Transcatheter Aortic Valve Replacement Prognostication with Augmented Mean Arterial Pressure
Abstract
1. Background/Introduction
2. Materials and Methods
2.1. Study Population, Baseline Demographics and Clinical Data
2.2. Baseline Transthoracic Echocardiography
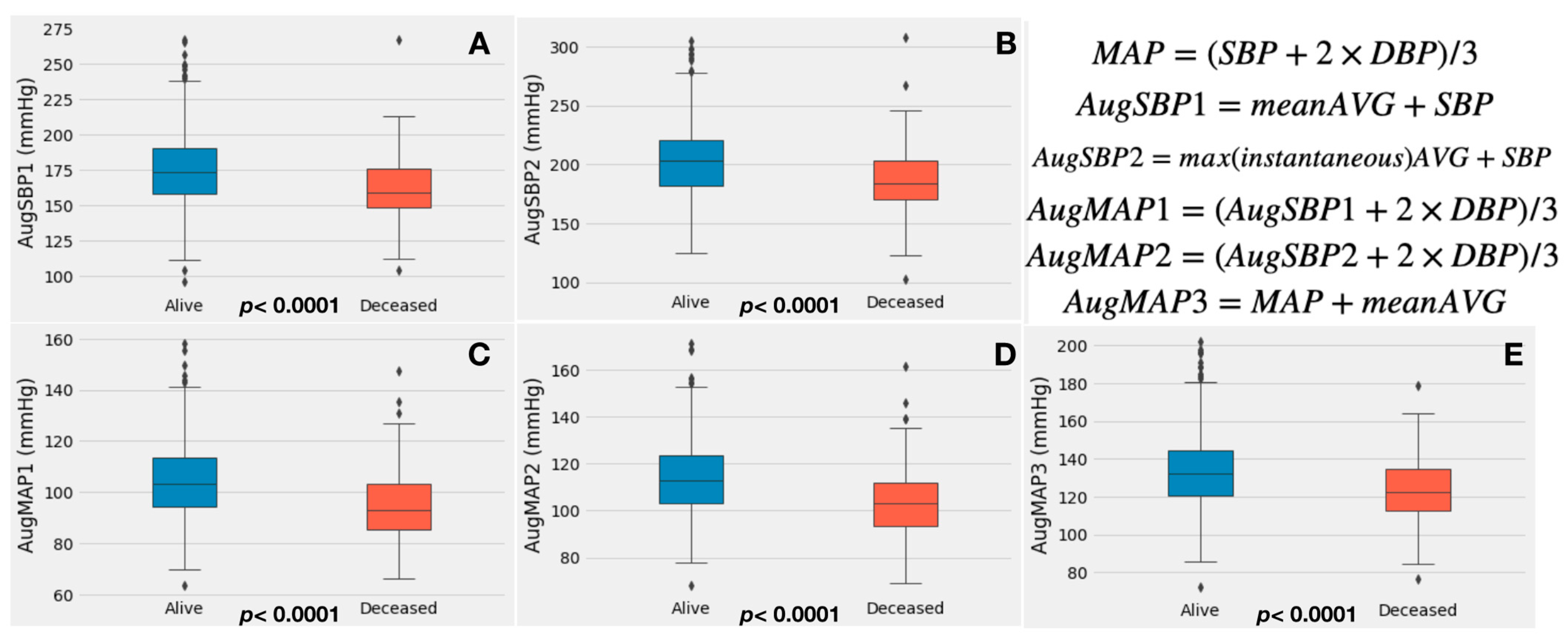
2.3. Calculation of Augmented Systolic Blood Pressure, Mean Arterial Pressure and Valvulo-Arterial Impedance
- (1)
- Augmented SBP1 (AugSBP1): the mean aortic valve gradient (mean AVG) was added to systolic blood pressure (Equation (2)), and augmented MAP1 (AugMAP1) was calculated by replacing the SBP with augmented SBP1 in the MAP formula (Equation (3));
- (2)
- Augmented SBP2 (AugSBP2): the aortic valve maximal instantaneous gradient was added to systolic blood pressure (Equation (4)), and augmented MAP2 (AugMAP2) was calculated by replacing the SBP with augmented SBP2 (Equation (5));
- (3)
2.4. Statistical Analysis
2.5. Patient and Public Involvement
3. Results
3.1. Study Population and Baseline Demographics
3.2. Augmented Blood Pressure, Echocardiography and Zva Measurements
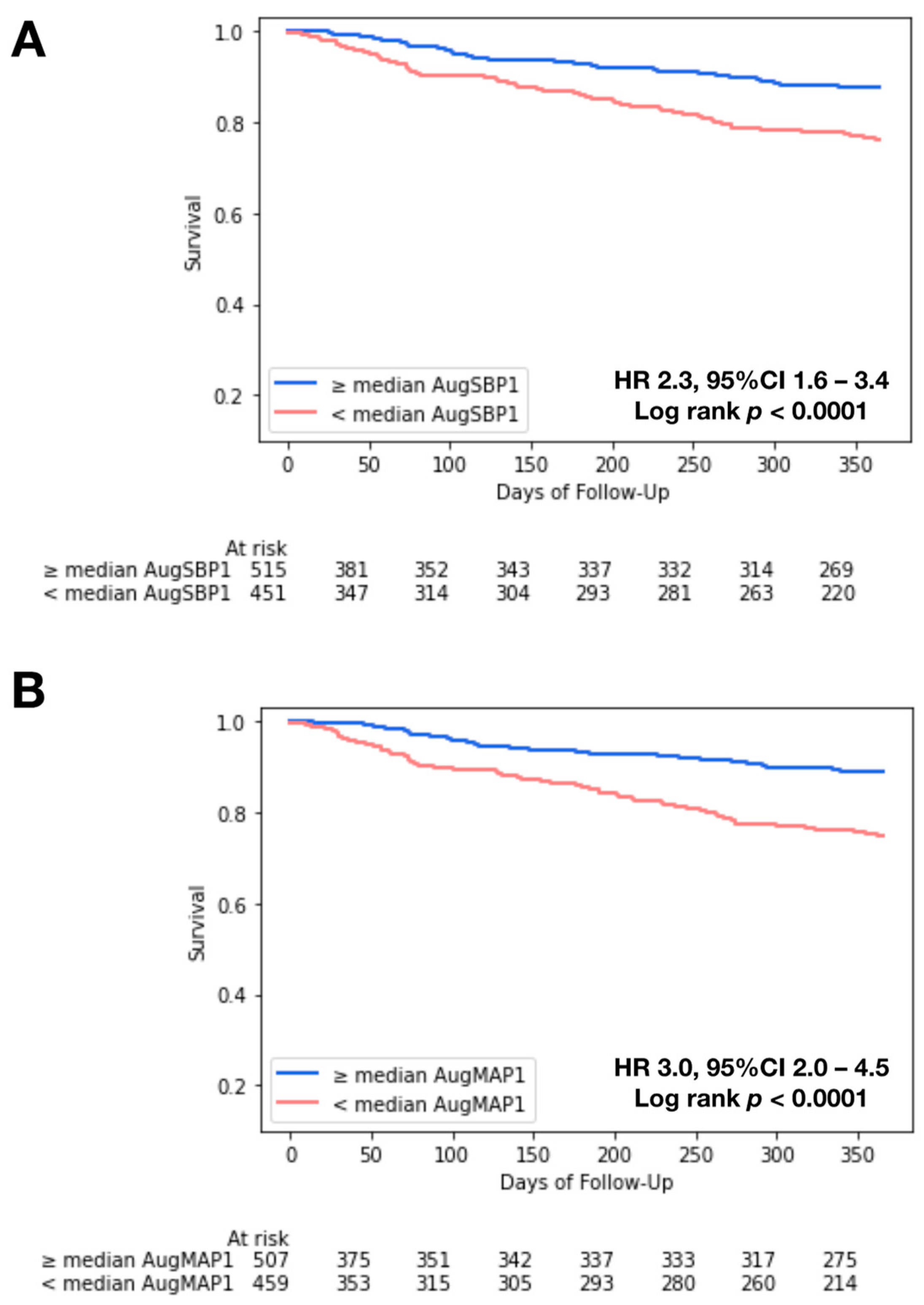
3.3. Kaplan–Meier Analysis and Cox Regression
3.4. Performance of Univariate Cox Regression Models
4. Discussion
4.1. The Physiological Significance of Augmented Blood Pressure
4.2. An Overlooked Outcome Predictor: Augmented Blood Pressure
4.3. Valvulo-Arterial Impedance (Zva) vs. AugMAP/AugSBP
4.4. Incorporating Augmented MAP in the Assessment of TAVR Patients
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AVG | aortic valve gradient |
| AugSBP | augmented systolic blood pressure |
| AugMAP | augmented mean arterial pressure |
| CO | cardiac output |
| DBP | diastolic blood pressure |
| MAP | mean arterial pressure |
| SBP | systolic blood pressure |
| Svi | stroke volume index |
| STS | Society of Thoracic Surgeons |
| SAVR | surgical aortic valve replacement |
| TTE | transthoracic echocardiography |
| TAVR | transcatheter aortic valve replacement |
| Zva | valvulo-arterial impedance |
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; Writing Committee Members; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.E.; Tseng, A.; Mookadam, F.; Venepally, N.R.; Buras, M.; Khetarpal, B.K.; Allam, M.; et al. Artificial intelligence trumps TAVI2-SCORE and CoreValve Score in predicting 1-year mortality post Transcatheter Aortic Valve Replacement. Cardiovasc. Revascularization Med. 2020, 24, 33–41. [Google Scholar] [CrossRef]
