Abstract
Background: Understanding and effectively treating dystrophin-deficient cardiomyopathy is of high importance for Duchenne muscular dystrophy (DMD) patients due to their prolonged lifespan. We used two-dimensional speckle tracking echocardiography to analyze more deeply the non-uniformity of myocardial strain within the left ventricle during the progression of cardiomyopathy in golden retriever muscular dystrophy (GRMD) dogs. Methods: The circumferential strain (CS) and longitudinal strain (LS) of left ventricular (LV) endocardial, middle and epicardial layers were analyzed from three parasternal short-axis views and three apical views, respectively, in GRMD (n = 22) and healthy control dogs (n = 7) from 2 to 24 months of age. Results: In GRMD dogs, despite normal global systolic function (normal LV fractional shortening and ejection fraction), a reduction in systolic CS was detected in the three layers of the LV apex but not in the LV middle-chamber and base at 2 months of age. This spatial heterogeneity in CS progressed with age, whereas a decrease in systolic LS could be detected early at 2 months of age in the three layers of the LV wall from three apical views. Conclusions: Analyzing the evolution of myocardial CS and LS in GRMD dogs reveals spatial and temporal non-uniform alterations of LV myocardial strain, providing new insights into the progression of dystrophin-deficient cardiomyopathy in this relevant model of DMD.
1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked hereditary disease affecting approximately 1 in 5000 male births worldwide and is caused by mutations in the gene that encodes the 427-kDa cytoskeletal protein dystrophin. Dystrophin forms a dystrophin protein complex with dystrophin-associated proteins including dystroglycans, sarcoglycans, integrins and caveolin in the sarcolemma, linking the internal cytoskeleton to the extracellular matrix in striated muscle and playing an important role in stabilizing the cell membrane and signal transduction during muscle contraction [1]. The vast majority of DMD patients lack the dystrophin protein, making the membrane susceptible to damage by mechanical stress which can lead to muscle degeneration. DMD is characterized by progressive muscle degeneration and weakness. Beginning as early as 2 to 3 years of age, the principal symptom of DMD is muscle weakness, affecting the proximal muscles first, then the distal limb muscles and usually the lower external muscles before the upper external muscles. Generally, DMD patients are wheelchair-bound by 12 years of age and die of respiratory failure in their late teens or early twenties. Although there is currently no effective treatment for this disease, patients with DMD live significantly longer thanks to improved respiratory ventilation and other supportive care. As a result, deaths from heart failure rather than respiratory failure have increased [2]. Therefore, understanding the development of dystrophin-deficient cardiomyopathy and effectively treating it has become very important for DMD patients.
Similar to DMD, golden retriever muscular dystrophy (GRMD) is also caused by the dystrophin gene mutation, a single nucleotide change that leads to exon skipping and an out-of-frame DMD transcript [3]. GRMD dogs develop symptoms and signs like DMD patients and are used to explore pathological changes and test novel therapeutic strategies for DMD [4,5,6,7,8,9,10,11].
Using image-processing algorithms for two-dimensional digital echocardiographic images, two-dimensional speckle tracking echocardiography (2D-STE) identifies small stable myocardial footprints called speckles generated by ultrasound-myocardial tissue interactions within a defined region of interest and tracks speckles frame-to-frame over the cardiac cycle. Distances between speckles or their spatiotemporal displacement provide information about global and segmental myocardial deformation. Despite some technical factors influencing strain value [12], 2D-STE has been developed into a reliable method to objectively quantify left ventricular (LV) regional and global myocardial function [12,13] since strain information is independent of the Doppler angle of incidence [13]. Studies have shown that 2D-STE can be used to reveal early pathological changes in the heart of DMD patients [14,15,16] and GRMD dogs and cardiac responses in GRMD therapeutic trials [8,9,10]. Interestingly, recent technological developments have enabled 2D-STE to analyze LV wall strain in more detail, allowing, for example, the analysis of endocardial, middle and epicardial strains in different regions of the LV wall. Therefore, the aim of this study was to evaluate the changes in myocardial circumferential strain (CS) and longitudinal strain (LS) of LV endocardial, middle and epicardial layers in GRMD dogs aged from 2 to 24 months. These GRMD dogs were compared to healthy control dogs to reveal alterations in LV myocardial strain during the pathological development of dystrophin-deficient cardiomyopathy. Our results showed that spatial and temporal non-uniform alterations of myocardial strain occur within the left ventricle during the progression of the disease. This finding may provide insight into the progression of cardiomyopathy due to dystrophin deficiency in this highly relevant canine model of DMD.
