A Systematic Approach for the Interpretation of Cardiopulmonary Exercise Testing in Children with Focus on Cardiovascular Diseases
Abstract
1. Introduction
2. Physiological Basis of CPET
3. Clinical Guidelines for Exercise Testing in Children
4. Parameters Obtained by Cardiopulmonary Exercise Tests
4.1. Respiratory Equivalent Ratio (RER)
4.2. Peak VO2 (PVO2)
4.3. Anaerobic Threshold (AT)
4.4. Ventilatory Equivalent for CO2 (VE/VCO2) and O2 (VE/VO2)
4.5. Oxygen (O2) Pulse
4.6. Heart Rate (HR) and Heart Rate Reserve (HRR)
4.7. Ventilatory Reserve (VR)
4.8. Oxygen Uptake Efficiency Slope (OUES)
4.9. Oxygen Saturation (SpO2)
4.10. End-Tidal CO2 Partial Pressure (PETCO2)
4.11. Dead Space Ventilation (VD) and Tidal Ventilation (VT)
4.12. Work Efficiency (VO2/WR)
4.13. Circulatory and Ventilatory Power
4.14. VO2 Kinetics
4.15. Periodic Breathing and Exercise Oscillatory Ventilation (EOV)
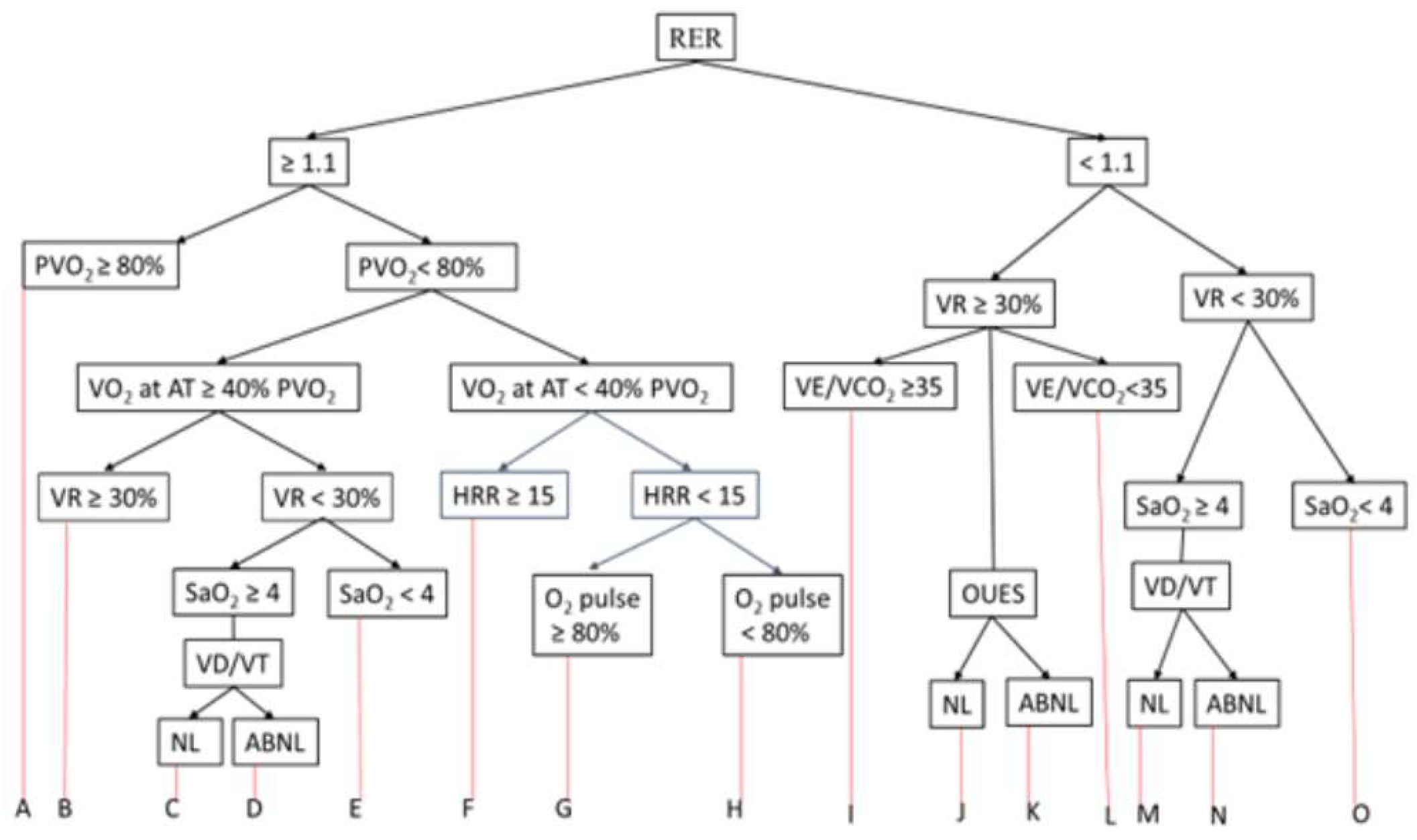
5. Interpretation of CPET
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: http://exerciseismedicine.org (accessed on 2 January 2023).
