Nkx2-5 Loss of Function in the His-Purkinje System Hampers Its Maturation and Leads to Mechanical Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Lines
2.2. Macroscopic and Histological Analyses
2.3. Cardiac Magnetic Resonance Imaging (MRI)
2.4. Echocardiography
2.5. Surface Electrocardiography
2.6. Statistical Analysis
3. Results
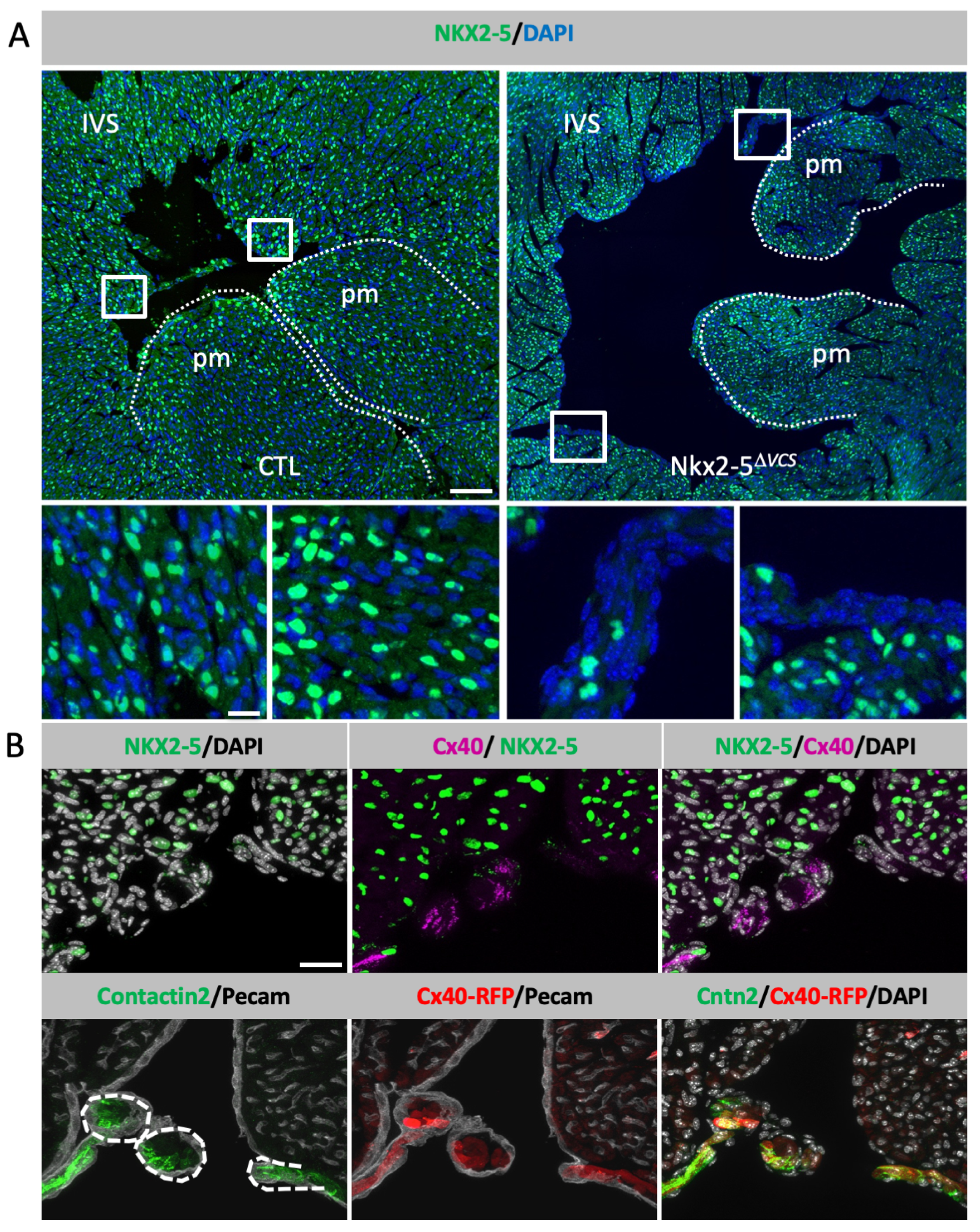
3.1. Conditional Deletion of Nkx2-5 in the Ventricular Conduction System
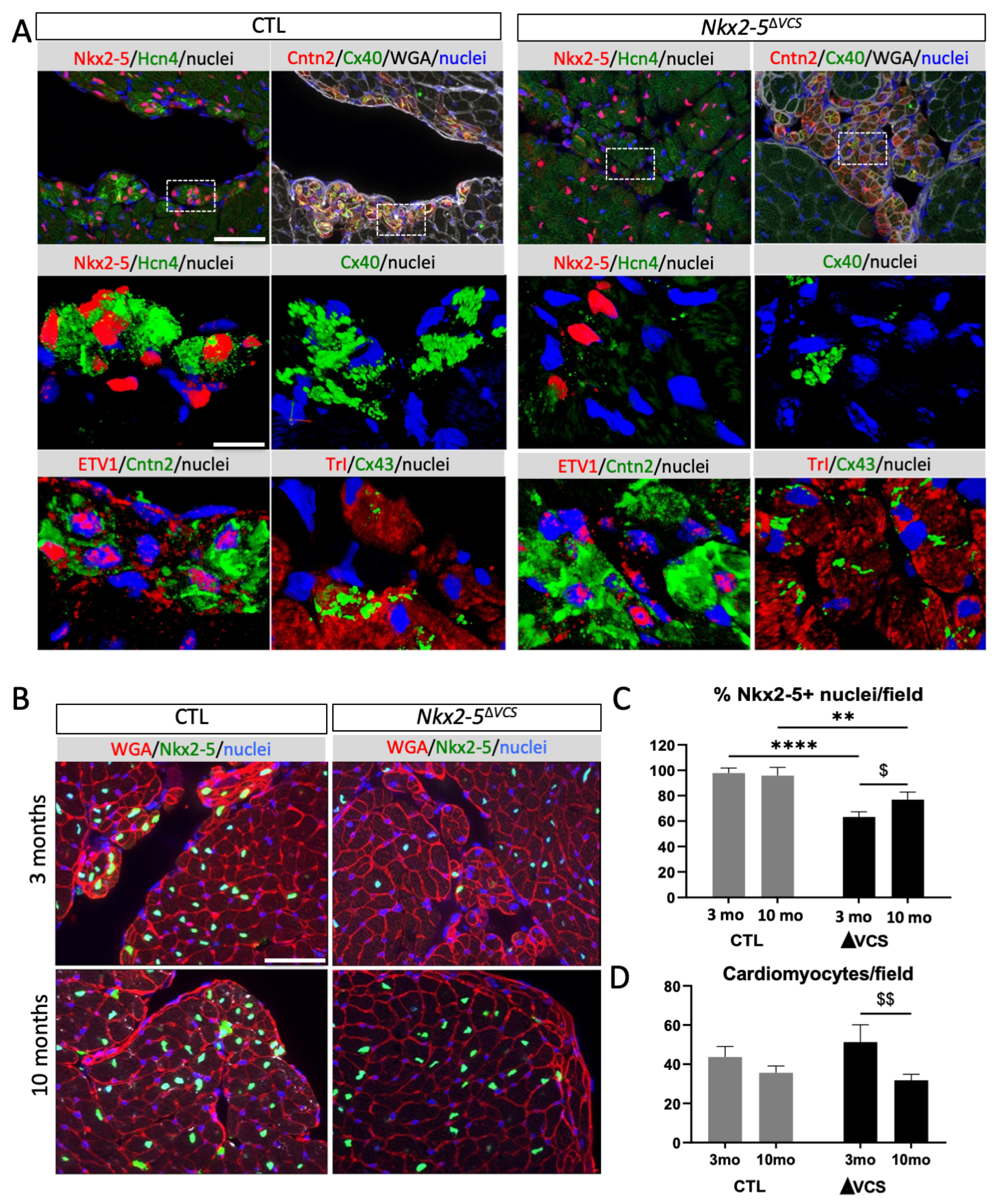
3.2. Neonatal Loss of Nkx2-5 in the VCS Disturbs Its Maturation and Provokes Apical PF Hypoplasia
3.3. Persistent Nkx2-5-Negative Conductive Cells Progressively Downregulate Fast-Conduction Markers
3.4. Conditional Deletion of Nkx2-5 in the VCS Leads to Cardiac Functional Defects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyett, M.R. ‘And the beat goes on.’ The cardiac conduction system: The wiring system of the heart. Exp. Physiol. 2009, 94, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Ho, S.Y. The morphology of the cardiac conduction system. Novartis Found. Symp. 2003, 250, 6–17; discussion 18–24, 276–279. [Google Scholar]
- Miquerol, L.; Moreno-Rascon, N.; Beyer, S.; Dupays, L.; Meilhac, S.M.; Buckingham, M.E.; Franco, D.; Kelly, R.G. Biphasic development of the mammalian ventricular conduction system. Circ. Res. 2010, 107, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zaglia, T.; Pianca, N.; Borile, G.; Da Broi, F.; Richter, C.; Campione, M.; Lehnart, S.E.; Luther, S.; Corrado, D.; Miquerol, L.; et al. Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of channelrhodopsin-2. Proc. Natl. Acad. Sci. USA 2015, 112, E4495–E4504. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, M.J.; ten Velde, I.; Wessels, A.; Oosthoek, P.W.; Gros, D.; Jongsma, H.J.; Moorman, A.F.; Lamers, W.H. Differential connexin distribution accommodates cardiac function in different species. Microsc. Res. Tech. 1995, 31, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Gros, D.B.; Jongsma, H.J. Connexins in mammalian heart function. Bioessays 1996, 18, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Marionneau, C.; Couette, B.; Liu, J.; Li, H.; Mangoni, M.E.; Nargeot, J.; Lei, M.; Escande, D.; Demolombe, S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J. Physiol. 