A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Origin of the Animals
2.1.2. Approval of the Ethics Committees
2.1.3. Conditions of Maintenance
2.1.4. Formation of Study Groups
2.2. Drug Administration
2.3. Echocardiography
2.4. Myocyte Necrosis Analysis
2.5. Mouse Cardiomyocytes Isolation
2.6. Tissue Harvesting, Histology, and Immunohistochemistry
2.7. Quantitative RT-PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Echocardiography Analysis Reveals Typical Features of Dilated Cardiomyopathy Resulting from Acute Isoproterenol Exposure Followed by 5-Fluorouracil Administration
3.2. Strain Analysis Reveals Typical Features of Dilated Cardiomyopathy Resulting from Isoproterenol + 5-Fluorouracil Administration
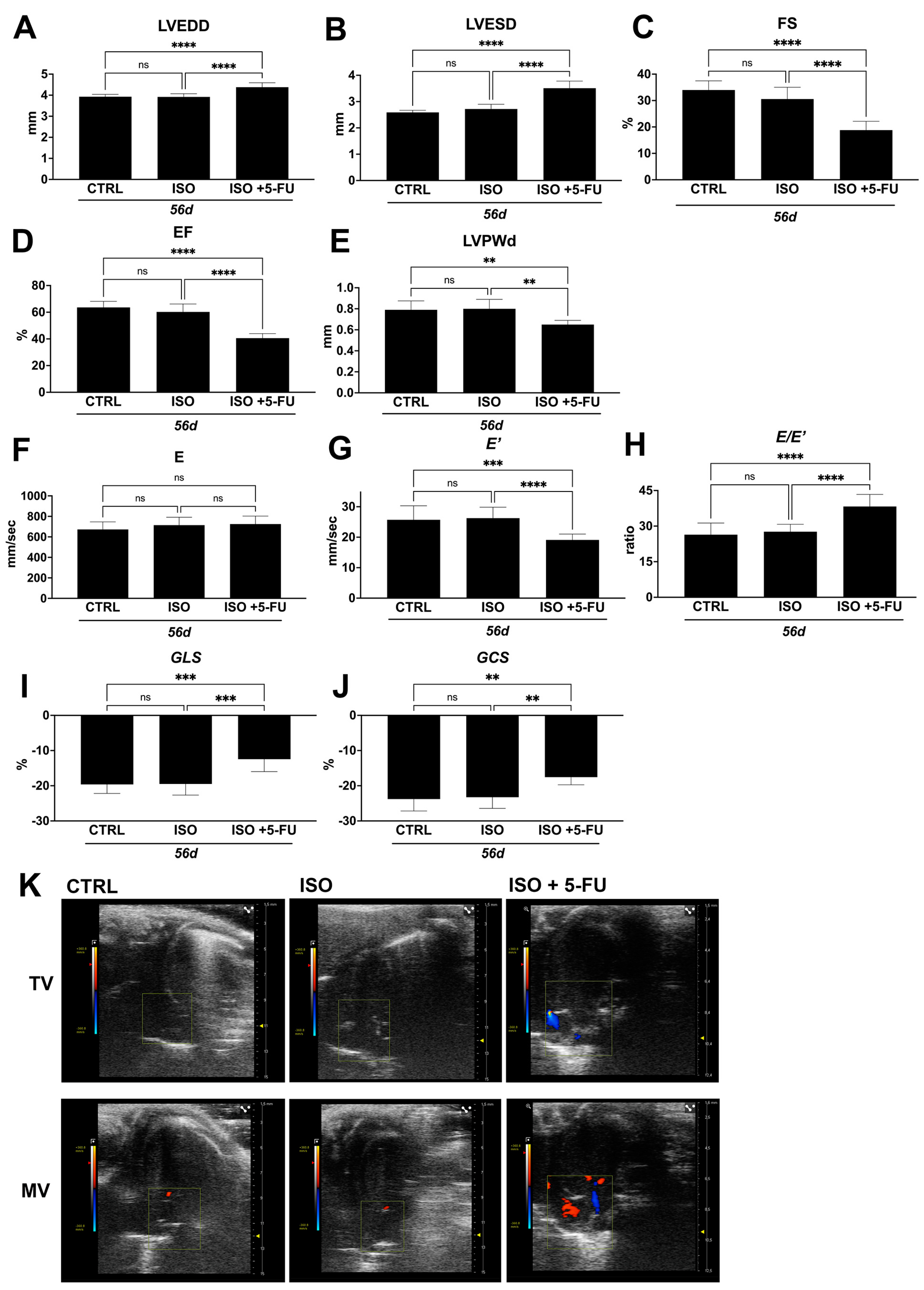
3.3. Dilated Cardiomyopathy Following ISO+ 5-FU Is Persistent at Long Follow-Up
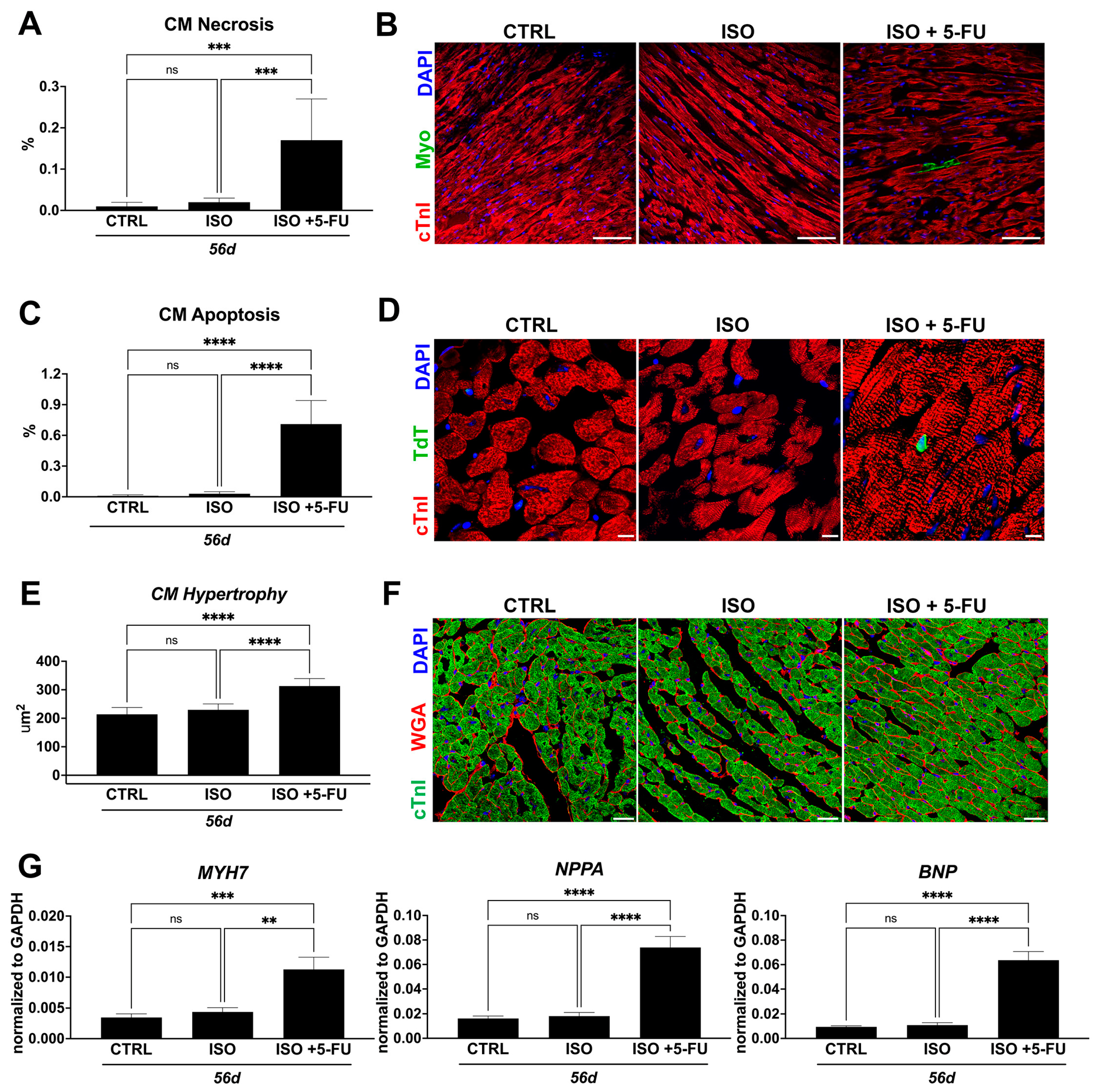
3.4. ISO + 5-FU Administration Causes Cardiomyocyte Remodeling Typical of Dilated Cardiomyopathy
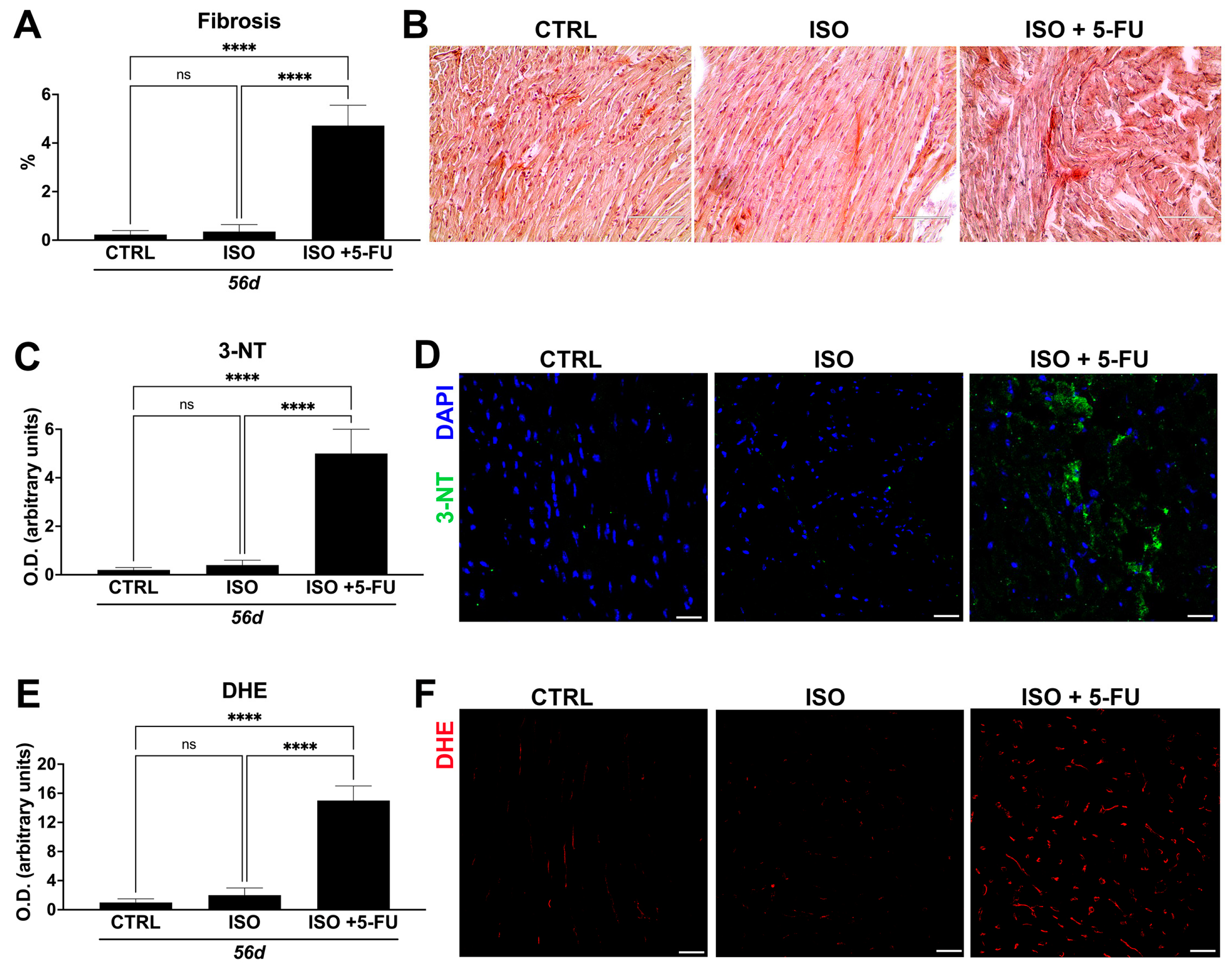
3.5. Acute Isoproterenol Exposure Followed by 5-Fluorouracil Administration Causes Myocardial Tissue Alterations Typical of Dilated Cardiomyopathy
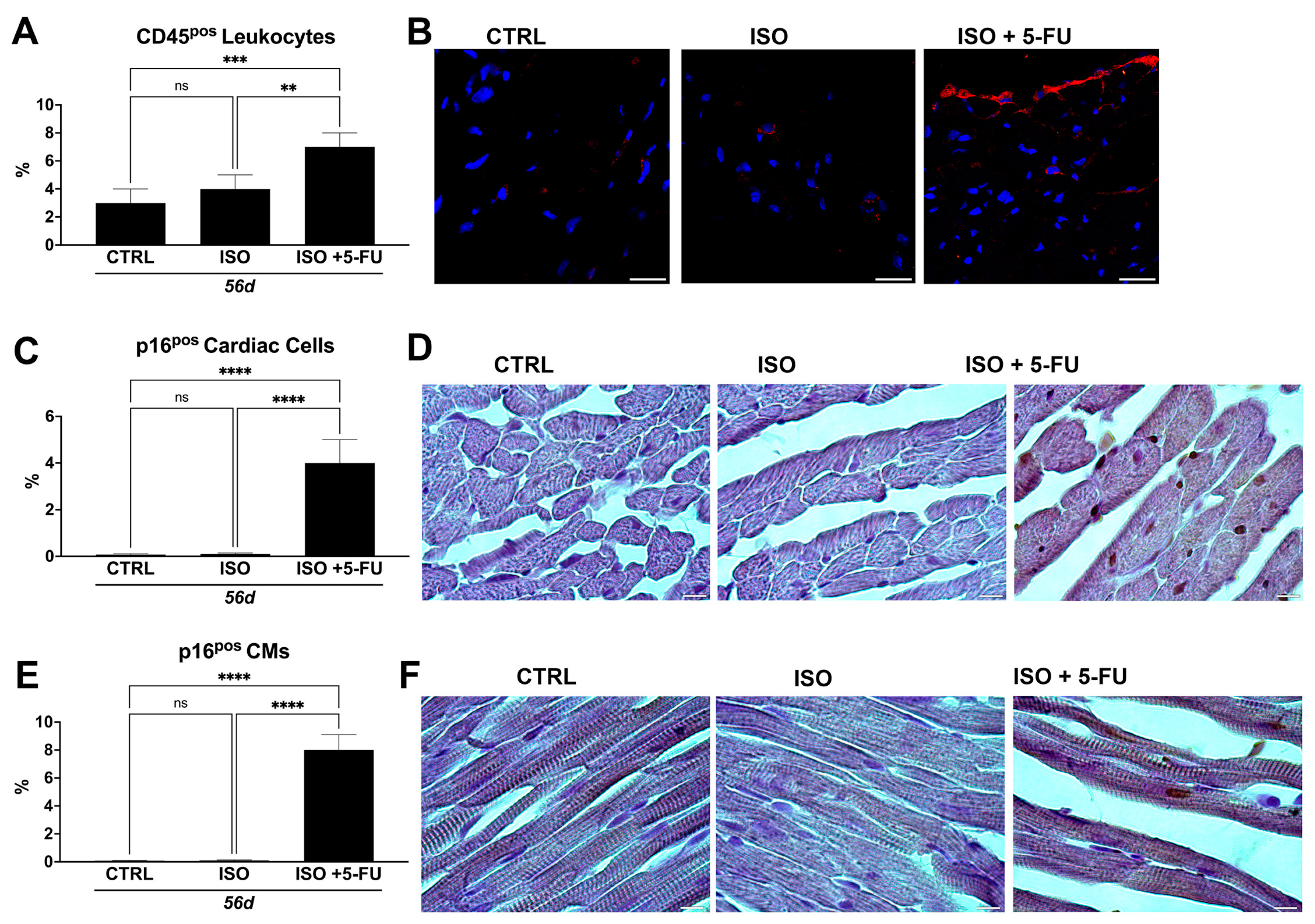
3.6. ISO + 5-FU Administration Produces Myocardial Tissue Inflammation Associated with Senescence Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salerno, N.; Salerno, L.; Marino, F.; Scalise, M.; Chiefalo, A.; Panuccio, G.; De Angelis, A.; Cianflone, E.; Urbanek, K.; Torella, D. Myocardial regeneration protocols towards the routine clinical scenario: An unseemly path from bench to bedside. EClinicalMedicine 2022, 50, 101530. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Palazzuoli, A.; Gronda, E. Recent advances in pharmacological treatment of heart failure. Eur. J. Clin. Investig. 2021, 51, e13624. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; Neri, G.; Macrì, L.M.; Salerno, N.; De Rosa, S.; Torella, D. Use of Impella device in cardiogenic shock and its clinical outcomes: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101007. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Solomon, S.D. Classification of Heart Failure According to Ejection Fraction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, M.; Coles, J.G.; Maynes, J.T. Rodent Models of Dilated Cardiomyopathy and Heart Failure for Translational Investigations and Therapeutic Discovery. Int. J. Mol. Sci. 2023, 24, 3162. [Google Scholar] [CrossRef] [PubMed]
- Orphanou, N.; Papatheodorou, E.; Anastasakis, A. Dilated cardiomyopathy in the era of precision medicine: Latest concepts and developments. Heart Fail. Rev. 2022, 27, 1173–1191. [Google Scholar] [CrossRef]
- Di Siena, S.; Gimmelli, R.; Nori, S.L.; Barbagallo, F.; Campolo, F.; Dolci, S.; Rossi, P.; Venneri, M.A.; Giannetta, E.; Gianfrilli, D.; et al. Activated c-Kit receptor in the heart promotes cardiac repair and regeneration after injury. Cell Death Dis. 2016, 7, e2317. [Google Scholar] [CrossRef][Green Version]