- Agasthi, P.; Pujari, S.H.; Mookadam, F.; Tseng, A.; Venepally, N.R.; Wang, P.; Allam, M.; Sweeney, J.; Eleid, M.; Fortuin, F.D.; et al. Does a Gradient-Adjusted Cardiac Power Index Improve Prediction of Post-Transcatheter Aortic Valve Replacement Survival Over Cardiac Power Index? Yonsei. Med. J. 2020, 61, 482. [Google Scholar] [CrossRef]
- Agasthi, P.; Arsanjani, R.; Mookadam, F.; Wang, P.; Venepally, N.R.; Sweeney, J.; Eleid, M.; Holmes, D.R., Jr.; Pollak, P.; Fortuin, F.D. Does Resting Cardiac Power Index Affect Survival Post Transcatheter Aortic Valve Replacement? J. Invasive Cardiol. 2020, 32, 129–137. [Google Scholar]
- Lindman, B.R.; Otto, C.M.; Douglas, P.S.; Hahn, R.T.; Elmariah, S.; Weissman, N.J.; Stewart, W.J.; Ayele, G.M.; Zhang, F.; Zajarias, A.; et al. Blood Pressure and Arterial Load After Transcatheter Aortic Valve Replacement for Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 10, e006308. [Google Scholar] [CrossRef]
- Lindman, B.R.; Goel, K.; Bermejo, J.; Beckman, J.; O’Leary, J.; Barker, C.M.; Kaiser, C.; Cavalcante, J.L.; Elmariah, S.; Huang, J.; et al. Lower Blood Pressure after Transcatheter or Surgical Aortic Valve Replacement is Associated with Increased Mortality. J. Am. Hear. Assoc. 2019, 8, e014020. [Google Scholar] [CrossRef]
- Nuis, R.-J.; Goudzwaard, J.A.; de Ronde-Tillmans, M.J.; Kroon, H.; Ooms, J.F.; van Wiechen, M.P.; Geleijnse, M.L.; Zijlstra, F.; Daemen, J.; Van Mieghem, N.M.; et al. Impact of Valvulo-Arterial Impedance on Long-Term Quality of Life and Exercise Performance After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2020, 13, e008372. [Google Scholar] [CrossRef]
- Fadahunsi, O.O.; Olowoyeye, A.; Ukaigwe, A.; Li, Z.; Vora, A.N.; Vemulapalli, S.; Elgin, E.; Donato, A. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: Analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc. Interv. 2016, 9, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.-P.; Bartko, P.; Hofer, F.; Zbiral, M.; Burger, A.; Ghanim, B.; Kastner, J.; Lang, I.M.; Mascherbauer, J.; Hengstenberg, C.; et al. Evolution of outcome and complications in TAVR: A meta-analysis of observational and randomized studies. Sci. Rep. 2020, 10, 15568. [Google Scholar] [CrossRef]