2005, 562, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, N.; Le Bouter, S.; Szuts, V.; Varro, A.; Escande, D.; Nattel, S.; Demolombe, S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007, 582, 675–693. [Google Scholar] [CrossRef]
- John, R.M.; Tedrow, U.B.; Koplan, B.A.; Albert, C.M.; Epstein, L.M.; Sweeney, M.O.; Miller, A.L.; Michaud, G.F.; Stevenson, W.G. Ventricular arrhythmias and sudden cardiac death. Lancet 2012, 380, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Varriale, P.; Chryssos, B.E. The RSR’ complex not related to right bundle branch block: Diagnostic value as a sign of myocardial infarction scar. Am. Heart J. 1992, 123, 369–376. [Google Scholar] [CrossRef]
- Harvey, R.P.; Lai, D.; Elliott, D.; Biben, C.; Solloway, M.; Prall, O.; Stennard, F.; Schindeler, A.; Groves, N.; Lavulo, L.; et al. Homeodomain factor Nkx2-5 in heart development and disease. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital heart disease caused by mutations in the transcription factor Nkx2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef]
- Tanaka, M.; Berul, C.I.; Ishii, M.; Jay, P.Y.; Wakimoto, H.; Douglas, P.; Yamasaki, N.; Kawamoto, T.; Gehrmann, J.; Maguire, C.T.; et al. A mouse model of congenital heart disease: Cardiac arrhythmias and atrial septal defect caused by haploinsufficiency of the cardiac transcription factor Csx/Nkx2.5. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Jay, P.Y.; Harris, B.S.; Maguire, C.T.; Buerger, A.; Wakimoto, H.; Tanaka, M.; Kupershmidt, S.; Roden, D.M.; Schultheiss, T.M.; O’Brien, T.X.; et al. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J. Clin. Investig. 2004, 113, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Pashmforoush, M.; Lu, J.T.; Chen, H.; Amand, T.S.; Kondo, R.; Pradervand, S.; Evans, S.M.; Clark, B.; Feramisco, J.R.; Giles, W.; et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 2004, 117, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Ellesoe, S.G.; Johansen, M.M.; Bjerre, J.V.; Hjortdal, V.E.; Brunak, S.; Larsen, L.A. Familial atrial