- Vicinanza, C.; Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Fumagalli, F.; Giovannone, E.D.; Cristiano, F.; Iaccino, E.; et al. Kitcre knock-in mice fail to fate-map cardiac stem cells. Nature 2018, 555, E1–E5. [Google Scholar] [CrossRef]
- Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Filardo, A.; Smith, A.J.; Cappetta, D.; De Angelis, A.; Urbanek, K.; et al. c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration. Cell Death Dis. 2019, 10, 436. [Google Scholar] [CrossRef][Green Version]
- Salerno, N.; Marino, F.; Scalise, M.; Salerno, L.; Molinaro, C.; Filardo, A.; Chiefalo, A.; Panuccio, G.; De Angelis, A.; Urbanek, K.; et al. Pharmacological clearance of senescent cells improves cardiac remodeling and function after myocardial infarction in female aged mice. Mech. Ageing Dev. 2022, 208, 111740. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Marino, F.; Salerno, L.; Mancuso, T.; Cappetta, D.; Barone, A.; Parrotta, E.I.; Torella, A.; Palumbo, D.; Veltri, P.; et al. In vitro CSC-derived cardiomyocytes exhibit the typical microRNA-mRNA blueprint of endogenous cardiomyocytes. Commun. Biol. 2021, 4, 1146. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cianflone, E.; Cristiano, M.C.; Salerno, N.; Tarsitano, M.; Marino, F.; Molinaro, C.; Fresta, M.; Torella, D.; Paolino, D. Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application. Pharmaceutics 2022, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Scalise, M.; Salerno, N.; Salerno, L.; Molinaro, C.; Cappetta, D.; Torella, M.; Greco, M.; Foti, D.; Sasso, F.C.; et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age. Diabetes 2022, 71, 1081–1098. [Google Scholar] [CrossRef]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarf, M.; et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef][Green Version]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef]
- Coppè, J.P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.Y.; Campisi, J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef][Green Version]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.K.; Halliday, B.P. Myocardial Fibrosis in Dilated Cardiomyopathy: Moving From Stratifying Risk to Improving Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Marino, F.; Salerno, L.; Cianflone, E.; Molinaro, C.; Salerno, N.; De Angelis, A.; Viglietto, G.; Urbanek, K.; Torella, D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021, 22, 13180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.X.; Kimura, S.; Nishiyama, A.; Shokoji, T.; Rahman, M.; Yao, L.; Nagai, Y.; Fujisawa, Y.; Miyatake, A.; Abe, Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc. Res. 2005, 65, 230–238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riehle, C.; Bauersachs, J. Small animal models of heart failure. Cardiovasc. Res. 2019, 115, 1838–1849. [Google Scholar] [CrossRef]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.J.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef]
- Hayashi, T.; Tiwary, S.K.; Lim, K.R.Q.; Rocha-Resende, C.; Kovacs, A.; Weinheimer, C.; Mann, D.L. Refining the reproducibility of a murine model of stress-induced reversible cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H229–H240. [Google Scholar] [CrossRef]
- Ren, S.; Chang, S.; Tran, A.; Mandelli, A.; Wang, Y.; Wang, J.J. Implantation of an Isoproterenol Mini-Pump to Induce Heart Failure in Mice. J. Vis. Exp. 2019, 152, e59646. [Google Scholar] [CrossRef]
- Maass, A.H.; Ikeda, K.; Oberdorf-Maass, S.; Maier, S.K.; Leinwand, L.A. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation 2004, 110, 2102–2109. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View From the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellison, G.M.; Torella, D.; Karakikes, I.; Purushothaman, S.; Curcio, A.; Gasparri, C.; Indolfi, C.; Cable, N.T.; Goldspink, D.F.; Nadal-Ginard, B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J. Biol. Chem. 2007, 282, 11397–11409. [Google Scholar] [CrossRef][Green Version]
- Hayashi, T.; Tiwary, S.K.; Lavine, K.J.; Acharya, S.; Brent, M.; Adamo, L.; Kovacs, A.; Mann, D.L. The Programmed Death-1 Signaling Axis Modulates Inflammation and LV Structure/Function in a Stress-Induced Cardiomyopathy Model. JACC Basic Transl. Sci. 2022, 7, 1120–1139. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chang, E.; Tang, X.; Watanabe, I.; Zhang, R.; Jeong, H.W.; Adams, R.H.; Jain, M.K. Cardiac macrophages regulate isoproterenol-induced Takotsubo-like cardiomyopathy. JCI Insight 2022, 7, e156236. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Salerno, N.; Scalise, M.; Salerno, L.; Torella, A.; Molinaro, C.; Chiefalo, A.; Filardo, A.; Siracusa, C.; Panuccio, G.; et al. Streptozotocin-Induced Type 1 and 2 Diabetes Mellitus Mouse Models Show Different Functional, Cellular and Molecular Patterns of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 1132. [Google Scholar] [CrossRef]
- Molinaro, C.; Salerno, L.; Marino, F.; Scalise, M.; Salerno, N.; Pagano, L.; De Angelis, A.; Cianflone, E.; Torella, D.; Urbanek, K. Unraveling and Targeting Myocardial Regeneration Deficit in Diabetes. Antioxidants 2022, 11, 208. [Google Scholar] [CrossRef] [PubMed]

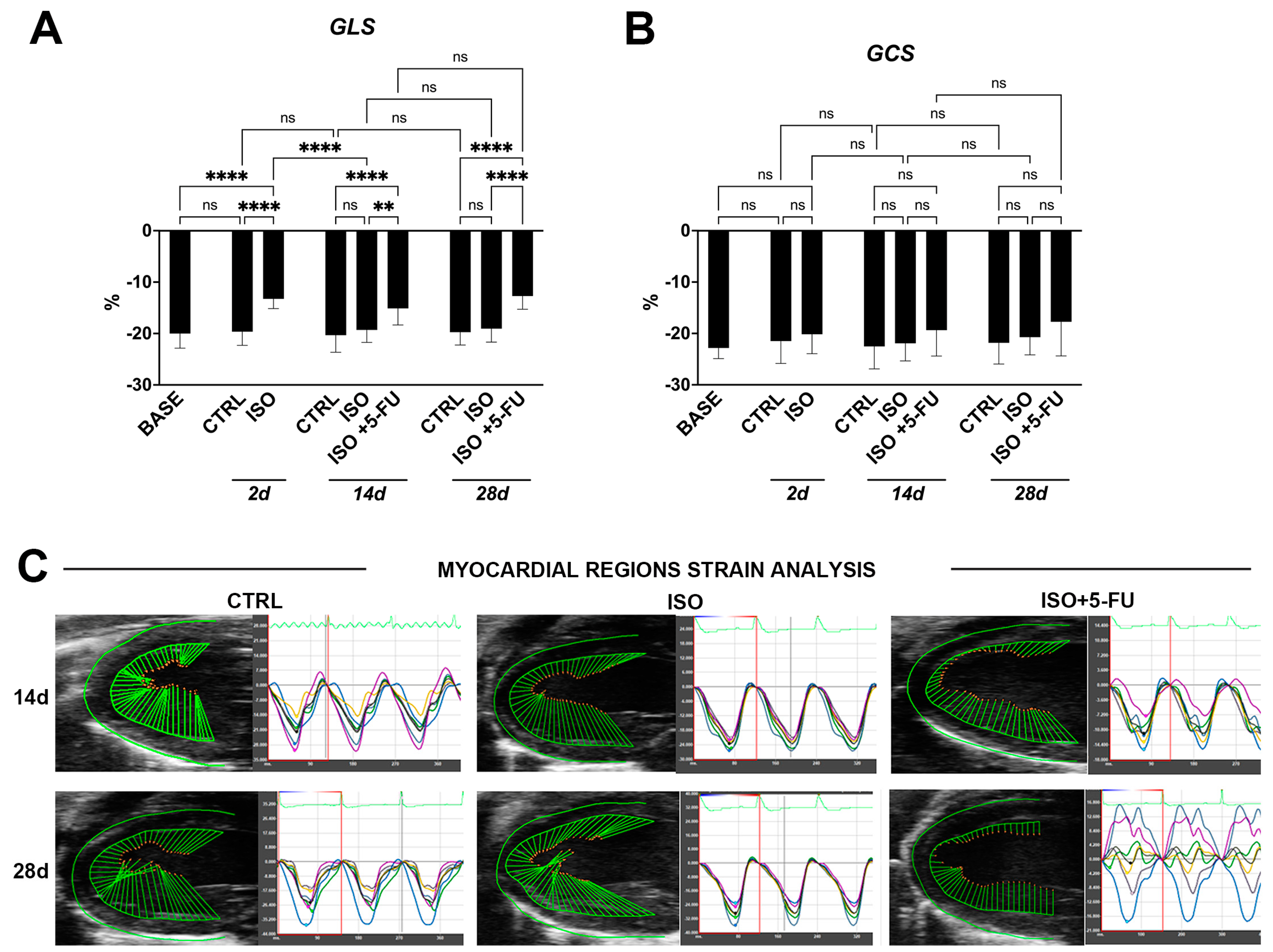
| Baseline (n = 26) | Ctrl 2d (n = 8) | ISO 2d (n = 18) | Ctrl 14d (n = 8) | ISO 14d (n = 8) | ISO + 5-FU 14d (n = 8) | Ctrl 28d (n = 8) | ISO 28d (n = 8) | ISO + 5-FU 28d (n = 8) | |
|---|---|---|---|---|---|---|---|---|---|
| LVEDD (mm) | 3.82 ± 0.24 | 3.9 ± 0.33 | 4.22 ± 0.41 | 3.91 ± 0.16 | 3.95 ± 0.14 | 4.3 ± 0.14 | 3.9 ± 0.1 | 3.92 ± 0.21 | 4.35 ± 0.2 |
| LVESD (mm) | 2.63 ± 0.25 | 2.62 ± 0.4 | 3.23 ± 0.38 | 2.64 ± 0.14 | 2.69 ± 0.16 | 3.23 ± 0.19 | 2.62 ± 0.13 | 2.7 ± 0.35 | 3.48 ± 0.12 |
| LVPWd (mm) | 0.75 ± 0.07 | 0.76 ± 0.05 | 0.8 ± 0.1 | 0.78 ± 0.04 | 0.78 ± 0.09 | 0.71 ± 0.04 | 0.79 ± 0.07 | 0.79 ± 0.04 | 0.68 ± 0.03 |
| EF (%) | 60.7 ± 3.92 | 62.1 ± 6.5 | 48.05 ± 2.92 | 59.62 ± 2.99 | 58.51 ± 3.28 | 48.47 ± 3.73 | 61.9 ± 5.35 | 59.5 ± 2.75 | 39.9 ± 4.53 |