2. Materials and Methods
2.1. Animal Model
Twenty-two GRMD and seven healthy control dogs were provided by the Centre d’Elevage du Domaine des Souches (Mezilles, France) and were cared for by veterinarians throughout the study. GRMD was diagnosed by DNA analysis at 1 month of age. The experimental protocol was approved by the animal ethics committee (ComEth ANSES-ENVA-UPEC agreement #20/12/12–18) and the experimental procedures were performed in accordance with the European Union Directive 2010/63/EU.
2.2. Echocardiographic Image Acquisition and Analysis
Echocardiographic image acquisition and analysis were performed by the same investigator in GRMD dogs and their healthy littermates at 2, 6, 9, 12, 18 and 24 months of age. Using a Vivid 7 ultrasound (General Electric Medical System, Horten, Norway), echocardiographic images were acquired from conscious (i.e., without the use of sedatives or anesthetics) 2-month-old puppies in a standing position using a 7S cardiac sector array probe and from dogs of other ages in a standing position in a sling using a 5S or an M4S cardiac sector array probe after an adaptive period of 20–30 min. The standing position allows for the acquisition of images from all commonly used LV views. An ECG was recorded during the echocardiographic examination to calculate heart rate.
M-mode parasternal short-axis (PSAX) images at the mid-chamber (papillary muscles) level were recorded to measure LV end-diastolic (EDD) and end-systolic (ESD) diameters, end-diastolic and end-systolic posterior and interventricular septal wall dimensions and fractional shortening (FS).
High-frame-rate videos (ranging from 60 to 119 frames/s) were recorded in apical 2-chamber, 3-chamber and 4-chamber views. In some cases, the apical 5-chamber view was used when an adequate 3-chamber image could not be obtained. The PSAX-base view was acquired at the level of mitral valve leaflets. The PSAX-mid-chamber view was recorded at the level of papillary muscles. The PSAX-apex view was recorded when the LV cavity was as small as possible without visible papillary muscles. For each view, a cine loop of at least three consecutive cardiac cycles was stored digitally for offline analysis. To ensure the image quality for strain analysis, the images of young dogs were generally acquired with the highest frame rate possible.
Echocardiographic images were analyzed as recommended [17]. FS (%) was calculated as [(LV EDD − LV ESD)/LV EDD × 100%]. LV end-diastolic volume (EDV, mL), end-systolic volume (ESV, mL) and ejection fraction (EF, %) were calculated by the biplane Simpson’s method using the apical 4-chamber and 2-chamber images.
Using dedicated software, 2D-STE was performed offline (EchoPac version 201, GE Healthcare, Horten, Norway). CS and LS were analyzed in endocardial (inner), middle and epicardial (outer) layers of GRMD and healthy control dogs of various ages using EchoPac version 201. The cardiac cycle length was measured from the beginning of systole. After delineating an area of interest within the LV wall and performing manual adjustments to include the entire LV wall, the software automatically divided the LV wall into 6 LV wall segments for each view. Each segment was divided into endocardial and epicardial parts and averaged to obtain endocardial, middle and epicardial strains. After viewing the validation results automatically generated by the software and adequately curve fitting the area of interest within the LV wall, the adequate tracking results were considered for analysis. It is often necessary to manually adjust the area of interest several times to obtain an adequate fitting. CS was analyzed in PSAX-apex, PSAX-mid-chamber and PSAX-base views. LS was analyzed in apical 3-chamber (or 5-chamber), 4-chamber and 2-chamber views.
2.3. Statistical Analysis
Continuous variables were expressed as mean ± SEM. Statistical analysis was performed with StatView (Version 5.0, Abacus Concepts Inc., Berkeley, CA, USA). The one-way ANOVA was used to test within-group differences over time (age). The ANOVA for repeated measurements over time was performed on echocardiographic data from 8 GRMD dogs and 4 healthy control dogs aged 2 to 24 months to test between-group differences. The two-tailed unpaired two-sample t-test was performed on data obtained in all dogs at each time point to determine the differences between GRMD and healthy control dogs. A difference was considered statistically significant at p < 0.05.
The assessment of inter-observer variability was not carried out in the study, because the acquisition and analysis of the echocardiographic images were carried out by a single investigator. To examine intra-observer variability, a set of images used for the calculation of EF, CS in the PSAX-apex view and LS in the 4-chamber view were taken from 6 randomly selected dogs used in the study and analyzed twice by the same sonographer in an interval of 24 h or more (3 heart beats were analyzed for each image). The interclass correlation coefficient (ICC) for these parameters was calculated using the Real Statistics Resource Pack. The coefficient of variation (CoV) for these parameters was calculated by the following equation: CoV (%) = standard deviation/mean × 100. The two measurements were used for the calculation of the ICC and the CoV for EF (0.908 and 3.1 ± 2.7%, respectively), endocardial CS (0.955 and −3.4 ± 2.9%), middle CS (0.941 and −3.8 ± 2.5%), epicardial CS (0.738 and −7.9 ± 5.7%), endocardial LS (0.958 and −3.1 ± 2.4%), middle LS (0.950 and −3.3 ± 2.7%) and epicardial LS (0.913 and −4.3 ± 4.2%).