septal defect and sudden cardiac death: Identification of a novel Nkx2-5 mutation and a review of the literature. Congenit. Heart Dis. 2016, 11, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Maury, P.; Gandjbakhch, E.; Baruteau, A.E.; Bessiere, F.; Kyndt, F.; Bouvagnet, P.; Rollin, A.; Bonnet, D.; Probst, V.; Maltret, A. Cardiac phenotype and long-term follow-up of patients with mutations in nkx2-5 gene. J. Am. Coll. Cardiol. 2016, 68, 2389–2390. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Kelly, R.G.; Miquerol, L. Nkx2-5 defines distinct scaffold and recruitment phases during formation of the murine cardiac Purkinje fiber network. Nat. Commun. 2020, 11, 5300. [Google Scholar] [CrossRef]
- Choquet, C.; Nguyen, T.H.M.; Sicard, P.; Buttigieg, E.; Tran, T.T.; Kober, F.; Varlet, I.; Sturny, R.; Costa, M.W.; Harvey, R.P.; et al. Deletion of Nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy. PLoS Genet. 2018, 14, e1007502. [Google Scholar]
- Furtado, M.B.; Wilmanns, J.C.; Chandran, A.; Tonta, M.; Biben, C.; Eichenlaub, M.; Coleman, H.A.; Berger, S.; Bouveret, R.; Singh, R.; et al. A novel conditional mouse model for Nkx2-5 reveals transcriptional regulation of cardiac ion channels. Differentiation 2016, 91, 29–41. [Google Scholar] [CrossRef]
- Beyer, S.; Kelly, R.G.; Miquerol, L. Inducible Cx40-cre expression in the cardiac conduction system and arterial endothelial cells. Genesis 2011, 49, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Watanabe, T.; Lin, C.S.; William, C.M.; Tanabe, Y.; Jessell, T.M.; Costantini, F. Cre reporter strains produced by targeted insertion of eYFP and eCFP into the Rosa26 locus. BMC Dev. Biol. 2001, 1, 4. [Google Scholar] [CrossRef]
- Miquerol, L.; Meysen, S.; Mangoni, M.; Bois, P.; van Rijen, H.V.; Abran, P.; Jongsma, H.; Nargeot, J.; Gros, D. Architectural and functional asymmetry of the his-purkinje system of the murine heart. Cardiovasc. Res. 2004, 63, 77–86. [Google Scholar] [CrossRef]
- Bhan, A.; Sirker, A.; Zhang, J.; Protti, A.; Catibog, N.; Driver, W.; Botnar, R.; Monaghan, M.J.; Shah, A.M. High-frequency speckle tracking echocardiography in the assessment of left ventricular function and remodeling after murine myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1371–H1383. [Google Scholar] [CrossRef]
