eerenberg F, Luermans J, Lumens J, Nguyên UC, Vernooy K, van Stipdonk A. Exploring QRS Area beyond Patient Selection in CRT—Can It Guide Left Ventricular Lead Placement? Journal of Cardiovascular Development and Disease. 2024; 11(1):18. https://doi.org/10.3390/jcdd11010018
Chicago/Turabian StyleEerenberg, Frederieke, Justin Luermans, Joost Lumens, Uyên Châu Nguyên, Kevin Vernooy, and Antonius van Stipdonk. 2024. "Exploring QRS Area beyond Patient Selection in CRT—Can It Guide Left Ventricular Lead Placement?" Journal of Cardiovascular Development and Disease 11, no. 1: 18. https://doi.org/10.3390/jcdd11010018
APA StyleEerenberg, F., Luermans, J., Lumens, J., Nguyên, U. C., Vernooy, K., & van Stipdonk, A. (2024). Exploring QRS Area beyond Patient Selection in CRT—Can It Guide Left Ventricular Lead Placement? Journal of Cardiovascular Development and Disease, 11(1), 18. https://doi.org/10.3390/jcdd11010018





