Characterization of Green Fluorescent Protein in Heart Valves of a Transgenic Swine Model for Partial Heart Transplant Research
Abstract
1. Introduction
2. Materials and Methods
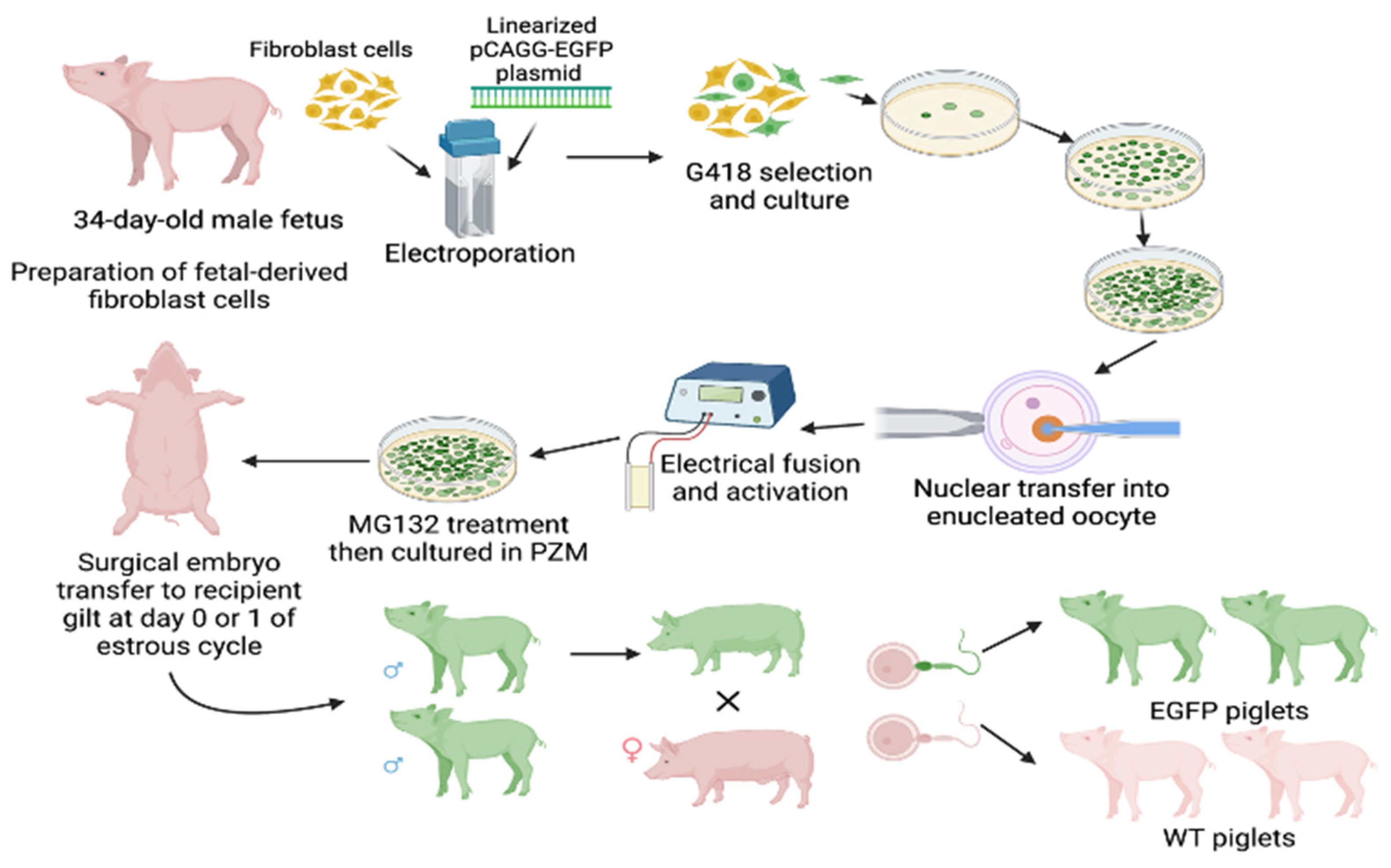
2.1. Propagation of GFP-Transgenic Pigs
2.2. Macroscopic GFP Visualization
2.3. Tissue Processing
2.3.1. Dissection
2.3.2. Tissue Fixation and Processing
2.4. Immunofluorescence
2.5. Microscopy
2.6. Statistics
3. Results
3.1. Propagation of GFP-Transgenic Pigs and Visualization of GFP Macroscopically
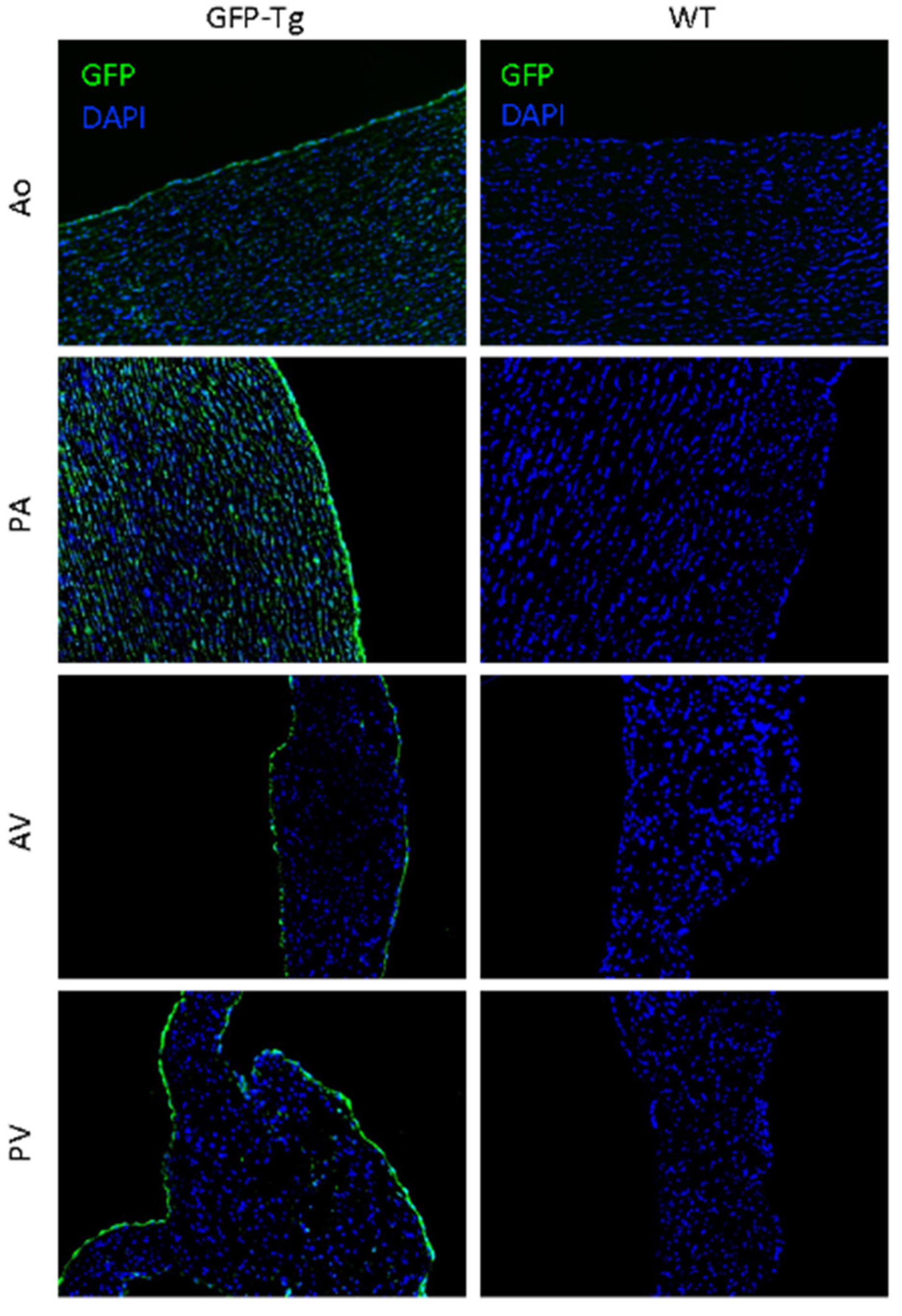
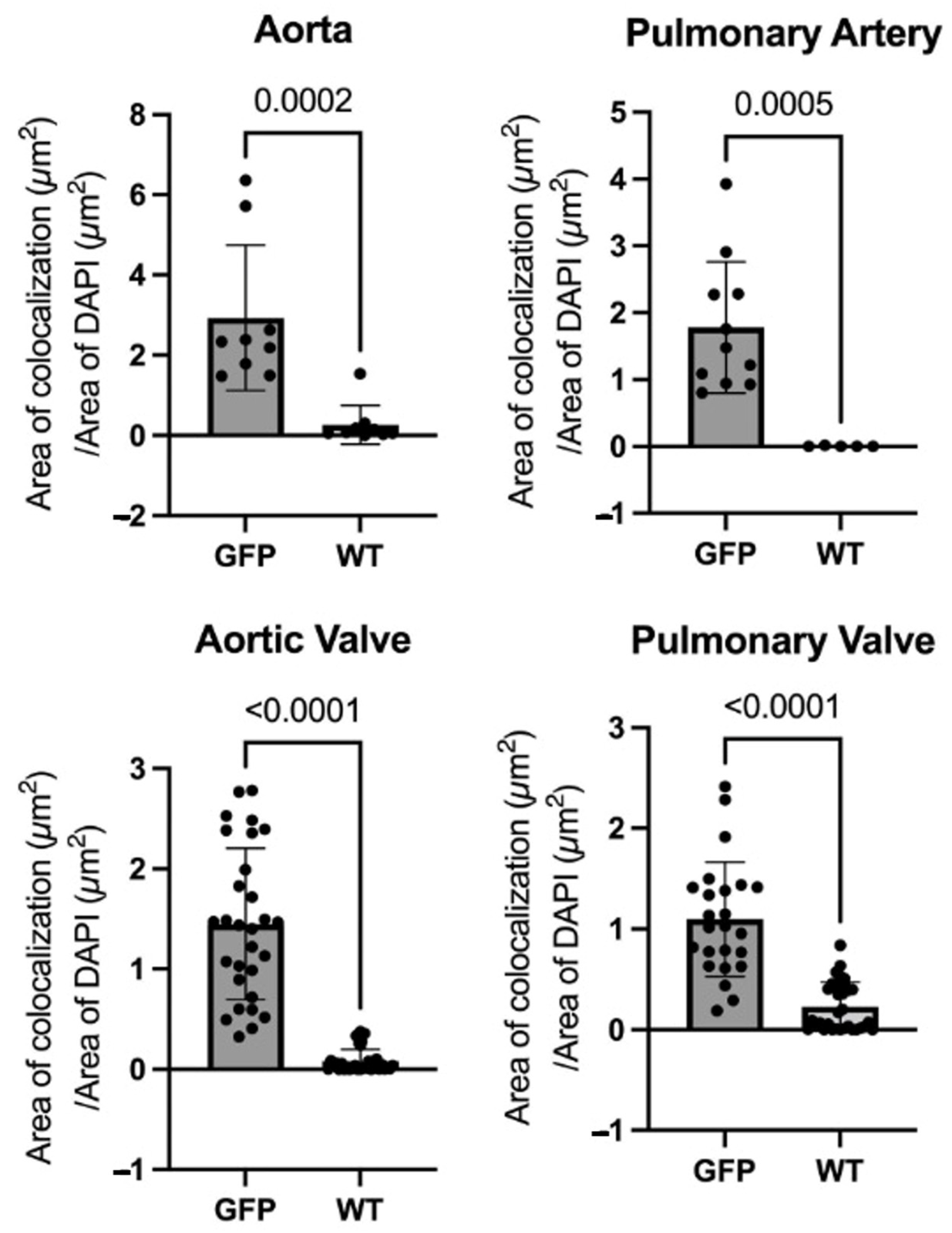
3.2. Microscopy of Immunofluorescence of GFP Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murakami, T.; Kobayashi, E. GFP-transgenic animals for in vivo imaging: Rats, rabbits, and pigs. Methods Mol. Biol. 2012, 872, 177–189. [Google Scholar] [CrossRef]
- Houdebine, L.M. Transgenic animal models in biomedical research. Methods Mol. Biol. 2007, 360, 163–202. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Gaston, R.; Eckhoff, D.; Ladowski, J.; Yamamoto, T.; Wang, L.; Iwase, H.; Hara, H.; Tector, M.; Tector, A.J. Xenotransplantation-the current status and prospects. Br. Med. Bull. 2018, 125, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Kostomitsopoulos, N.G.; Xanthos, T.T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 432–438. [Google Scholar] [PubMed]