- Balan, P.; Zhao, Y.; Johnson, S.; Arain, S.; Dhoble, A.; Estrera, A.; Smalling, R.; Nguyen, T.C. The Society of Thoracic Surgery Risk Score as a Predictor of 30-Day Mortality in Transcatheter vs Surgical Aortic Valve Replacement: A Single-Center Experience and its Implications for the Development of a TAVR Risk-Prediction Model. J. Invasive Cardiol. 2017, 29, 109–114. [Google Scholar] [PubMed]
- Hemmann, K.; Sirotina, M.; De Rosa, S.; Ehrlich, J.R.; Fox, H.; Weber, J.; Moritz, A.; Zeiher, A.M.; Hofmann, I.; Schächinger, V.; et al. The STS score is the strongest predictor of long-term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact. Cardiov. Thorac. Surg. 2013, 17, 359–364. [Google Scholar] [CrossRef]
- Pilgrim, T.; Franzone, A.; Stortecky, S.; Nietlispach, F.; Haynes, A.G.; Tueller, D.; Toggweiler, S.; Muller, O.; Ferrari, E.; Noble, S.; et al. Predicting Mortality after Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2018, 10, e005481. [Google Scholar] [CrossRef] [PubMed]
- Pagoulatou, S.; Stergiopulos, N.; Bikia, V.; Rovas, G.; Licker, M.-J.; Müller, H.; Noble, S.; Adamopoulos, D. Acute effects of transcatheter aortic valve replacement on the ventricular-aortic interaction. J. Physiol.—Heart Circ. Physiol. 2020, 319, H1451–H1458. [Google Scholar] [CrossRef]
- Katsanos, S.; Yiu, K.H.; Clavel, M.-A.; Rodés-Cabau, J.; Leong, D.; van der Kley, F.; Marsan, N.A.; Bax, J.J.; Pibarot, P.; Delgado, V. Impact of Valvuloarterial Impedance on 2-Year Outcome of Patients Undergoing Transcatheter Aortic Valve Implantation. J. Am. Soc. Echocardiogr. 2013, 26, 691–698. [Google Scholar] [CrossRef]
- Nagura, F.; Kataoka, A.; Hara, M.; Kozuma, K.; Watanabe, Y.; Nakashima, M.; Hioki, H.; Kawashima, H.; Nara, Y.; The OCEAN-TAVI Investigators; et al. Association between valvuloarterial impedance after transcatheter aortic valve implantation and 2-year mortality in elderly patients with severe symptomatic aortic stenosis: The OCEAN-TAVI registry. Heart Vessel. 2019, 34, 1031–1039. [Google Scholar] [CrossRef]
- Giannini, C.; Petronio, A.S.; De Carlo, M.; Guarracino, F.; Benedetti, G.; Donne, M.G.D.; Dini, F.L.; Marzilli, M.; Di Bello, V. The Incremental Value of Valvuloarterial Impedance in Evaluating the Results of Transcatheter Aortic Valve Implantation in Symptomatic Aortic Stenosis. J. Am. Soc. Echocardiog. 2012, 25, 444–453. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, M.; He, W.; Churchill, T.W.; Capoulade, R.; Liu, S.; Lee, H.; Danik, J.S.; Picard, M.H.; Pibarot, P.; Levine, R.A.; et al. Transvalvular Flow Rate Determines Prognostic Value of Aortic Valve Area in Aortic Stenosis. JACC 2020, 75, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Haibe-Kains, B.; Desmedt, C.; Sotiriou, C.; Bontempi, G. A comparative study of survival models for breast cancer prognostication based on microarray data: Does a single gene beat them all? Bioinformatics 2008, 24, 2200–2208. [Google Scholar] [CrossRef]
- Perlman, G.Y.; Loncar, S.; Pollak, A.; Gilon, D.; Alcalai, R.; Planer, D.; Lotan, C.; Danenberg, H.D. Post-Procedural Hypertension Following Transcatheter Aortic Valve Implantation Incidence and Clinical Significance. JACC Cardiovasc. Interv. 2013, 6, 472–478. [Google Scholar] [CrossRef]
- Mancusi, C.; de Simone, G.; Hitij, J.B.; Sudano, I.; Mahfoud, F.; Parati, G.; Kahan, T.; Barbato, E.; Pierard, L.A.; Garbi, M.; et al. Management of patients with combined arterial hypertension and aortic valve stenosis: A consensus document from the Council on Hypertension and Council on Valvular Heart Disease of the European Society of Cardiology, the European Association of Cardiovascular Imaging (EACVI), the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J.—Cardiovasc. Pharmacother. 2020, 7, 242–250. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the Valvuloarterial Impedance to Predict Adverse Outcome in Asymptomatic Aortic Stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef]
- Harada, K.; Saitoh, T.; Tanaka, J.; Shibayama, K.; Berdejo, J.; Shiota, T. Valvuloarterial Impedance, But Not Aortic Stenosis Severity, Predicts Syncope in Patients with Aortic Stenosis. Circ. Cardiovasc. Imaging 2013, 6, 1024–1031. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Ramanan, T.; Khalil, C.; Pham, M.; Sinibaldi, E.; Hansen, R.; Baldo, S.; Colern, G.; Sawant, A.C.; Corbelli, J.; et al. Valvuloarterial Impedance Predicts Heart Failure Readmissions in Patients Undergoing Transcatheter Aortic Valve Replacement. Struct. Heart 2017, 1, 277–284. [Google Scholar] [CrossRef]
- Shih, T.; Paone, G.; Theurer, P.F.; McDonald, D.; Shahian, D.M.; Prager, R.L. The Society of Thoracic Surgeons Adult Cardiac Surgery Database Version 2.73: More Is Better. Ann. Thorac. Surg. 2015, 100, 516–521. [Google Scholar] [CrossRef]