- Tipton, C.M. Historical perspective: The antiquity of exercise, exercise physiology, and the exercise prescription for health. In Nutrition and Fitness: Cultural, Genetic, and Metabolic Aspects; Imopoulos, S.P., Ed.; Karger: Basel, Switzerland, 2008; pp. 198–245. [Google Scholar]
- Hippocrates (5th century BCE). Ancient medicine. In Oeuvres Completes d’ Hippocrate, vol Traduction Nouvelle Avec le Texte Grec en Regard, Collatione Sur Les Manuscrits et Toutes les Editions; Accompagnee d’ une Introduction, de Commentaries Medicaux, de Variants et de Notes Philologiques; Suivie d’ une Table Generale des Matieres; Littré, M.P.E., Ed.; J-B Bailliere: Paris, France, 1839; p. 572. [Google Scholar]
- Wong, K.C.; Lien-The, W. History of Chinese Medicine: A Chronicle of Medical Happenings in China from Ancient Times to Present, 2nd ed.; National Quarantine Service: Shanghai, China, 1933. [Google Scholar] [CrossRef]
- Robinson, R.S. Sources for the History of Greek Athletics; Ares Publishers: Chicago, IL, USA, 1981; pp. 1–112. [Google Scholar]
- Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022 (accessed on 2 January 2023).
- Bryan, S.; Afful, J.; Carroll, M.; Te-Ching, C.; Orlando, D.; Fink, S.; Fryar, C. National health and nutrition examination survey 2017-March 2020 pre-pandemic data files-development of files and prevalence estimates for selected health outcomes. Natl. Health Stat. Rep. 2021, 15, 1629. [Google Scholar]
- Berenson, G.S. Bogalusa Heart Study Investigators Bogalusa Heart Study: A long-term community study of a rural biracial (Black/White) population. Am. J. Med. Sci. 2001, 322, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.B.; Liu, K.; Kiefe, C.; Greenland, P. Cardiovascular disease risk factor knowledge in young adults and 10-year change in risk factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Epidemiol. 2006, 164, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K. Coupling of external to cellular respiration during exercise: The wisdom of the body revisited. Am. J. Physiol. Metab. 1994, 266, E519–E539. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Sato, T.; Yoshihisa, A.; Kanno, Y.; Suzuki, S.; Yamaki, T.; Sugimoto, K.; Kunii, H.; Nakazato, K.; Suzuki, H.; Saitoh, S.-I.; et al. Cardiopulmonary exercise testing as prognostic indicators: Comparisons among heart failure patients with reduced, mid-range and preserved ejection fraction. Eur. J. Prev. Cardiol. 2017, 24, 1979–1987. [Google Scholar] [CrossRef]
- Sahlin, K.; Tonkonogi, M.; Söderlund, K. Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 1998, 162, 261–266. [Google Scholar] [CrossRef]
- Paridon, S.M.; Alpert, B.S.; Boas, S.R.; Cabrera, M.E.; Caldarera, L.L.; Daniels, S.R.; Kimball, T.R.; Knilans, T.K.; Nixon, P.A.; Rhodes, J.; et al. Clinical stress testing in the pediatric age group: A statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation 2006, 113, 1905–1920. [Google Scholar] [CrossRef]
- Washington, R.L.; van Gundy, J.C.; Sondheimer, H.M.; Wolfe, R.R. Normal aerobic and anaerobic exercise data for North American School-age Children. J. Pediatr. 1988, 112, 223–233. [Google Scholar] [CrossRef]
- Blais, S.; Berbari, J.; Counil, F.P.; Dallaire, F. A Systematic Review of Reference Values in Pediatric Cardiopulmonary Exercise Testing. Pediatr. Cardiol. 2015, 36, 1553. [Google Scholar] [CrossRef]
- Jan Ten Harkel, A.D.; Takken, T.; Van Osch-Gevers, M.; Helbing, W.A. Normal values for cardiopulmonary exercise testing in children. Eur. J. Cardiovasc. Prev. Rehab. 2011, 18, 48–54. [Google Scholar] [CrossRef] [PubMed]
- van der Cammen-van Zijp, M.H.M.; Ijsselstijn, H.; Takken, T.; Willemsen, S.P.; Tibboel, D.; Stam, H.J.; Berg-Emons, R.J.G.V.D. Exercise testing of pre-school children using the Bruce treadmill protocol: New reference values. Eur. J. Appl. Physiol. 2009, 108, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Takken, T.; Mylius, C.; Paap, D.; Broeders, W.; Hulzebos, H.; Van Brussel, M.; Bongers, B. Reference values for cardiopulmonary exercise testing in healthy subjects—an updated systematic review. Expert Rev. Cardiovasc. Ther. 2019, 17, 413–426. [Google Scholar] [CrossRef]
