Echocardiographic Assessment in Patients Recovered from Acute COVID-19 Illness
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
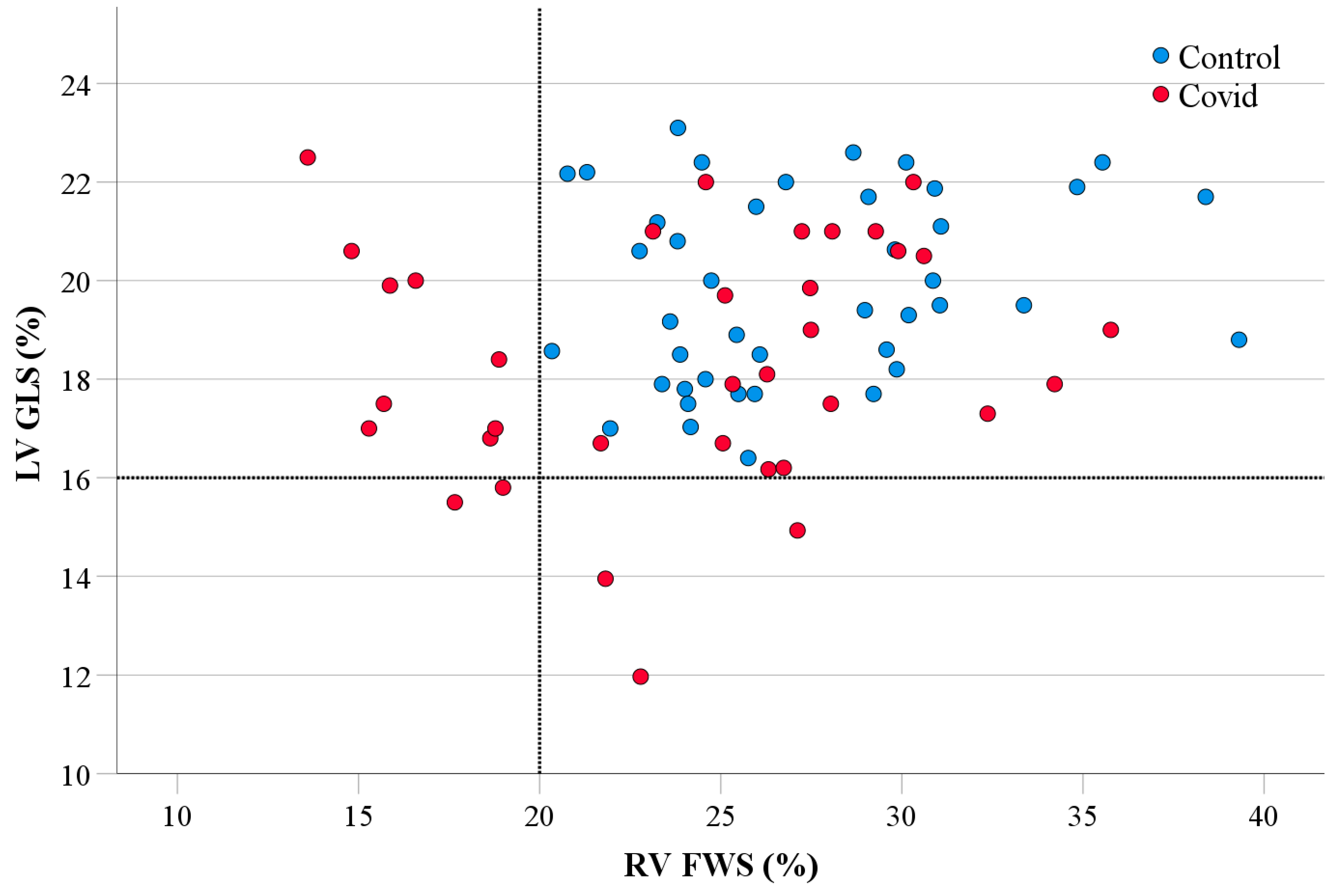
- The COVID-19 cohort, even without cardiovascular history or risk factors, had lower LV GLS when compared to the healthy controls.
- Abnormal LV GLS and RV FWS were exhibited almost evenly between hospitalized and non-hospitalized patients.
- There is a modest correlation between COVID-19 infection and both LV GLS and RV FWS (Tables S1 and S2).