3. Results
Of the 22 GRMD dogs included in the study, three were euthanized around the age of 6 months due to loss of ambulation; four between 9 and 12 months due to severely reduced mobility (n = 2), poor general conditions (n = 1) and severe bronchopneumonia (n = 1); three between 12 and 18 months of age due to reduced mobility (n = 2) and respiratory failure (n = 1); and two died of heart failure. No death occurred in healthy dogs throughout the study, and three of the seven healthy control dogs were rehomed after the 6-month examination.
GRMD dogs had significantly lower body weights than healthy control dogs at different ages (Table 1), notably reflecting a loss in muscle mass. In GRMD and healthy control dogs, the heart rate decreased with age, and the majority of GRMD dogs had slightly higher heart rates than healthy control dogs at the same age (Table 1).

Table 1.
Body weight and LV dimensional and functional parameters measured by echocardiography in GRMD and healthy control dogs.
3.1. Changes in LV Dimensional and Functional Parameters Measured by Echocardiography in GRMD and Healthy Control Dogs at Different Ages
As shown in Table 1, the LV EDD of healthy control dogs increased with age until 9 months old. The LV EDD of GRMD dogs increased with age but was smaller than that of healthy control dogs until 9 months of age. Thereafter, LV EDD was similar in both groups. LV ESD increased with age in healthy control and GRMD dogs. As a result, these changes in LV EDD and ESD of GRMD dogs resulted in a significant reduction in FS at 12, 18 and 24 months (p < 0.05 or p < 0.01). The decrease in FS in some GRMD dogs reached a pathological value (i.e., less than 28%) at 18 and 24 months, whereas healthy control dogs had a stable FS throughout all ages examined. In healthy control dogs, interventricular septal wall end-diastolic thickness (IVSWEDT) and LV posterior wall end-diastolic thickness (PWEDT) increased with age and reached a stable thickness at 12 months. In contrast, IVSWEDT and PWEDT of GRMD dogs were smaller than those of healthy control dogs starting from 6 and 9 months of age, respectively. However, interventricular septal wall systolic thickening (% IVSWTh) was not significantly changed over time in healthy control dogs and decreased, without reaching significance, in GRMD dogs. Similarly, LV posterior wall systolic thickening (% PWTh) did not significantly change throughout the protocol in healthy control dogs, whereas it significantly decreased over time in GRMD dogs.
LV end-diastolic volume (LVEDV) increased with age in both healthy control and GRMD dogs, but GRMD dogs had smaller LVEDV at each age examined, which is certainly due to the marked smaller size and body weight of GRMD dogs. LV end-systolic volume (LVESV) increased with age in healthy control dogs whereas GRMD dogs had a smaller LVESV at 2 and 6 months of age than healthy control dogs but a similar LVESV from 9 months of age. Consequently, GRMD dogs had smaller EF and, contrasted with healthy control dogs, EF decreased progressively with age, starting at 9 months whereas healthy control dogs had stable EF at all ages examined (Table 1). The decrease in EF in some GRMD dogs reached a pathological value (i.e., less than 50%) at 18 and 24 months old.
These data obtained by conventional echocardiography indicated that significant alterations in LV global function (FS and EF) can be detected rather tardily (12–18 months of age) in GRMD dogs.
3.2. Changes in CS in the Three LV Wall Layers Analyzed by 2D-STE from the Three Short-Axis Views in GRMD and Healthy Control Dogs through Time
Figure 1 shows two typical examples of LV endocardial, middle and epicardial CS from a 2-month-old healthy control dog (panel A) and a 2-month-old GRMD dog (panel B) analyzed by 2D-STE from the PSAX-apex view. As shown in Table 2, in the LV apical region, the peak CS of endocardial, middle and epicardial layers remained rather stable with age in healthy control dogs. In contrast, GRMD dogs showed significant reduction in peak CS in the three examined layers of the LV apex (values being less negative) at 2 months, which were mainly caused by the significantly smaller peak CS of LV anterior and antero-lateral regions than those of healthy control dogs. It is worth noting that this smaller peak CS of epicardial and middle layers in the LV apical region of GRMD dogs could be seen at the different ages examined.