- Chang, R.-K.; Gurvitz, M.; Rodriguez, S.; Hong, E.; Klitzner, T.S. Current practice of exercise stress testing among pediatric cardiology and pulmonology centers in the United States. Pediatr. Cardiol. 2006, 27, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef]
- Godfrey, S. Exercise Testing in Children: Applications in Health and Disease; Saunders: London, UK, 1974; pp. 1–168. [Google Scholar]
- Takken, T.; Bongers, B.C.; van Brussel, M.; Haapala, E.A.; Hulzebos, E.H.J. Cardiopulmonary Exercise Testing in Pediatrics. Ann. Am. Thorac. Soc. 2017, 14, S123–S128. [Google Scholar] [CrossRef]
- Bongers, B.; Hulzebos, H.; Blank, A.; van Brussel, M.; Takken, T. The oxygen uptake efficiency slope in children with congenital heart disease: Construct and group validity. Eur. J. Prev. Cardiol. 2011, 18, 384–392. [Google Scholar] [CrossRef]
- Wasserman, K.; Whipp, B.J. Exercise physiology in health and disease. Am. Rev. Respir. Dis. 1975, 112, 219–249. [Google Scholar]
- Mezzani, A.; Corrà, U.; Bosimini, E.; Giordano, A.; Giannuzzi, P. Contribution of peak respiratory exchange ratio to peak VO2 prognostic reliability in patients with chronic heart failure and severely reduced exercise capacity. Am. Heart J. 2003, 145, 1102–1107. [Google Scholar] [CrossRef]
- Weber, K.T.; Kinasewitz, G.T.; Janicki, J.S.; Fishman, A.P. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 1982, 65, 1213–1223. [Google Scholar] [CrossRef]
- Wagner, J.; Agostoni, P.; Arena, R.; Belardinelli, R.; Dumitrescu, D.; Hager, A.; Myers, J.; Rauramaa, R.; Riley, M.; Takken, T.; et al. The role of gas exchange variables in cardiopulmonary exercise testing for risk stratification and management of heart failure with reduced ejection fraction. Am. Heart J. 2018, 202, 116–126. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.O.; Young, J.B.; Pothier, C.E.; Lauer, M.S. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005, 111, 2313–2338. [Google Scholar] [CrossRef] [PubMed]
- Cattadori, G.; Agostoni, P.; Corrà, U.; Di Lenarda, A.; Sinagra, G.; Veglia, F.; Salvioni, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; et al. Severe heart failure prognosis evaluation for transplant selection in the era of beta-blockers: Role of peak oxygen consumption. Int. J. Cardiol. 2013, 168, 5078–5081. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. EACPR/AHA scientific statement: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Das, B.B.; Taylor, A.L.; Boucek, M.M.; Wolfe, R.W.; Yetman, A.T. Exercise capacity in pediatric heart transplant candidates: Is there any role for the 14 mL/kg/min guideline? Pediatr. Cardiol. 2006, 27, 226–229. [Google Scholar] [CrossRef]
- Das, B.B.; Young, M.-L.; Niu, J.; Mendoza, L.E.; Chan, K.-C.; Roth, T. Relation between New York heart association functional class and objective measures of cardiopulmonary exercise in adults with congenital heart disease. Am. J. Cardiol. 2019, 123, 1868–1873. [Google Scholar] [CrossRef]
- van Genuchten, W.; Helbing, W.; Harkel, A.; Fejzic, Z.; Kuipers, I.; Slieker, M.; van der Ven, J.; Boersma, E.; Takken, T.; Bartelds, B. Exercise capacity in a cohort of children with congenital heart disease. Eur. J. Pediatr. 2023, 182, 295–306. [Google Scholar] [CrossRef]
- Amedro, P.; Gavotto, A.; Guillaumont, S.; Bertet, H.; Vincenti, M.; De La Villeon, G.; Bredy, C.; Acar, P.; Ovaert, C.; Picot, M.-C.; et al. Cardiopulmonary fitness in children with congenital heart diseases versus healthy children. Heart 2017, 104, 1026–1036. [Google Scholar] [CrossRef]
- Villaseca-Rojas, Y.; Varela-Melo, J.; Torres-Castro, R.; Vasconcello-Castillo, L.; Mazzucco, G.; Vilaró, J.; Blanco, I. Exercise Capacity in children and adolescents with congenital heart disease: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 874700. [Google Scholar] [CrossRef]