- Baumgartner, H.; Otto, C.M. Aortic Stenosis Severity Do We Need a New Concept? Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. J. Am. Coll. Cardiol. 2009, 54, 1012–1013. [Google Scholar] [CrossRef] [PubMed]

| Variables | Alive | Deceased | Total | p-Value |
|---|---|---|---|---|
| N = 835 | N = 139 | N = 974 | ||
| Baseline Demographics | ||||
| Age (year) | 81.5 ± 8.1 | 80.7 ± 9.4 | 81.4 ± 8.3 | 0.302 |
| Male sex (%) | 469 (56.2%) | 82 (59.0%) | 551 (56.6%) | 0.824 |
| Caucasian race | 814 (97.5%) | 133 (95.7%) | 947 (97.3%) | 0.792 |
| STS risk score | 7.9 ± 5.1 | 9.8 ± 5.3 | 8.2 ± 5.2 | <0.0001 |
| Hypertension | 702 (84.1%) | 119 (85.6%) | 821 (84.3%) | 0.899 |
| Diabetes mellitus | 300 (35.9%) | 54 (38.8%) | 354 (36.3%) | 0.803 |
| Prior MI | 200 (24.0%) | 37 (26.6%) | 237 (24.3%) | 0.794 |
| Prior CABG | 208 (24.9%) | 42 (30.2%) | 250 (25.7%) | 0.415 |
| Prior stroke | 75 (9.0%) | 20 (14.4%) | 95 (9.8%) | 0.138 |
| Prior PAD | 421 (50.4%) | 75 (54.0%) | 496 (50.9%) | 0.742 |
| Current dialysis | 26 (3.1%) | 13 (9.4%) | 39 (4.0%) | 0.002 |
| Atrial fibrillation/atrial flutter | 322 (38.6%) | 84 (60.4%) | 406 (41.7%) | <0.0001 |
| Permanent pacemaker | 119 (14.3%) | 25 (18.0%) | 144 (14.8%) | 0.517 |
| Previous implantable cardioverter device | 32 (3.8%) | 6 (4.3%) | 38 (3.9%) | 0.963 |
| NYHA class within 2 weeks | 0.656 | |||
| I | 23 (2.8%) | 7 (5.0%) | 30 (3.1%) | |
| II | 184 (22.0%) | 27 (19.4%) | 211 (21.7%) | |
| III | 513 (61.4%) | 80 (57.6%) | 593 (60.9%) | |
| IV | 115 (13.8%) | 25 (18.0%) | 140 (14.4%) | |
| Device type | 0.315 | |||
| Balloon-expandable valve | 677 (81.1%) | 105 (75.5%) | 782 (80.3%) | |
| Self-expanding valve | 158 (18.9%) | 34 (24.5%) | 192 (19.7%) | |
| Aortic valve morphology | 0.029 | |||
| Tricuspid | 818 (98.0%) | 138 (99.3%) | 956 (99.0%) | |
| Bicuspid | 8 (1.0%) | 0 (0.0%) | 0 (0.8%) | |
| Augmented Blood Pressure Parameters | ||||
| Heart rate (bpm) | 70.3 ± 13.1 | 69.1 ± 12.7 | 70.2 ± 13.0 | 0.218 |
| Systolic blood pressure (mmHg) | 130.9 ± 21.4 | 119.1 ± 20.0 | 129.3 ± 21.6 | <0.0001 |
| Diastolic blood pressure (mmHg) | 68.9 ± 13.1 | 62.3 ± 12.3 | 68.0 ± 13.2 | <0.0001 |
| Mean arterial blood pressure (mmHg) | 89.5 ± 13.4 | 81.2 ± 13.0 | 88.3 ± 13.6 | <0.0001 |
| Aortic valve systolic mean gradient (mmHg) | 44.2 ± 13.1 | 42.7 ± 12.6 | 44.0 ± 13.1 | 0.199 |