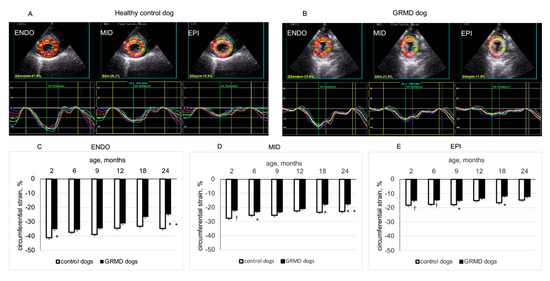
Figure 1.
Circumferential strain (CS) of left ventricular (LV) endocardial (ENDO), middle (MID) and epicardial (EPI) layers analyzed from the parasternal short-axis-apex view. Panels (A,B) show the values and curves of 6 segments (showed by different colors) analyzed in each LV wall layer obtained in a 2-month-old healthy control dog and in an age-matched GRMD (golden retriever muscular dystrophy) dog. The curve in dotted line in each part of the LV wall is the average of the 6 curves. Panels (C–E) compare the peak systolic circumferential strain values of ENDO, MID and EPI layers of healthy control and GRMD dogs at different ages. Data were expressed as mean ± SEM. The one-way ANOVA was performed to test the within-group differences. The two-tailed unpaired two-sample t-test was performed on data obtained in all dogs at each time point to determine the difference between GRMD and healthy control dogs. n = 22, 22, 17, 13, 8 and 8 for GRMD dogs, and 7, 7, 4, 4, 4 and 4 for healthy control dogs at 2, 6, 9, 12, 18 and 24 months of age, respectively. * p < 0.05 and † p < 0.01 versus healthy control dogs of the same age.

Table 2.
Circumferential strain in myocardial layers of the left ventricle analyzed by 2-dimensional speckle tracking echocardiography in healthy control and GRMD dogs at different ages.
At the PSAX-mid-chamber level, GRMD had similar peak CS in the endocardial layer as healthy control dogs except at 2 and 24 months of age and had smaller peak CS in epicardial and middle layers at 18 and 24 months of age. In the endocardial layer, this parameter significantly decreased with age in both groups. However, in the middle and epicardial layer, peak CS significantly decreased with age in GRMD dogs, whereas no significant change was detected in healthy control dogs. At the base of the left ventricle, the peak CS of endocardial, middle and epicardial layers was basically similar for GRMD and healthy control dogs except for the epicardial layer at 9, 18 and 24 months and the middle and endocardial layers at 24 months, where GRMD dogs had smaller peak CS than healthy control dogs. In the epicardial layer, basal peak CS decreased significantly with age in GRMD dogs, whereas this parameter remained stable over time in healthy control dogs.
These results indicated that GRMD dogs exhibited a non-uniform spatial alteration over time in CS, characterized by an early and persistent decrease in peak CS in the epicardial and middle layers of the LV apical region, mainly due to decreased peak CS in the apical anterior and antero-lateral regions. At the middle and basal levels of the left ventricle, decreased peak CS in GRMD dogs occurred later (at 18–24 months of age). In these regions of the left ventricle, the decrease in peak CS occurred earlier in epicardial and middle layers than in the endocardial layer.
3.3. Changes in LS in the Three LV Wall Layers Analyzed by 2D-STE from the Three Apical Views in GRMD and Healthy Control Dogs at Different Ages
Figure 2 shows two typical examples of LV endocardial, middle and epicardial LS from a 2-month-old healthy control dog (panel A) and a 2-month-old GRMD dog (panel B) analyzed by 2D-STE from the apical four-chamber view. As shown in Table 3, at the apical three-chamber view, GRMD dogs had significantly smaller peak LS than healthy control dogs at 2, 6, 9 and 12 months of age in the three layers of the LV wall. In the apical four-chamber view, peak LS of the three layers of LV wall were slightly but not significantly decreased with age in healthy control dogs. Contrastingly, GRMD dogs displayed a reduction with age and significantly smaller peak LS in the three layers of the LV wall at 2, 6, 9 and 24 months of age (Figure 2 and Table 3). In the apical two-chamber view, GRMD had significantly smaller peak LS in the endocardial layer than healthy control dogs at 2, 6, 9 and 12 months and significantly smaller peak LS in epicardial and middle layer at 2, 6 and 12 months (Table 3).
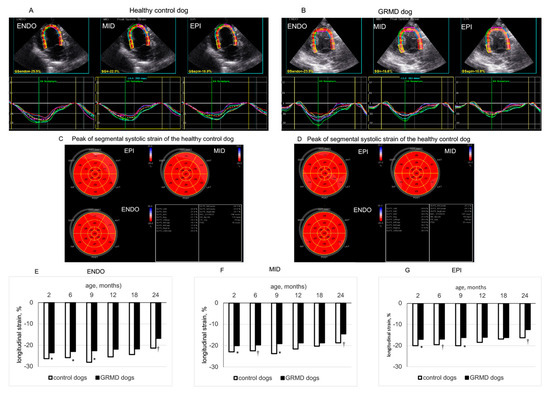
Figure 2.