- Wang, W.; Zhou, L.; Lee, S.J.; Liu, Y.; Fernandez de Castro, J.; Emery, D.; Vukmanic, E.; Kaplan, H.J.; Dean, D.C. Swine cone and rod precursors arise sequentially and display sequential and transient integration and differentiation potential following transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 301–309. [Google Scholar] [CrossRef]
- Kurome, M.; Ueda, H.; Tomii, R.; Naruse, K.; Nagashima, H. Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgenic Res. 2006, 15, 229–240. [Google Scholar] [CrossRef]
- Liu, Z.; Hatayama, N.; Xie, L.; Kato, K.; Zhu, P.; Ochiya, T.; Nagahara, Y.; Hu, X.; Li, X.K. Eicosapentenoic Acid Attenuates Allograft Rejection in an HLA-B27/EGFP Transgenic Rat Cardiac Transplantation Model. Cell Med. 2012, 3, 63–74. [Google Scholar] [CrossRef]
- Kawarasaki, T.; Uchiyama, K.; Hirao, A.; Azuma, S.; Otake, M.; Shibata, M.; Tsuchiya, S.; Enosawa, S.; Takeuchi, K.; Konno, K.; et al. Profile of new green fluorescent protein transgenic Jinhua pigs as an imaging source. J. Biomed. Opt. 2009, 14, 054017. [Google Scholar] [CrossRef]
- Brunetti, D.; Perota, A.; Lagutina, I.; Colleoni, S.; Duchi, R.; Calabrese, F.; Seveso, M.; Cozzi, E.; Lazzari, G.; Lucchini, F.; et al. Transgene expression of green fluorescent protein and germ line transmission in cloned pigs derived from in vitro transfected adult fibroblasts. Cloning Stem Cells 2008, 10, 409–419. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Li, R.; Spate, L.D.; Wax, D.M.; Rieke, A.; Whyte, J.J.; Manandhar, G.; Sutovsky, M.; Green, J.A.; Sutovsky, P.; et al. Method of oocyte activation affects cloning efficiency in pigs. Mol. Reprod. Dev. 2009, 76, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.; Warfvinge, K.; Schwartz, P.H.; Kiilgaard, J.F.; Shamie, N.; Jiang, C.; Samuel, M.; Scherfig, E.; Prather, R.S.; Young, M.J. Isolation of progenitor cells from GFP-transgenic pigs and transplantation to the retina of allorecipients. Cloning Stem Cells 2008, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sekiya, I.; Muneta, T.; Hatsushika, D.; Horie, M.; Tsuji, K.; Kawarasaki, T.; Watanabe, A.; Hishikawa, S.; Fujimoto, Y.; et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012, 14, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Everhart, M.B.; Han, W.; Parman, K.S.; Polosukhin, V.V.; Zeng, H.; Sadikot, R.T.; Li, B.; Yull, F.E.; Christman, J.W.; Blackwell, T.S. Intratracheal administration of liposomal clodronate accelerates alveolar macrophage reconstitution following fetal liver transplantation. J. Leukoc. Biol. 2005, 77, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bredow, L.; Schwartzkopff, J.; Reinhard, T. Host-derived endothelial regeneration of corneal transplants in a rat keratoplasty model. Ophthalmic Res. 2014, 52, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rajab, T.K. Evidence-based surgical hypothesis: Partial heart transplantation can deliver growing valve implants for congenital cardiac surgery. Surgery 2021, 169, 983–985. [Google Scholar] [CrossRef]