| Aortic valve systolic maximal instantaneous gradient (mmHg) | 72.1 ± 21.1 | 69.1 ± 21.0 | 71.7 ± 21.1 | 0.080 |
| Aortic valve systolic peak velocity (m/s) | 4.2 ± 0.6 | 4.1 ± 0.6 | 4.2 ± 0.6 | 0.139 |
| Aortic valve systolic area (cm2) | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.410 |
| AugSBP1 (mmHg) | 174.9 ± 25.6 | 161.5 ± 23.9 | 173.0 ± 25.8 | <0.0001 |
| AugSBP2 (mmHg) | 203.0 ± 30.4 | 187.2 ± 30.0 | 200.7 ± 30.8 | <0.0001 |
| AugMAP1 (mmHg) | 104.1 ± 14.3 | 95.3 ± 13.7 | 102.9 ± 14.6 | <0.0001 |
| AugMAP2 (mmHg) | 113.3 ± 15.3 | 103.8 ± 15.1 | 111.9 ± 15.6 | <0.0001 |
| AugMAP3 (mmHg) | 133.5 ± 19.3 | 123.6 ± 18.1 | 132.1 ± 19.5 | <0.0001 |
| Zva (mmHg mL−1 m−2) | 4.2 ± 1.1 | 3.9 ± 0.9 | 4.2 ± 1.1 | 0.023 |
| Medications | ||||
| Aspirin | 122 (14.6%) | 21 (15.1%) | 143 (14.7%) | 0.988 |
| Beta blocker | 583 (69.9%) | 82 (63.1%) | 665 (69.0%) | 0.619 |
| ACE inhibitor | 147 (17.6%) | 24 (18.5%) | 171 (17.8%) | 0.932 |
| ARB | 76 (9.1%) | 11 (8.5%) | 87 (9.0%) | 0.403 |
| Labs | ||||
| Hemoglobin (g/dL) | 12.2 ± 1.8 | 11.4 ± 1.9 | 12.1 ± 1.8 | <0.0001 |
| Creatinine (mg/dL) | 1.3 ± 1.0 | 1.7 ± 1.6 | 1.4 ± 1.1 | <0.0001 |
| Total albumin (g/dL) | 4.1 ± 0.3 | 4.0 ± 0.4 | 4.1 ± 0.4 | 0.001 |
| Echocardiography Parameters | ||||
| Left ventricular mass index (g/m2) | 118.2 ± 32.0 | 121.1 ± 32.4 | 118.6 ± 32.1 | 0.173 |
| Left ventricular ejection fraction(%) | 57.8 ± 12.8 | 55.9 ± 14.5 | 57.5 ± 13.1 | 0.221 |
| Estimated right atrial pressure (mmHg) | 7.2 ± 3.8 | 8.9 ± 5.2 | 7.4 ± 4.1 | 0.0004 |
| Right ventricular systolic pressure (mm Hg) | 41.0 ± 13.2 | 45.4 ± 18.5 | 41.7 ± 14.2 | 0.010 |
| Left ventricular internal systolic dimension (mm) | 32.4 ± 8.3 | 34.2 ± 10.3 | 32.7 ± 8.7 | 0.092 |
| Left ventricular internal end diastolic dimension (mm) | 48.8 ± 7.0 | 49.6 ± 8.8 | 48.9 ± 7.3 | 0.203 |
| Left ventricular stroke volume index (mL/m2) | 43.7 ± 9.8 | 42.8 ± 9.7 | 43.6 ± 9.8 | 0.123 |
| Transvalvular flow rate (Q, mL/s) | 259.4 ± 71.2 | 261.9 ± 86.3 | 259.7 ± 73.4 | 0.309 |
| Left ventricular cardiac output (L/min) | 5.7 ± 1.3 | 5.3 ± 1.2 | 5.6 ± 1.3 | 0.003 |
| Aortic valve systolic TVI (cm) | 102.9 ± 19.8 | 98.8 ± 21.3 | 102.3 ± 20.0 | 0.024 |
| ≥Moderate aortic regurgitation | 113 (14.9%) | 14 (10.7%) | 127 (14.3%) | 0.530 |
| ≥Moderate mitral valve regurgitation | 203 (24.3%) | 38 (27.7%) | 241(24.8%) | 0.864 |