Longitudinal strain of left ventricular (LV) endocardial (ENDO), middle (MID) and epicardial (EPI) layers analyzed from the apical 4-chamber view. Panels (A,B) show the values and curves of 6 segments (showed by different colors) analyzed at each layer of the LV wall obtained in a 2-month-old healthy control dog and an age-matched GRMD (golden retriever muscular dystrophy) dog. The curve in dotted line in each part of the LV wall is the average of the 6 curves. Panels (C,D) are the bulls-eye presentations of segmental peak LS values obtained in each LV myocardial layer from 3 apical views of the above dogs. Panels (E–G) compare the longitudinal strain values of the ENDO, MID and EPI layers of healthy control and GRMD dogs at different ages. Data were expressed as mean ± SEM. The one-way ANOVA was performed to test within-group differences. The two-tailed unpaired two-sample t-test was performed on data obtained in all dogs at each time point to determine the differences between GRMD and healthy control dogs. n = 22, 22, 17, 13, 8 and 8 for GRMD dogs, and 7, 7, 4, 4, 4 and 4 for healthy control dogs at 2, 6, 9, 12, 18 and 24 months of age, respectively. * p < 0.05 and † p < 0.01 versus healthy control dogs of the same age.

Table 3.
Longitudinal strain in myocardial layers of the left ventricle analyzed by 2-dimensional speckle tracking echocardiography in healthy control and GRMD dogs at different ages.
Thus, from a young age, a significantly smaller peak LS could be detected in the three layers of the LV wall of GRMD dogs from three apical views. The difference in peak LS between GRMD and healthy control dogs became less evident at 18 and 24 months of age.
4. Discussion
In this longitudinal study, significant alteration in global LV systolic function (i.e., decreased FS and EF) was revealed late by conventional echocardiography in GRMD dogs, as previously reported [6,8]. The decline in LV systolic function with age reflects the natural progression of cardiomyopathy in GRMD dogs in the absence of specific treatment targeting cardiomyopathy.
Myocardial regional mechanics can be assessed by echocardiography in terms of four principal types of strain or deformation: circumferential, longitudinal, radial and rotational. A combined analysis of these strains can give a complete description of LV myocardial strain. At present, simultaneously analyzing myocardial strains of LV endocardial, middle and epicardial layers by echocardiography is technically available for CS and LS. This study analyzed myocardial strain in terms of the CS and LS of different LV wall layers of different regions, with the hope that assessing LV myocardial strain from two aspects gives a more detailed description of the changes in LV myocardial regional strain during the development of cardiomyopathy in GRMD dogs. In fact, more detailed analysis of LV myocardial CS and LS revealed different change trends in LV myocardial strain in both the circumferential and longitudinal directions, showing earlier alterations consistent with previous findings [6,8]. More importantly, these alterations in LV function were not uniform within the LV wall, showing differential progression of cardiomyopathy from the apex to the base and from the epicardium to the endocardium.
Clinical studies using cardiac magnetic resonance image [18], tissue Doppler imaging (TDI) [19] and 2D-STE [16] showed that DMD boys (less than 10 years old) had a decreased peak CS which worsened with age. Interestingly, a study in DMD boys (aged 14.8 ± 3.1 years old) showed that peak CS in the posterior wall analyzed by TDI exhibited smaller values, more frequently, in the outer layer than in the inner layer [20]. In the present study, an analysis of endocardial, middle and epicardial CS by 2D-STE from PSAX-apex, PSAX-mid-chamber and PSAX-base views demonstrated a non-uniform change in peak CS in GRMD dogs, which occurred early (2 months of age) and manifested as a significant and persistently smaller peak CS in the epicardial and middle layers of the LV apical anterior and antero-lateral wall (Figure 2 and Table 2). Meanwhile, at the mid-chamber and basal levels of the left ventricle, a smaller CS was detected relatively later in GRMD dogs (Table 2). These results suggest that analysis of CS in the LV apical region may be a useful tool to reveal early manifestations of cardiomyopathy in GRMD dogs, paralleling the pattern described in DMD patients. This non-uniform distribution of functional alteration between the apical and basal areas was complemented by differential timing of reduced contractile function from the epicardial to the endocardial layer.
Previous studies have shown that a reduction in global systolic LS can be detected in young DMD boys [14,16,19,21] and GRMD dogs [8,10]. Going further, this study analyzed LS more deeply in the three layers of the LV wall from three apical views and showed that systolic LS was lower in the three LV wall layers in GRMD dogs than in healthy control dogs from a young age, which is consistent with previous studies showing a worse global systolic LS in GRMD dogs [8,10]. These results suggest that analysis of LS remains a sensitive tool to detect early alterations in LV systolic function in GRMD dogs.