- Konsek, H.; Sherard, C.; Bisbee, C.; Kang, L.; Turek, J.W.; Rajab, T.K. Growing heart valve implants for children. J Cardiovasc Dev Dis. 2023, 10, 148. [Google Scholar] [CrossRef]
- Zafar, F.; Castleberry, C.; Khan, M.S.; Mehta, V.; Bryant, R., 3rd; Lorts, A.; Wilmot, I.; Jefferies, J.L.; Chin, C.; Morales, D.L.S. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 82–88. [Google Scholar] [CrossRef]
- Williams, R.J.; Lu, M.; Sleeper, L.A.; Blume, E.D.; Esteso, P.; Fynn-Thompson, F.; Vanderpluym, C.J.; Urbach, S.; Daly, K.P. Pediatric heart transplant waiting times in the United States since the 2016 allocation policy change. Am. J. Transplant. 2022, 22, 833–842. [Google Scholar] [CrossRef]
- Kim, Y.H. Pediatric heart transplantation: How to manage problems affecting long-term outcomes? Clin. Exp. Pediatr. 2021, 64, 49–59. [Google Scholar] [CrossRef]
- Hill, M.A.; Kwon, J.H.; Gerry, B.; Hardy, W.A.; Walkowiak, O.A.; Kavarana, M.N.; Nadig, S.N.; Rajab, T.K. Immune Privilege of Heart Valves. Front. Immunol. 2021, 12, 731361. [Google Scholar] [CrossRef]
- Kwon, J.H.; Hill, M.; Gerry, B.; Kubalak, S.W.; Mohiuddin, M.; Kavarana, M.N.; Rajab, T.K. Surgical techniques for aortic valve xenotransplantation. J. Cardiothorac. Surg. 2021, 16, 358. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bishara, K.; Kwon, J.H.; Hill, M.A.; Helke, K.L.; Norris, R.A.; Whitworth, K.; Prather, R.S.; Rajab, T.K. Characterization of Green Fluorescent Protein in Heart Valves of a Transgenic Swine Model for Partial Heart Transplant Research. J. Cardiovasc. Dev. Dis. 2023, 10, 254. https://doi.org/10.3390/jcdd10060254
Bishara K, Kwon JH, Hill MA, Helke KL, Norris RA, Whitworth K, Prather RS, Rajab TK. Characterization of Green Fluorescent Protein in Heart Valves of a Transgenic Swine Model for Partial Heart Transplant Research. Journal of Cardiovascular Development and Disease. 2023; 10(6):254. https://doi.org/10.3390/jcdd10060254
Chicago/Turabian StyleBishara, Katherine, Jennie H. Kwon, Morgan A. Hill, Kristi L. Helke, Russell A. Norris, Kristin Whitworth, Randall S. Prather, and Taufiek Konrad Rajab. 2023. "Characterization of Green Fluorescent Protein in Heart Valves of a Transgenic Swine Model for Partial Heart Transplant Research" Journal of Cardiovascular Development and Disease 10, no. 6: 254. https://doi.org/10.3390/jcdd10060254
APA StyleBishara, K., Kwon, J. H., Hill, M. A., Helke, K. L., Norris, R. A., Whitworth, K., Prather, R. S., & Rajab, T. K. (2023). Characterization of Green Fluorescent Protein in Heart Valves of a Transgenic Swine Model for Partial Heart Transplant Research. Journal of Cardiovascular Development and Disease, 10(6), 254. https://doi.org/10.3390/jcdd10060254