- Pallante, B.A.; Giovannone, S.; Fang-Yu, L.; Zhang, J.; Liu, N.; Kang, G.; Dun, W.; Boyden, P.A.; Fishman, G.I. Contactin-2 expression in the cardiac Purkinje fiber network. Circ. Arrhythm. Electrophysiol. 2010, 3, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.W. Genetic origins of pediatric heart disease. Pediatr. Cardiol. 2010, 31, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Prendiville, T.; Jay, P.Y.; Pu, W.T. Insights into the genetic structure of congenital heart disease from human and murine studies on monogenic disorders. Cold Spring Harb. Perspect. Med. 2014, 4, 100262. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.W. Genetics of atrioventricular conduction disease in humans. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 934–939. [Google Scholar] [CrossRef]
- Ashraf, H.; Pradhan, L.; Chang, E.I.; Terada, R.; Ryan, N.J.; Briggs, L.E.; Chowdhury, R.; Zarate, M.A.; Sugi, Y.; Nam, H.J.; et al. A mouse model of human congenital heart disease: High incidence of diverse cardiac anomalies and ventricular noncompaction produced by heterozygous Nkx2-5 homeodomain missense mutation. Circ. Cardiovasc. Genet. 2014, 7, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.E.; Takeda, M.; Cuadra, A.E.; Wakimoto, H.; Marks, M.H.; Walker, A.J.; Seki, T.; Oh, S.P.; Lu, J.T.; Sumners, C.; et al. Perinatal loss of Nkx2-5 results in rapid conduction and contraction defects. Circ. Res. 2008, 103, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Terada, R.; Warren, S.; Lu, J.T.; Chien, K.R.; Wessels, A.; Kasahara, H. Ablation of Nkx2-5 at mid-embryonic stage results in premature lethality and cardiac malformation. Cardiovasc. Res. 2011, 91, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Boukens, B.J.; Hoogendijk, M.G.; Verkerk, A.O.; Linnenbank, A.; van Dam, P.; Remme, C.A.; Fiolet, J.W.; Opthof, T.; Christoffels, V.M.; Coronel, R. Early repolarization in mice causes overestimation of ventricular activation time by the QRS duration. Cardiovasc. Res. 2013, 97, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, S.; Nelles, E.; Hagendorff, A.; Kruger, O.; Traub, O.; Willecke, K. Reduced cardiac conduction velocity and predisposition to arrhythmias in Connexin40-deficient mice. Curr. Biol. 1998, 8, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.M.; Goodenough, D.A.; Paul, D.L. Mice lacking Connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 1998, 8, 295–298. [Google Scholar] [CrossRef] [PubMed]
- van Rijen, H.V.; van Veen, T.A.; van Kempen, M.J.; Wilms-Schopman, F.J.; Potse, M.; Krueger, O.; Willecke, K.; Opthof, T.; Jongsma, H.J.; de Bakker, J.M. Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein Connexin40. Circulation 2001, 103, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Schrickel, J.W.; Kreuzberg, M.M.; Ghanem, A.; Kim, J.S.; Linhart, M.; Andrie, R.; Tiemann, K.; Nickenig, G.; Lewalter, T.; Willecke, K. Normal impulse propagation in the atrioventricular conduction system of Cx30.2/Cx40 double deficient mice. J. Mol. Cell. Cardiol. 2009, 46, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, H.S.; Vaidya, D.; Simon, A.M.; Paul, D.L.; Jalife, J.; Morley, G.E. High-resolution optical mapping of the right bundle branch in Connexin40 knockout mice reveals slow conduction in the specialized conduction system. Circ. Res. 2000, 87, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Song, Y.; Yu, D.; Sun, C.; Liu, C.; Chen, F.; Zhang, Y.; Wang, F.; Harvey, R.P.; et al. A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015, 142, 2521–2532. [Google Scholar] [PubMed]
- Mesirca, P.; Alig, J.; Torrente, A.G.; Muller, J.C.; Marger, L.; Rollin, A.; Marquilly, C.; Vincent, A.; Dubel, S.; Bidaud, I.; et al. Cardiac arrhythmia induced by genetic silencing of ‘funny’ (f) channels is rescued by girk4 inactivation. Nat. Commun. 2014, 5, 4664. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, A.; Lin, X.; Liu, F.Y.; Zhang, J.; Mo, H.; Bastarache, L.; Denny, J.C.; Cox, N.J.; Delmar, M.; Roden, D.M.; et al. Transcription factor Etv1 is essential for rapid conduction in the heart. J. Clin. Investig. 2016, 126, 4444–4459. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, A.; Lin, X.; Lin, B.; Liu, F.Y.; Zhang, J.; Khodadadi-Jamayran, A.; Tsirigos, A.; Bu, L.; Fishman, G.I.; Park, D.S. Etv1 activates a rapid conduction transcriptional program in rodent and human cardiomyocytes. Sci. Rep. 2018, 8, 9944. [Google Scholar] [CrossRef]