4.1. Clinical Perspectives
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, T.; Mui, D.; Ferrari, V.; Jagasia, D.; Scherrer-Crosbie, M.; Chen, Y.; Han, Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020, 106, 1132–1141. [Google Scholar] [CrossRef]
- Ruan, S. Likelihood of survival of coronavirus disease 2019. Lancet Infect. Dis. 2020, 20, 630–631. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Di Salvo, G.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef]
- Boldrini, M.; Cappelli, F.; Chacko, L.; Restrepo-Cordoba, M.A.; Lopez-Sainz, A.; Giannoni, A.; Aimo, A.; Baggiano, A.; Martinez-Naharro, A.; Whelan, C.; et al. Multiparametric Echocardiography Scores for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 909–920. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Sanderson, J.E.; Marwick, T.H.; Oh, J.K. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J. Am. Coll. Cardiol. 2007, 49, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11 Pt 1, 260–274. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Baruch, G.; Rothschild, E.; Sadon, S.; Szekely, Y.; Lichter, Y.; Kaplan, A.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; et al. Evolution of right and left ventricle routine and speckle-tracking echocardiography in patients recovering from coronavirus disease 2019: A longitudinal study. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 1055–1065. [Google Scholar] [CrossRef]
- Mahajan, S.; Kunal, S.; Shah, B.; Garg, S.; Palleda, G.M.; Bansal, A.; Batra, V.; Yusuf, J.; Mukhopadhyay, S.; Kumar, S.; et al. Left ventricular global longitudinal strain in COVID-19 recovered patients. Echocardiography 2021, 38, 1722–1730. [Google Scholar] [CrossRef]
- Wu, X.; Deng, K.-Q.; Li, C.; Yang, Z.; Hu, H.; Cai, H.; Zhang, C.; He, T.; Zheng, F.; Wang, H.; et al. Cardiac Involvement in Recovered Patients From COVID-19: A Preliminary 6-Month Follow-Up Study. Front. Cardiovasc. Med. 2021, 8, 654405. [Google Scholar] [CrossRef] [PubMed]
- Özer, S.; Candan, L.; Özyıldız, A.G.; Turan, O.E. Evaluation of left ventricular global functions with speckle tracking echocardiography in patients recovered from COVID-19. Int. J. Cardiovasc. Imaging 2021, 37, 2227–2233. [Google Scholar] [CrossRef]
- Nuzzi, V.; Castrichini, M.; Collini, V.; Roman-Pognuz, E.; Di Bella, S.; Luzzati, R.; Berlot, G.; Confalonieri, M.; Merlo, M.; Stolfo, D.; et al. Impaired Right Ventricular Longitudinal Strain Without Pulmonary Hypertension in Patients Who Have Recovered From COVID-19. Circ. Cardiovasc. Imaging 2021, 14, e012166. [Google Scholar] [CrossRef]
- Baykiz, D.; Govdeli, E.A.; Ozer, P.K.; Karaayvaz, E.B.; Catma, Y.; Medetalibeyoglu, A.; Cagatay, A.; Umman, B.; Tukek, T.; Bugra, Z. Evaluation the relationship of left ventricular global longitudinal strain and laboratory parameters in discharged patients with COVID-19: A follow-up study. Int. J. Cardiovasc. Imaging 2021, 37, 2451–2464. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.; Silverwood, R.J.; Gessa, G.D.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.K.; Prins, J.B. The link between abdominal obesity and the metabolic syndrome. Curr. Hypertens. Rep. 2008, 10, 156–164. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Izzo, C.; Visco, V.; Gambardella, J.; Ferruzzi, G.J.; Rispoli, A.; Rusciano, M.R.; Toni, A.L.; Virtuoso, N.; Carrizzo, A.; Di Pietro, P.; et al. Cardiovascular Implications of microRNAs in Coronavirus Disease 2019. J. Pharmacol. Exp. Ther. 2023, 384, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef]
- Halushka, M.K.; Heide, R.S.V. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2020, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Mrudula, A.S.S.; Agarwal, P.M.; Vempati, R.M.; Alla, D.M.; Balusu, K.M.; Tarannum, S.M.; Patel, K.M.; Devaragudi, S.M.; Patel, D.M.; Sultana, Q.M.; et al. Clinical outcome of established diagnostic and treatment modalities of COVID-19-associated myocarditis: A systematic review. Ann. Med. Surg. 2023, 85, 3583–3594. [Google Scholar] [CrossRef]
- Meindl, C.; Paulus, M.; Poschenrieder, F.; Zeman, F.; Maier, L.S.; Debl, K. Patients with acute myocarditis and preserved systolic left ventricular function: Comparison of global and regional longitudinal strain imaging by echocardiography with quantification of late gadolinium enhancement by CMR. Clin. Res. Cardiol. 2021, 110, 1792–1800. [Google Scholar] [CrossRef]
- D’andrea, A.; Cante, L.; Palermi, S.; Carbone, A.; Ilardi, F.; Sabatella, F.; Crescibene, F.; Di Maio, M.; Giallauria, F.; Messalli, G.; et al. COVID-19 Myocarditis: Prognostic Role of Bedside Speckle-Tracking Echocardiography and Association with Total Scar Burden. Int. J. Environ. Res. Public Health 2022, 19, 5898. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Plein, S.; Wong, T.C.; Tao, Q.; Raisi-Estabragh, Z.; Jain, S.S.; Han, Y.; Ojha, V.; Bluemke, D.A.; Hanneman, K.; et al. Cardiovascular magnetic resonance for evaluation of cardiac involvement in COVID-19: Recommendations by the Society for Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Webb, S. CMR in patients with COVID-19—American College of Cardiology. American College of Cardiology. Available online: https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2022/04/19/19/30/cardiovascular-magnetic-resonance-for-patients (accessed on 19 April 2022).