A preferential alteration in subendocardial contractile function has been demonstrated in many pathological conditions such as exercise under acute increase in afterload [22], LV hypertrophy [23], coronary heart disease [24], and heart failure [25]. Previous studies have shown an early alteration of subendocardial systolic velocity analyzed by TDI in GRMD dogs [4,8]. This non-uniform change in subendocardial-subepicardial function has been considered to contribute to the progression of the disease. Interestingly, the present study showed that the left ventricle of GRMD dogs exhibits spatial non-uniform alteration in myocardial strain that occurs particularly in the apical region for CS. Because this non-uniform alteration in myocardial strain occurs early, it is reasonable to speculate that it is an important factor contributing to the progression of LV global contractile dysfunction.
The study may have some limitations. (1). Due to the obvious physical differences between GRMD and healthy control dogs, it is impossible for the investigator to be blind to the study group, which may lead to study bias. (2). This study characterized the temporal changes of LV myocardial strain in GRMD dogs but did not provide cellular and molecular mechanisms to explain this early change in myocardial strain. Thus, further studies of cellular and molecular mechanisms are needed. (3). The LV myocardial wall is a three-dimensional object and has strain that can occur along three planes. During contraction, the heart is not only shortening along its long and short axis, but also rotating, tilting and displacing inside the chest. Thus, in addition to technical factors that may influence the analysis of strain [12], this may also affect linear strain analyzed by 2D-STE, which is based on a simplistic sense of lengthening, shortening, thickening or rotating. In addition, the small thickness of the myocardial wall of young dogs (to be further divided into different layers by the software) and the high heart rate may increase the difficulty for accurate analysis of myocardial strain and possibly affect the accuracy of results. Therefore, caution should be taken when interpreting these results. However, the results obtained in young and adult dogs regardless of their conditions (healthy or GRMD) provide consistent myocardial strain information which is consistent with classic notions about LV contractile function. For example, both CS and LS data showed that the myocardial strain of the subendocardial layer is stronger than that in the subepicardial layer, which is consistent with the classic concept that the subendocardial layer contracts more strongly than the subepicardial layer. Further development of three-dimensional STE may give a more physiological description of LV myocardial strain reflecting the complexity of the heart’s three-dimensionality, and a recent study showed early impairment of LV 3-dimensional global LS in young patients with DMD [26].
5. Conclusions
In young dogs with DMD who have a normal global systolic function, a non-uniform reduction in systolic CS can be detected, especially in the LV apex, while decreased systolic LS can be detected in the three LV wall layers from three apical views. Our results suggest that analysis of myocardial strain by multi-layer 2D-STE may be a useful tool for monitoring the spatial evolution of alterations in LV systolic function and their progression with age in GRMD dogs. Given the similarity in pathogenesis, pathological changes, and clinical signs and symptoms between GRMD and DMD, our results suggest that assessing LV myocardial strain mechanics may be useful in detecting the early alteration of LV systolic function in early childhood of DMD patients and monitor its progression. The significance of these findings may warrant further investigation in DMD patients.
Author Contributions
Conceptualization, B.G., S.B. and J.B.S.; Methodology, J.B.S.; Validation, B.G. and J.B.S.; Formal Analysis, J.B.S.; Investigation, L.S., A.B. and J.B.S.; Resources, B.G., I.B. and S.B.; Data Curation, J.B.S.; Writing—Original Draft Preparation, J.B.S.; Writing—Review and Editing, B.G., I.B., A.B., D.C., L.H. and S.B., J.B.S.; Visualization, J.B.S.; Supervision, B.G.; Project Administration, B.G. and S.B.; Funding Acquisition, B.G., S.B. and J.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Association Française Contre les Myopathies (Grant No. 18778) and EspeRare Foundation (Switzerland, Bale) who were not involved in the study design, data collection or analysis, nor manuscript preparation and submission.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of ANSES-ENVA-UPEC (ComEth ANSES-ENVA-UPEC agreement #20/12/12–18).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data generated in this study are available in this article.