| FS (%) | 31.15 ± 2.63 | 32.82 ± 6.4 | 23.46 ± 1.5 | 32.48 ± 2.04 | 31.9 ± 2.26 | 24.59 ± 2.49 | 32.82 ± 3.87 | 31.12 ± 1.8 | 20 ± 4 |
| MV E (mm/s) | 705.57 ± 103.14 | 691.6 ± 74.44 | 718.62 ± 97.67 | 701.65 ± 111.74 | 734.07 ± 92.78 | 803.01 ± 66.79 | 700.18 ± 72.42 | 750.07 ± 40.01 | 756.11 ± 99.85 |
| E′ (mm/s) | 26.79 ± 4.23 | 26.51 ± 3.37 | 20.66 ± 4.16 | 27.38 ± 1.86 | 25.99 ± 3.14 | 22.6 ± 4.01 | 26.97 ± 3.65 | 27.55 ± 4.17 | 20.18 ± 1.55 |
| E/E′ | 26.72 ± 4.33 | 26.4 ± 3.96 | 35.32 ± 3.8 | 25.6 ± 25.6 | 28.45 ± 4.02 | 36.19 ± 5.2 | 26.25 ± 3.41 | 27.73 ± 3.23 | 37.58 ± 4.98 |
| GLS (%) | −20.01 ± 2.83 | −19.64 ± 2.66 | −13.25 ± 1.9 | −20.33 ± 3.32 | −19.31 ± 2.42 | −15.12 ± 3.21 | −19.74 ± 2.49 | −19.06 ± 2.62 | −12.7 ± 2.58 |
| GCS (%) | −22.83 ± 2.06 | −21.49 ± 4.35 | −20.15 ± 3.8 | −22.53 ± 4.38 | −21.92 ± 3.42 | −19.36 ± 5.04 | −21.82 ± 4.13 | −20.73 ± 3.44 | −17.72 ± 6.67 |
| Ctrl 56d (n = 8) | ISO 56d (n = 8) | ISO + 5-FU 56d (n = 8) | |
|---|---|---|---|
| LVEDD (mm) | 3.93 ± 0.11 | 3.92 ± 0.15 | 4.38 ± 0.21 |
| LVESD (mm) | 2.59 ± 0.08 | 2.72 ± 0.18 | 3.51 ± 0.27 |
| LVPWd (mm) | 0.79 ± 0.085 | 0.8 ± 0.09 | 0.65 ± 0.04 |
| EF (%) | 63.61 ± 4.55 | 60.25 ± 5.95 | 40.61 ± 3.39 |
| FS (%) | 33.98 ± 3.48 | 30.57 ± 4.45 | 18.8 ± 3.35 |
| MV E (mm/s) | 672.8 ± 74 | 714.68 ± 77.18 | 725.26 ± 77.5 |
| E′ (mm/s) | 25.72 ± 4.59 | 26.25 ± 3.6 | 19.1 ± 1.92 |
| E/E′ | 26.39 ± 4.92 | 27.65 ± 3.14 | 38.26 ± 5.08 |
| GLS (%) | −19.62 ± 2.56 | −19.48 ± 3.17 | −12.44 ± 3.55 |