- Beitzke, D.; Salgado, R.; Francone, M.; Kreitner, K.-F.; Natale, L.; Bremerich, J.; Gutberlet, M.; Mousseaux, E.; Nikolaou, K.; Peebles, C.; et al. Cardiac imaging procedures and the COVID-19 pandemic: Recommendations of the European Society of Cardiovascular Radiology (ESCR). Int. J. Cardiovasc. Imaging 2020, 36, 1801–1810. [Google Scholar] [CrossRef]
| Controls n = 42 | COVID-19 n = 42 | |
|---|---|---|
| Age (y) | 46.6 ± 16.4 | 46.8 ± 16.9 |
| Female | 27 (64) | 27 (64) |
| Height (cm) | 166.3 ± 8.3 | 166.3 ± 11.3 |
| Weight (kg) | 70 ± 12.5 | 78.3 ± 21.3 * |
| BMI (kg/m2) | 25.3 ± 3.9 | 28.0 ± 5.4 * |
| Body surface area (m2) | 1.78 ± 0.18 | 1.86 ± 0.29 |
| Systolic blood pressure (mmHg) | 122.8 ± 11.2 | 122.6 ± 15.8 |
| Diastolic blood pressure (mmHg) | 74.4 ± 6.9 | 76.9 ± 9.7 |
| Diabetic | 0 | 2 (4.7) |
| Hypertensive | 0 | 6 (14.3) |
| Hypertensive and diabetic | 0 | 4 (9.5) |
| Ischemic heart disease | 0 | 1 (2.4) |
| Atrial fibrillation | 0 | 2 (4.7) |
| Admitted to hospital | 0 | 23 (54.8) |
| Length in hospital | N/A | 7 [2–12] |
| Admitted to ICU | 0 | 6 (14.3) |
| Length in ICU | N/A | 11.5 [5–49.75] |
| Required respiratory support (O2) | 0 | 10 (23.8) |
| Endotracheal tube | 0 | 1 (2.4) |
| Tracheostomy | 0 | 2 (4.8) |
| Time from infection to echo follow up | N/A | 112 [68.75–178] |
| Entire Cohort | No Cardiovascular Risk Factors | |||||
|---|---|---|---|---|---|---|
| Echocardiographic Parameter | Controls n = 42 | COVID-19 n = 42 | p Value | Controls n = 30 | COVID-19 n = 30 | p Value |
| LV systolic function and volumes | ||||||
| LVEDD (mm) | 47.15 ± 4.44 | 45.45 ± 5.57 | 0.036 | 48.27 ± 4.33 | 45.77 ± 6.25 | 0.077 |
| LVESD (mm) | 30.26 ± 4.82 | 29.60 ± 6.08 | 0.315 | 31.79 ± 4.34 | 30.27 ± 6.75 | 0.302 |
| PW (mm) | 8.12 ± 1.64 | 8.76 ± 1.56 | 0.069 | 7.89 ± 1.72 | 8.43 ± 1.59 | 0.206 |
| IVS (mm) | 8.17 ± 1.82 | 9.17 ± 1.89 | 0.016 | 7.90 ± 1.85 | 8.83 ± 1.90 | 0.059 |
| LV mass (g/m2) | 70.90 ± 16.95 | 73.54 ± 23.09 | 0.741 | 70.26 ± 18.60 | 69.03 ± 24.05 | 0.826 |
| LVEDV (mL/m2) | 47.10 ± 10.89 | 46.70 ± 17.28 | 0.989 | 47.89 ± 10.72 | 47.65 ± 17.73 | 0.951 |
| LVESV (mL/m2) | 19.25 ± 5.14 | 18.91 ± 9.21 | 0.499 | 20.05 ± 4.96 | 19.69 ± 10.09 | 0.859 |
| LVEF (%) | 59.40 ± 5.27 | 60.19 ± 5.42 | 0.503 | 58.43 ± 5.22 | 59.80 ± 5.72 | 0.338 |