- Meysen, S.; Marger, L.; Hewett, K.W.; Jarry-Guichard, T.; Agarkova, I.; Chauvin, J.P.; Perriard, J.C.; Izumo, S.; Gourdie, R.G.; Mangoni, M.E.; et al. Nkx2.5 cell-autonomous gene function is required for the postnatal formation of the peripheral ventricular conduction system. Dev. Biol. 2007, 303, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.L.; Tajik, A.J.; Wilansky, S.; Steidley, D.E.; Mookadam, F. Isolated noncompaction of the left ventricular myocardium in adults: A systematic overview. J. Card. Fail. 2011, 17, 771–778. [Google Scholar] [CrossRef]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Takenaka, K.; Ebihara, A.; Uno, K.; Morita, H.; Nakajima, T.; Ozawa, T.; Aida, I.; Yonemochi, Y.; Higuchi, S.; et al. Prognostic impact of left ventricular noncompaction in patients with Duchenne/Becker muscular dystrophy—Prospective multicenter cohort study. Int. J. Cardiol. 2013, 2, 214. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Cheng, S.; Unno, K.; Lin, F.C.; Liao, R. Regional cardiac dysfunction and dyssynchrony in a murine model of afterload stress. PLoS ONE 2013, 8, e59915. [Google Scholar]
- de Lucia, C.; Wallner, M.; Eaton, D.M.; Zhao, H.; Houser, S.R.; Koch, W.J. Echocardiographic strain analysis for the early detection of left ventricular systolic/diastolic dysfunction and dyssynchrony in a mouse model of physiological aging. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 3, 2441. [Google Scholar] [CrossRef] [PubMed]
- Crendal, E.; Dutheil, F.; Naughton, G.; McDonald, T.; Obert, P. Increased myocardial dysfunction, dyssynchrony, and epicardial fat across the lifespan in healthy males. BMC Cardiovasc. Disord. 2014, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Wu, C.K.; Juang, J.M.; Wang, Y.C.; Su, M.Y.; Lai, L.P.; Hwang, J.J.; Chiang, F.T.; Tseng, W.Y.; Lin, J.L. Myocardial regional interstitial fibrosis is associated with left intra-ventricular dyssynchrony in patients with heart failure: A cardiovascular magnetic resonance study. Sci. Rep. 2016, 6, 20711. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Ahmad, M. Left ventricular dyssynchrony by 3 dimensional echocardiography: Current understanding and potential future clinical applications. Echocardiography 2015, 32, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.B.; Mafi-Rad, M.; van Stipdonk, A.M.; Vernooy, K.; Prinzen, F.W. Why qrs duration should be replaced by better measures of electrical activation to improve patient selection for cardiac resynchronization therapy. J. Cardiovasc. Transl. Res. 2016, 9, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Suffoletto, M.S.; Dohi, K.; Cannesson, M.; Saba, S.; Gorcsan, J., 3rd. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006, 113, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Gorcsan, J., 3rd; Bleeker, G.B.; Zhang, Q.; Schalij, M.J.; Suffoletto, M.S.; Fung, J.W.; Schwartzman, D.; Chan, Y.S.; Tanabe, M.; et al. Usefulness of tissue doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am. J. Cardiol. 2007, 100, 1263–1270. [Google Scholar] [CrossRef] [PubMed]

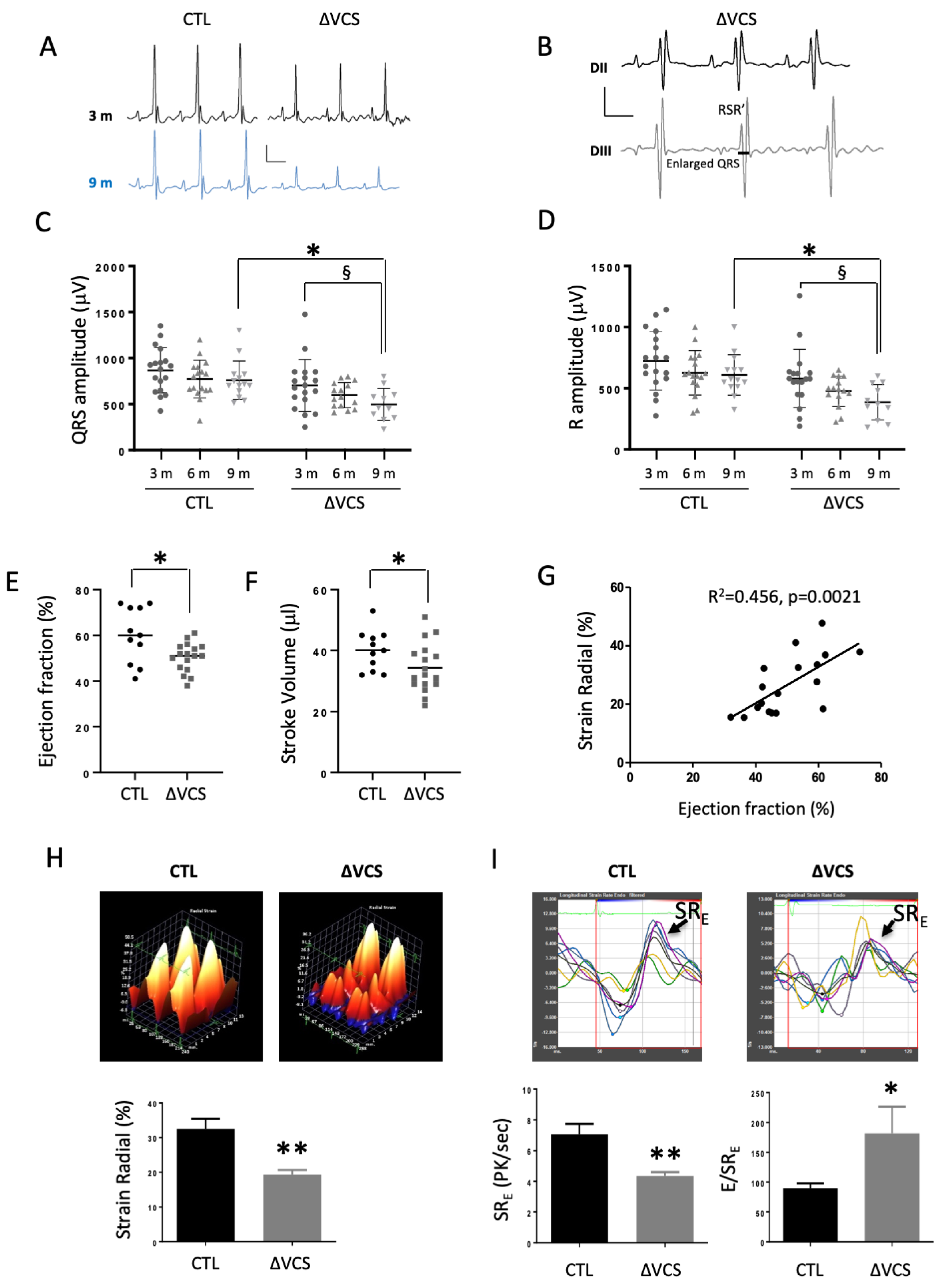
| 3 Months Old | 6 Months Old | 9 Months Old | 2-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Groups | Ctrl | ∆VCS | Ctrl | ∆VCS | Ctrl | ∆VCS | Age | Group |
| N | 18 | 18 | 17 | 15 | 14 | 11 | ||
| Heart Rate (BPM) | 519 ± 83 | 507 ± 61 | 493 ± 63 | 490 ± 54 | 495 ± 69 | 504 ± 57 | NS | NS |
| PR (ms) | 31.8 ± 3.3 | 32.0 ± 3.6 | 34.3 ± 4.3 | 33.3 ± 3.2 | 34.4 ± 3.3 | 33.0 ± 3.1 | ¶ | NS |
| QRS lead III (ms) | 12.3 ± 1.3 | 13.4 ± 2.8 | 11.6 ± 1.1 | 13.6 ± 2.8 * | 11.7 ± 1.5 | 13.3 ± 3.3 | NS | ¶¶ |
| RSR’-QRS (ms) | 15.6 ± 3.4 (28%) | 15.9 ± 2.5 (47%) | 15.2 ± 3.0 (55%) | |||||
| QT (ms) | 41.7 ± 1.7 | 42.8 ± 4.0 | 42.7 ± 5.3 | 43.8 ± 7.2 | 47.5 ± 7.7 § | 45.6 ± 8.2 | ¶ | NS |
| T (µV) | 65.6 ± 34.2 | 63.9 ± 38.1 | 78.2 ± 34.5 | 70.7 ± 21.3 | 87.7 ± 25.5 | 63.6 ± 25.3 | NS | NS |
| QRS (µV) | 868.1 ± 245.3 | 702.7 ± 282.0 | 772.1 ± 205.3 | 597.7 ± 136.7 | 760.1 ± 209.6 | 497.2 ± 174.1 *,§ | ¶ | ¶¶¶¶ |
| S (µV) | 144.9 ± 72.1 | 122.2 ± 85.6 | 144.8 ± 91.1 | 120.8 ± 82.3 | 150.8 ± 96.5 | 110.9 ± 62.9 | NS | NS |