| GCS (%) | −23.79 ± 3.38 | −23.25 ± 3.19 | −17.56 ± 2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, N.; Scalise, M.; Marino, F.; Filardo, A.; Chiefalo, A.; Panuccio, G.; Torella, M.; De Angelis, A.; De Rosa, S.; Ellison-Hughes, G.M.; et al. A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration. J. Cardiovasc. Dev. Dis. 2023, 10, 225. https://doi.org/10.3390/jcdd10060225
Salerno N, Scalise M, Marino F, Filardo A, Chiefalo A, Panuccio G, Torella M, De Angelis A, De Rosa S, Ellison-Hughes GM, et al. A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration. Journal of Cardiovascular Development and Disease. 2023; 10(6):225. https://doi.org/10.3390/jcdd10060225
Chicago/Turabian StyleSalerno, Nadia, Mariangela Scalise, Fabiola Marino, Andrea Filardo, Antonio Chiefalo, Giuseppe Panuccio, Michele Torella, Antonella De Angelis, Salvatore De Rosa, Georgina M. Ellison-Hughes, and et al. 2023. "A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration" Journal of Cardiovascular Development and Disease 10, no. 6: 225. https://doi.org/10.3390/jcdd10060225
APA StyleSalerno, N., Scalise, M., Marino, F., Filardo, A., Chiefalo, A., Panuccio, G., Torella, M., De Angelis, A., De Rosa, S., Ellison-Hughes, G. M., Urbanek, K., Viglietto, G., Torella, D., & Cianflone, E. (2023). A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration. Journal of Cardiovascular Development and Disease, 10(6), 225. https://doi.org/10.3390/jcdd10060225