| LV GLS (%) | 19.86 ± 1.90 | 18.33 ± 2.43 | 0.004 | 19.57 ± 1.80 | 18.10 ± 2.45 | 0.010 |
| Abnormal LV GLS (<16%) | 0 | 6 (14.3) | 0.011 | 0 | 5 (16.7) | 0.020 |
| LV diastolic function | ||||||
| Peak E (cm/s) | 75.23 ± 15.99 | 73.14 ± 18.08 | 0.577 | 78.44 ± 15.93 | 77.57 ± 19.26 | 0.848 |
| Peak A (cm/s) | 60.02 ± 15.99 | 67.55 ± 16.92 | 0.166 | 55.73 ± 12.57 | 61.90 ± 15.92 | 0.101 |
| Septal e’ (cm/s) | 8.75 ± 2.34 | 7.98 ± 2.88 | 0.176 | 9.54 ± 2.10 | 8.83 ± 2.91 | 0.286 |
| Lateral e’ (cm/s) | 11.88 ± 3.12 | 10.69 ± 4.68 | 0.089 | 12.77 ± 3.00 | 12.17 ± 4.67 | 0.557 |
| E/mean e’ | 7.55 ± 1.81 | 8.68 ± 2.69 | 0.026 | 7.25 ± 1.84 | 8.16 ± 2.53 | 0.116 |
| LAVImax (mL/m2) | 28.20 ± 6.72 | 30.00 ± 9.25 | 0.604 | 28.38 ± 6.25 | 29.27 ± 8.55 | 0.646 |
| RV systolic function, volumes, and pressure | ||||||
| TAPSE (mm) | 22.40 ± 3.77 | 21.48 ± 4.65 | 0.425 | 22.47 ± 3.87 | 21.83 ± 3.77 | 0.523 |
| RV S’ (m/s) | 11.51 ± 1.60 | 11.93 ± 2.09 | 0.427 | 11.70 ± 1.67 | 12.10 ± 2.01 | 0.399 |
| RVEDV (mL/m2) | 36.67 ± 13.08 | 40.70 ± 20.04 | 0.784 | 39.50 ± 13.55 | 44.52 ± 20.02 | 0.263 |
| RVESV (mL/m2) | 17.29 ± 7.36 | 19.30 ± 10.08 | 0.446 | 18.67 ± 7.56 | 21.31 ± 10.49 | 0.270 |
| RVEF (%) | 52.98 ± 9.21 | 52.30 ± 10.15 | 0.753 | 53.07 ± 7.94 | 51.97 ± 10.96 | 0.659 |
| RVEDA (cm2) | 17.46 ± 3.92 | 18.61 ± 5.37 | 0.626 | 18.41 ± 3.93 | 19.80 ± 5.29 | 0.255 |
| RVESA (cm2) | 10.35 ± 2.69 | 11.15 ± 3.36 | 0.339 | 10.90 ± 2.73 | 11.87 ± 3.35 | 0.227 |
| RV FAC (%) | 40.81 ± 6.70 | 39.79 ± 7.98 | 0.533 | 41.02 ± 5.81 | 39.77 ± 8.33 | 0.505 |
| RVSP (mmHg) | 20.41 ± 5.18 | 22.63 ± 5.17 | 0.129 | 19.80 ± 3.35 | 21.55 ± 5.03 | 0.237 |
| RV septal strain (%) | 18.15 ± 3.47 | 17.18 ± 3.50 | 0.216 | 18.31 ± 3.28 | 17.88 ± 3.10 | 0.609 |
| RV FWS (%) | 27.32 ± 4.56 | 24.05 ± 5.89 | 0.008 | 26.86 ± 3.68 | 25.08 ± 5.40 | 0.151 |
| RV global strain (%) | 22.73 ± 3.27 | 20.62 ± 4.03 | 0.013 | 22.58 ± 2.85 | 21.46 ± 3.47 | 0.188 |
| Abnormal RV FWS (<20%) | 0 | 11 (31.4) | <0.001 | 0 | 6 (23.1) | 0.005 |
| Abnormal RV global strain (<17%) | 0 | 9 (25.7) | <0.001 | 0 | 4 (15.4) | 0.026 |
| Parameters | LV GLS | ||
|---|---|---|---|
| ≥16% (Normal) n = 36 | <16% (Abnormal) n = 6 | p Value | |
| Age (y) | 47.92 ± 17.50 | 39.83 ± 11.79 | 0.284 |
| Female | 25 (69.4) | 2 (33.3) | 0.087 |
| BMI (kg/m2) | 27.97 ± 5.55 | 28.41 ± 4.95 | 0.857 |