| ≥Moderate tricuspid valve regurgitation | 193 (23.1%) | 50 (36.0%) | 243 (24.9%) | 0.005 |
| Covariate | HR (Per Unit Increase) | Lower 95%CI | Upper 95%CI | p-Value |
|---|---|---|---|---|
| Age | 0.990 | 0.972 | 1.009 | 0.317 |
| Sex | 0.871 | 0.616 | 1.229 | 0.431 |
| HTN | 1.130 | 0.695 | 1.837 | 0.621 |
| DM | 1.085 | 0.766 | 1.537 | 0.647 |
| Stroke | 1.490 | 0.906 | 2.448 | 0.116 |
| PriorMI | 1.179 | 0.804 | 1.728 | 0.399 |
| PAD | 1.062 | 0.755 | 1.492 | 0.731 |
| Current hemodialysis | 2.383 | 1.316 | 4.313 | 0.004 |
| Afib/Aflutter | 2.213 | 1.564 | 3.131 | <0.0001 |
| Hgb (per unit increase) | 0.800 | 0.725 | 0.882 | <0.0001 |
| RAP (per unit increase) | 1.076 | 1.033 | 1.121 | 0.0004 |
| RVSP (per unit increase) | 1.020 | 1.009 | 1.031 | 0.0003 |
| Creatinine (per unit increase) | 1.172 | 1.066 | 1.289 | 0.001 |
| Albumin (per unit increase) | 0.420 | 0.280 | 0.632 | <0.0001 |
| ≥Moderate MR | 1.195 | 0.815 | 1.752 | 0.361 |
| ≥Moderate TR | 1.885 | 1.321 | 2.690 | 0.0005 |
| LVDD | 1.097 | 0.921 | 1.306 | 0.300 |
| STS risk score (per unit increase) | 1.027 | 1.009 | 1.046 | 0.003 |
| Q (per unit increase) | 1.001 | 0.998 | 1.003 | 0.535 |
| LVEF (per unit increase) | 0.994 | 0.982 | 1.006 | 0.320 |
| SBP (per unit increase) | 0.971 | 0.962 | 0.981 | <0.0001 |
| DBP (per unit increase) | 0.960 | 0.946 | 0.975 | <0.0001 |
| mean AVG (per unit increase) | 0.990 | 0.977 | 1.003 | 0.145 |
| AugMAP1 (per unit increase) | 0.956 | 0.943 | 0.969 | <0.0001 |
| AugMAP2 (per unit increase) | 0.960 | 0.948 | 0.972 | <0.0001 |
| AugMAP3 (per unit increase) | 0.973 | 0.964 | 0.983 | <0.0001 |
| AugSBP1 (per unit increase) | 0.978 | 0.971 | 0.986 | <0.0001 |
| AugSBP2 (per unit increase) | 0.983 | 0.977 | 0.989 | <0.0001 |
| Zva (per unit increase) | 0.819 | 0.674 | 0.996 | 0.046 |
| Stroke volume index (per unit increase) | 0.991 | 0.972 | 1.010 | 0.347 |
| Multivariate Cox Regression | ||||
| Covariate | HR (Per Unit Increase) | Lower 95%CI | Upper 95%CI | p-Value |
| Model 1 * | ||||
| AugMAP1 (per unit increase) | 0.958 | 0.944 | 0.973 | <0.0001 |
| AugMAP2 (per unit increase) | 0.960 | 0.947 | 0.974 | <0.0001 |
| AugMAP3 (per unit increase) | 0.975 | 0.965 | 0.985 | <0.0001 |
| AugSBP1 (per unit increase) | 0.979 | 0.970 | 0.987 | <0.0001 |
| AugSBP2 (per unit increase) | 0.982 | 0.975 | 0.989 | <0.0001 |