Acknowledgments
We thank the CEDS for providing GRMD and healthy dogs. We acknowledge Xavier Cauchois, Pablo Aguilar and the entire BNMS group in the ENVA for providing excellent cares to our canine patients. We thank Yi Su for her excellent technical support in the preparation of the figures.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CS: circumferential strain; DMD: Duchenne muscular dystrophy; EDD: end-diastolic diameter; EDV: end-diastolic volume; EF: ejection fraction; ESD: end-systolic diameter; ESV: end-systolic volume; FS: fractional shortening; GRMD: golden retriever muscular dystrophy; LS: longitudinal strain; LV: left ventricular; IVSWEDT: interventricular septal wall end-diastolic thickness; IVSWTh: interventricular septal wall systolic thickening; % IVSWTh: percentage of interventricular septal wall systolic thickening: PSAX: parasternal short-axis; PWEDT: posterior wall end-diastolic thickness; PWTh: posterior wall systolic thickening; % PWTh: percentage of posterior wall systolic thickening; STE: speckle tracking echocardiography; TDI: tissue Doppler imaging; 2C, 3C and 4C: 2-chamber, 3-chamber and 4-chamber.
References
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, F.; Garry, D.J. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, J.N. The Golden Retriever Model of Duchenne Muscular Dystrophy. Skelet. Muscle 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Chetboul, V. Tissue Doppler Imaging Detects Early Asymptomatic Myocardial Abnormalities in a Dog Model of Duchenne’s Cardiomyopathy. Eur. Heart J. 2004, 25, 1934–1939. [Google Scholar] [CrossRef]
- Townsend, D.; Turner, I.; Yasuda, S.; Martindale, J.; Davis, J.; Shillingford, M.; Kornegay, J.N.; Metzger, J.M. Chronic Administration of Membrane Sealant Prevents Severe Cardiac Injury and Ventricular Dilatation in Dystrophic Dogs. J. Clin. Investig. 2010, 120, 1140–1150. [Google Scholar] [CrossRef]
- Su, J.B.; Cazorla, O.; Blot, S.; Blanchard-Gutton, N.; Mou, Y.A.; Barthélémy, I.; Sambin, L.; Sampedrano, C.C.; Gouni, V.; Unterfinger, Y.; et al. Bradykinin Restores Left Ventricular Function, Sarcomeric Protein Phosphorylation, and e/NNOS Levels in Dogs with Duchenne Muscular Dystrophy Cardiomyopathy. Cardiovasc. Res. 2012, 95, 86–96. [Google Scholar] [CrossRef]
- Dabiré, H.; Barthélémy, I.; Blanchard-Gutton, N.; Sambin, L.; Sampedrano, C.C.; Gouni, V.; Unterfinger, Y.; Aguilar, P.; Thibaud, J.-L.; Ghaleh, B.; et al. Vascular Endothelial Dysfunction in Duchenne Muscular Dystrophy Is Restored by Bradykinin through Upregulation of ENOS and NNOS. Basic Res. Cardiol. 2012, 107, 240. [Google Scholar] [CrossRef]
- Ghaleh, B.; Barthélemy, I.; Sambin, L.; Bizé, A.; Hittinger, L.; Blot, S.; Su, J.B. Alteration in Left Ventricular Contractile Function Develops in Puppies With Duchenne Muscular Dystrophy. J. Am. Soc. Echocardiogr. 2020, 33, 120–129.e1. [Google Scholar] [CrossRef] [PubMed]
- Ghaleh, B.; Barthélemy, I.; Wojcik, J.; Sambin, L.; Bizé, A.; Hittinger, L.; Tran, T.D.; Thomé, F.P.; Blot, S.; Su, J.B. Protective Effects of Rimeporide on Left Ventricular Function in Golden Retriever Muscular Dystrophy Dogs. Int. J. Cardiol. 2020, 312, 89–95. [Google Scholar] [CrossRef]
- Cazorla, O.; Barthélémy, I.; Su, J.B.; Meli, A.C.; Chetboul, V.; Scheuermann, V.; Gouni, V.; Anglerot, C.; Richard, S.; Blot, S.; et al. Stabilizing Ryanodine Receptors Improves Left Ventricular Function in Juvenile Dogs with Duchenne Muscular Dystrophy. J. Am. Coll. Cardiol. 2021, 78, 2439–2453. [Google Scholar] [CrossRef]
- Martin, P.T.; Zygmunt, D.A.; Ashbrook, A.; Hamilton, S.; Packer, D.; Birch, S.M.; Bettis, A.K.; Balog-Alvarez, C.J.; Guo, L.-J.; Nghiem, P.P.; et al. Short-Term Treatment of Golden Retriever Muscular Dystrophy (GRMD) Dogs with RAAVrh74.MHCK7.GALGT2 Induces Muscle Glycosylation and Utrophin Expression but Has No Significant Effect on Muscle Strength. PLoS ONE 2021, 16, e0248721. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Gorcsan, J.; Tanaka, H. Echocardiographic Assessment of Myocardial Strain. J. Am. Coll. Cardiol. 2011, 58, 1401–1413. [Google Scholar] [CrossRef]