| Diabetes | 1 (2.8) | 1 (16.7) | 0.139 |
| Hypertensive | 6 (16.7) | 0 | 0.280 |
| Hypertensive and diabetic | 4 (11.1) | 0 | 0.391 |
| Atrial fibrillation | 2 (5.6) | 0 | 0.554 |
| Ischemic heart disease | 1 (2.8) | 0 | 0.679 |
| Admitted to hospital | 20 (55.6) | 3 (50) | 0.800 |
| Admitted to ICU | 3 (8.3) | 3 (50) | 0.007 |
| Required oxygen | 7 (19.4) | 3 (50) | 0.104 |
| Invasive respiratory support | 1 (2.8) | 2 (33.3) | 0.007 |
| PW (mm) | 8.67 ± 1.60 | 9.33 ± 1.21 | 0.281 |
| IVS (mm) | 8.92 ± 1.75 | 10.67 ± 2.16 | 0.034 |
| LV mass (g/m2) | 70.11 ± 17.76 | 94.08 ± 39.63 | 0.069 |
| LVEDV (mL/m2) | 44.16 ± 15.10 | 61.97 ± 22.93 | 0.017 |
| LVESV (mL/m2) | 17.34 ± 6.49 | 28.33 ± 16.63 | 0.053 |
| LVEF (%) | 60.81 ± 4.71 | 56.50 ± 8.19 | 0.208 |
| TAPSE (mm) | 21.48 ± 4.91 | 21.50 ± 2.88 | 0.847 |
| RV S’ (m/s) | 11.88 ± 1.97 | 12.17 ± 2.93 | 0.763 |
| RVEDA (cm2) | 17.94 ± 5.02 | 22.42 ± 6.19 | 0.065 |
| RVESA (cm2) | 10.59 ± 2.90 | 14.32 ± 4.25 | 0.027 |
| FAC (%) | 40.43 ± 8.19 | 36.17 ± 5.98 | 0.232 |
| RV FWS (%) | 24.44 ± 6.13 | 21.68 ± 3.68 | 0.338 |
| Peak E (cm/s) | 73.69 ± 19.12 | 69.83 ± 10.25 | 0.634 |
| Peak A (cm/s) | 68.81 ± 16.91 | 60.00 ± 16.31 | 0.297 |
| Septal e’ (cm/s) | 7.89 ± 2.94 | 8.50 ± 2.59 | 0.636 |
| Lateral e’ (cm/s) | 10.58 ± 4.31 | 11.33 ± 7.00 | 0.875 |
| E/mean e’ | 8.69 ± 2.59 | 8.67 ± 3.50 | 0.987 |
| LAVImax (mL/m2) | 29.17 ± 8.81 | 35.00 ± 11.10 | 0.155 |
| Parameters | RV FWS | ||
|---|---|---|---|
| ≥20% (Normal) n = 24 | <20% (Abnormal) n = 11 | p Value | |
| Age (y) | 41.46 ± 15.85 | 53.09 ± 16.96 | 0.057 |
| Female | 15 (62.5) | 7 (63.6) | 1.000 |
| BMI (kg/m2) | 26.44 ± 4.65 | 29.31 ± 4.411 | 0.094 |
| Diabetes | 1 (4.2) | 1 (9.1) | 0.536 |
| Hypertensive | 2 (8.3) | 3 (27.3) | 0.297 |
| Hypertensive and diabetic | 1 (4.2) | 1 (9.1) | 0.536 |
| Atrial fibrillation | 0 (0) | 1 (9.1) | 0.314 |
| Ischemic heart disease | 1 (4.2) | 0 (0) | 1.000 |
| Admitted to hospital | 11 (45.8) | 6 (54.5) | 0.725 |
| Admitted to ICU | 2 (8.3) | 2 (18.2) | 0.575 |
| Required oxygen | 3 (12.5) | 3 (27.3) | 0.352 |
| Invasive respiratory support | 0 (0) | 2 (18.2) | 0.092 |
| PW (mm) | 8.54 ± 1.69 | 9.00 ± 1.55 | 0.451 |
| IVS (mm) | 8.75 ± 2.03 | 9.64 ± 1.29 | 0.194 |
| LV mass (g/m2) | 74.06 ± 27.81 | 71.26 ± 18.02 | 0.986 |