| Zva (per unit increase) | 0.760 | 0.618 | 0.934 | 0.009 |
| Model 2 ** | ||||
| AugMAP1 (per unit increase) | 0.961 | 0.945 | 0.976 | <0.0001 |
| AugMAP2 (per unit increase) | 0.961 | 0.948 | 0.976 | <0.0001 |
| AugMAP3 (per unit increase) | 0.976 | 0.965 | 0.987 | <0.0001 |
| AugSBP1 (per unit increase) | 0.979 | 0.971 | 0.988 | <0.0001 |
| AugSBP2 (per unit increase) | 0.982 | 0.975 | 0.989 | <0.0001 |
| Zva (per unit increase) | 0.756 | 0.610 | 0.936 | 0.010 |
| Univariate Model | C-Index | Lower 95%CI | Upper 95%CI | p-Value * |
|---|---|---|---|---|
| STS risk score | 0.585 | 0.554 | 0.653 | -- |
| AugMAP1 | 0.681 | 0.644 | 0.705 | 0.001 |
| AugMAP2 | 0.679 | 0.625 | 0.708 | 0.003 |
| AugMAP3 | 0.647 | 0.545 | 0.704 | 0.031 |
| AugSBP1 | 0.651 | 0.594 | 0.686 | 0.032 |
| AugSBP2 | 0.645 | 0.584 | 0.662 | 0.050 |
| Zva | 0.552 | 0.499 | 0.653 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-J.; Agasthi, P.; Seri, A.R.; Barry, T.; Shanbhag, A.; Wang, Y.; Eleid, M.F.; Fortuin, D.; Sweeney, J.P.; Pollak, P.; et al. Transcatheter Aortic Valve Replacement Prognostication with Augmented Mean Arterial Pressure. J. Cardiovasc. Dev. Dis. 2023, 10, 192. https://doi.org/10.3390/jcdd10050192
Chao C-J, Agasthi P, Seri AR, Barry T, Shanbhag A, Wang Y, Eleid MF, Fortuin D, Sweeney JP, Pollak P, et al. Transcatheter Aortic Valve Replacement Prognostication with Augmented Mean Arterial Pressure. Journal of Cardiovascular Development and Disease. 2023; 10(5):192. https://doi.org/10.3390/jcdd10050192
Chicago/Turabian StyleChao, Chieh-Ju, Pradyumna Agasthi, Amith R. Seri, Timothy Barry, Anusha Shanbhag, Yuxiang Wang, Mackram F. Eleid, David Fortuin, John P. Sweeney, Peter Pollak, and et al. 2023. "Transcatheter Aortic Valve Replacement Prognostication with Augmented Mean Arterial Pressure" Journal of Cardiovascular Development and Disease 10, no. 5: 192. https://doi.org/10.3390/jcdd10050192
APA StyleChao, C.-J., Agasthi, P., Seri, A. R., Barry, T., Shanbhag, A., Wang, Y., Eleid, M. F., Fortuin, D., Sweeney, J. P., Pollak, P., El Sabbagh, A., Lester, S. J., Freeman, W. K., Naqvi, T. Z., Holmes, D. R., Appleton, C. P., & Arsanjani, R. (2023). Transcatheter Aortic Valve Replacement Prognostication with Augmented Mean Arterial Pressure. Journal of Cardiovascular Development and Disease, 10(5), 192. https://doi.org/10.3390/jcdd10050192