| R (µV) | 723.3 ± 238.0 | 580.6 ± 238.8 | 627.2 ± 181.1 | 476.9 ± 124.4 | 609.3 ± 164.8 | 386.3 ± 144.9 *,§ | ¶¶ | ¶¶¶¶ |
| 6 Months Old | ||
|---|---|---|
| Groups | Ctrl | ∆VCS |
| Physiological parameters | ||
| N | 11 | 17 |
| Body weight (g) | 46.5 ± 7.7 | 43.1 ± 8.6 |
| Heart rate (BPM) | 466 ± 61 | 503 ± 47 |
| Morphological parameters | ||
| EDV (μL) | 67.6 ± 9.4 | 68.2 ± 13.1 |
| ESV (μL) | 27.9 ± 11.3 | 33.9 ± 7.7 |
| LV mass (mg) | 100.4 ± 11.5 | 95.9 ± 19.5 |
| LV mass sys (mg) | 121.4 ± 18.3 | 118.2 ± 26.4 |
| sWTn (%) | 41.4 ± 18.3 | 36.8 ± 10.6 |
| Functional parameters | ||
| EF (%) | 60 ± 12 | 50 ± 6 * |
| SV (μL) | 40.1 ± 6.6 | 34.4 ± 8.0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choquet, C.; Sicard, P.; Vahdat, J.; Nguyen, T.H.M.; Kober, F.; Varlet, I.; Bernard, M.; Richard, S.; Kelly, R.G.; Lalevée, N.; et al. Nkx2-5 Loss of Function in the His-Purkinje System Hampers Its Maturation and Leads to Mechanical Dysfunction. J. Cardiovasc. Dev. Dis. 2023, 10, 194. https://doi.org/10.3390/jcdd10050194
Choquet C, Sicard P, Vahdat J, Nguyen THM, Kober F, Varlet I, Bernard M, Richard S, Kelly RG, Lalevée N, et al. Nkx2-5 Loss of Function in the His-Purkinje System Hampers Its Maturation and Leads to Mechanical Dysfunction. Journal of Cardiovascular Development and Disease. 2023; 10(5):194. https://doi.org/10.3390/jcdd10050194
Chicago/Turabian StyleChoquet, Caroline, Pierre Sicard, Juliette Vahdat, Thi Hong Minh Nguyen, Frank Kober, Isabelle Varlet, Monique Bernard, Sylvain Richard, Robert G. Kelly, Nathalie Lalevée, and et al. 2023. "Nkx2-5 Loss of Function in the His-Purkinje System Hampers Its Maturation and Leads to Mechanical Dysfunction" Journal of Cardiovascular Development and Disease 10, no. 5: 194. https://doi.org/10.3390/jcdd10050194
APA StyleChoquet, C., Sicard, P., Vahdat, J., Nguyen, T. H. M., Kober, F., Varlet, I., Bernard, M., Richard, S., Kelly, R. G., Lalevée, N., & Miquerol, L. (2023). Nkx2-5 Loss of Function in the His-Purkinje System Hampers Its Maturation and Leads to Mechanical Dysfunction. Journal of Cardiovascular Development and Disease, 10(5), 194. https://doi.org/10.3390/jcdd10050194