| LVEDV (mL/m2) | 50.95 ± 17.40 | 41.84 ± 16.95 | 0.072 |
| LVESV (mL/m2) | 21.25 ± 10.41 | 16.63 ± 6.78 | 0.142 |
| LVEF (%) | 59.50 ± 6.12 | 60.18 ± 3.92 | 0.738 |
| LV GLS (%) | 18.42 ± 2.62 | 18.27 ± 2.21 | 0.877 |
| TAPSE (mm) | 22.22 ± 5.73 | 19.82 ± 2.48 | 0.027 |
| RV S’ (m/s) | 12.46 ± 2.13 | 10.60 ± 1.84 | 0.025 |
| RVEDA (cm2) | 19.48 ± 5.67 | 17.34 ± 5.02 | 0.299 |
| RVESA (cm2) | 11.43 ± 3.62 | 10.56 ± 3.08 | 0.713 |
| FAC (%) | 41.18 ± 7.53 | 38.49 ± 9.98 | 0.383 |
| Peak E (cm/s) | 79.21 ± 17.78 | 61.36 ± 10.38 | 0.004 |
| Peak A (cm/s) | 63.63 ± 16.96 | 70.27 ± 16.61 | 0.287 |
| Septal e’ (cm/s) | 8.88 ± 2.97 | 7.00 ± 2.53 | 0.078 |
| Lateral e’ (cm/s) | 11.83 ± 4.79 | 9.27 ± 4.73 | 0.107 |
| E/mean e’ | 8.41 ± 2.54 | 8.27 ± 2.10 | 0.820 |
| LAVImax (mL/m2) | 31.96 ± 9.65 | 28.45 ± 9.92 | 0.224 |
| OR | Confidence Interval | |
|---|---|---|
| OR for abnormal LV GLS to predict LVEF < 55% | 8.50 | 0.93–78.02 |
| OR for abnormal RV FWS to predict RV S’ < 9.5 m/s | 9.86 | 0.88–110.43 |
| OR for abnormal global RV strain to predict RV S’ < 9.5 m/s | 4.00 | 0.46–34.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefani, L.; Brown, P.; Gerges, M.; Emerson, P.; Ferkh, A.; Kairaitis, K.; Gilroy, N.; Altman, M.; Thomas, L. Echocardiographic Assessment in Patients Recovered from Acute COVID-19 Illness. J. Cardiovasc. Dev. Dis. 2023, 10, 349. https://doi.org/10.3390/jcdd10080349
Stefani L, Brown P, Gerges M, Emerson P, Ferkh A, Kairaitis K, Gilroy N, Altman M, Thomas L. Echocardiographic Assessment in Patients Recovered from Acute COVID-19 Illness. Journal of Cardiovascular Development and Disease. 2023; 10(8):349. https://doi.org/10.3390/jcdd10080349
Chicago/Turabian StyleStefani, Luke, Paula Brown, Monica Gerges, Peter Emerson, Aaisha Ferkh, Kristina Kairaitis, Nicole Gilroy, Mikhail Altman, and Liza Thomas. 2023. "Echocardiographic Assessment in Patients Recovered from Acute COVID-19 Illness" Journal of Cardiovascular Development and Disease 10, no. 8: 349. https://doi.org/10.3390/jcdd10080349
APA StyleStefani, L., Brown, P., Gerges, M., Emerson, P., Ferkh, A., Kairaitis, K., Gilroy, N., Altman, M., & Thomas, L. (2023). Echocardiographic Assessment in Patients Recovered from Acute COVID-19 Illness. Journal of Cardiovascular Development and Disease, 10(8), 349. https://doi.org/10.3390/jcdd10080349