- Cho, M.-J.; Lee, J.-W.; Lee, J.; Shin, Y.B. Evaluation of Early Left Ventricular Dysfunction in Patients with Duchenne Muscular Dystrophy Using Two-Dimensional Speckle Tracking Echocardiography and Tissue Doppler Imaging. Pediatr. Cardiol. 2018, 39, 1614–1619. [Google Scholar] [CrossRef]
- Guo, L.-J.; Soslow, J.H.; Bettis, A.K.; Nghiem, P.P.; Cummings, K.J.; Lenox, M.W.; Miller, M.W.; Kornegay, J.N.; Spurney, C.F. Natural History of Cardiomyopathy in Adult Dogs With Golden Retriever Muscular Dystrophy. J. Am. Heart Assoc. 2019, 8, e012443. [Google Scholar] [CrossRef] [PubMed]
- Amedro, P.; Vincenti, M.; De La Villeon, G.; Lavastre, K.; Barrea, C.; Guillaumont, S.; Bredy, C.; Gamon, L.; Meli, A.C.; Cazorla, O.; et al. Speckle-Tracking Echocardiography in Children with Duchenne Muscular Dystrophy: A Prospective Multicenter Controlled Cross-Sectional Study. J. Am. Soc. Echocardiogr. 2019, 32, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Hor, K.N.; Wansapura, J.; Markham, L.W.; Mazur, W.; Cripe, L.H.; Fleck, R.; Benson, D.W.; Gottliebson, W.M. Circumferential Strain Analysis Identifies Strata of Cardiomyopathy in Duchenne Muscular Dystrophy. J. Am. Coll. Cardiol. 2009, 53, 1204–1210. [Google Scholar] [CrossRef]
- Ryan, T.D.; Taylor, M.D.; Mazur, W.; Cripe, L.H.; Pratt, J.; King, E.C.; Lao, K.; Grenier, M.A.; Jefferies, J.L.; Benson, D.W.; et al. Abnormal Circumferential Strain Is Present in Young Duchenne Muscular Dystrophy Patients. Pediatr. Cardiol. 2013, 34, 1159–1165. [Google Scholar] [CrossRef]
- Mori, K.; Hayabuchi, Y.; Inoue, M.; Suzuki, M.; Sakata, M.; Nakagawa, R.; Kagami, S.; Tatara, K.; Hirayama, Y.; Abe, Y. Myocardial Strain Imaging for Early Detection of Cardiac Involvement in Patients with Duchenne’s Progressive Muscular Dystrophy. Echocardiography 2007, 24, 598–608. [Google Scholar] [CrossRef]
- Mertens, L.; Ganame, J.; Claus, P.; Goemans, N.; Thijs, D.; Eyskens, B.; Van Laere, D.; Bijnens, B.; D’hooge, J.; Sutherland, G.R.; et al. Early Regional Myocardial Dysfunction in Young Patients with Duchenne Muscular Dystrophy. J. Am. Soc. Echocardiogr. 2008, 21, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Su, J.B.; Hittinger, L.; Le Franc, P.; Crozatier, B. Limited Left Ventricular Inotropic Response to Exercise in Early Phase of Pressure Overload in Dogs. Am. J. Physiol. 1992, 263, H1011–H1016. [Google Scholar] [CrossRef] [PubMed]
- Hittinger, L.; Ghaleh, B.; Chen, J.; Edwards, J.G.; Kudej, R.K.; Iwase, M.; Kim, S.-J.; Vatner, S.F.; Vatner, D.E. Reduced Subendocardial Ryanodine Receptors and Consequent Effects on Cardiac Function in Conscious Dogs with Left Ventricular Hypertrophy. Circ. Res. 1999, 84, 999–1006. [Google Scholar] [CrossRef]
- Nagao, M.; Hatakenaka, M.; Matsuo, Y.; Kamitani, T.; Higuchi, K.; Shikata, F.; Nagashima, M.; Mochizuki, T.; Honda, H. Subendocardial Contractile Impairment in Chronic Ischemic Myocardium: Assessment by Strain Analysis of 3T Tagged CMR. J. Cardiovasc. Magn. Reson. 2012, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Rahman, H.; Webb, A.; Shah, A.M.; Perera, D. Untangling the Pathophysiologic Link between Coronary Microvascular Dysfunction and Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2021, 42, 4431–4441. [Google Scholar] [CrossRef]
- Yu, H.-K.; Xia, B.; Liu, X.; Han, C.; Chen, W.; Li, Z. Initial Application of Three-Dimensional Speckle-Tracking Echocardiography to Detect Subclinical Left Ventricular Dysfunction and Stratify Cardiomyopathy Associated with Duchenne Muscular Dystrophy in Children. Int. J. Cardiovasc. Imaging 2019, 35, 67–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).