- Kempny, A.; Dimopoulos, K.; Uebing, A.; Moceri, P.; Swan, L.; Gatzoulis, M.A.; Diller, G.-P. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life—Single center experience and review of published data. Eur. Heart J. 2012, 33, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Washington, R. Cardiorespiratory testing: Anaerobic threshold/respiratory threshold. Pediatr. Cardiol. 1999, 20, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Frank, M.H.; Whipp, B.J.; Wasserman, K. Anaerobic threshold alterations caused by endurance training in middle-aged men. J. Appl. Physiol. 1979, 46, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Weatherald, J.; Sattler, C.; Garcia, G.; Laveneziana, P. Ventilatory response to exercise in cardiopulmonary disease: The role of chemosensitivity and dead space. Eur. Respir. J. 2018, 51, 1700860. [Google Scholar] [CrossRef]
- Stickland, M.K.; Butcher, S.J.; Marciniuk, D.D.; Bhutani, M. Assessing exercise limitation using cardiopulmonary exercise testing. Pulm. Med. 2012, 2012, 824091. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Zak, V.; Goldstein, B.H.; Schumacher, K.R.; Rhodes, J.; Penny, D.J.; Petit, C.; Ginde, S.; Menon, S.; Kim, S.-H.; et al. Results of the FUEL Trial. Circulation 2020, 141, 641–651. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary exercise testing: What is its value? J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Da Silva, C.L.; Castro Souza, F.C.; De Souza Leao Lima, R. Value of the oxygen pulse curve for the diagnosis of coronary artery disease. Physiol Res. 2018, 67, 679–686. [Google Scholar] [CrossRef]
- Washington, R.L.; Bricker, T.; Alpert, B.S.; Daniels, S.R.; Deckelbaum, R.J.; Fisher, E.A.; Gidding, S.; Isabel-Jones, J.; Kavey, R.; Marx, G. Guidelines for exercise testing in the pediatric age group. From the Committee on Atherosclerosis and Hypertension in Children, Council on Cardiovascular Disease in the Young, the American Heart Association. Circulation 1994, 90, 20166–20179. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.M.; Haskell, W. The exercise stress test needs for standardization. In Cardiology: Current Topics and Progress; Eliakim, M., Neufeld, H.N., Eds.; Academic Press: New York, NY, USA, 1970; Volume 6, pp. 149–154. [Google Scholar]
- American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 114–156.
- Nanas, S.; Anastasiou-Nana, M.; Dimopoulos, S.; Sakellariou, D.; Alexopoulos, G.; Kapsimalakou, S.; Papazoglou, P.; Tsolakis, E.; Papazachou, O.; Roussos, C.; et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int. J. Cardiol. 2006, 110, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Neder, J.; Andreoni, S.; Lerario, M.; Nery, L. Reference values for lung function tests: II. Maximal respiratory pressures and voluntary ventilation. Braz. J. Med. Biol. Res. 1999, 32, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.; Francis, D.P.; Mayet, J.; Ewert, R.; Obst, A.; Mason, M.; Elkin, S.; Hughes, A.D.; Wensel, R. Oxygen uptake efficiency slope and breathing reserve, not anaerobic threshold, discriminate between patients with cardiovascular disease over chronic obstructive pulmonary disease. JACC Heart Fail. 2016, 4, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, M.; Tager, I.B. Oxygen uptake efficiency slope: An index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J. Am. Coll. Cardiol. 2000, 36, 194–201. [Google Scholar] [CrossRef]
- Giardini, A.; Specchia, S.; Gargiulo, G.; Sangiorgi, D.; Picchio, F.M. Accuracy of oxygen uptake efficiency slope in adults with congenital heart disease. Int. J. Cardiol. 2009, 133, 74–79. [Google Scholar] [CrossRef]
- Cooper, D.M.; Weiler-Ravell, D.; Whipp, B.J.; Wasserman, K. Aerobic parameters of exercise as a function of body size during growth in children. J. Appl. Physiol. 1984, 56, 628–634. [Google Scholar] [CrossRef]
- Gavotto, A.; Vandenberghe, D.; Abassi, H.; Bertet, H.; Macioce, V.; Picot, M.C.; Guillaumont, S.; Matecki, S.; Amedro, P. Oxygen uptake efficiency slope in children with congenital heart disease versus healthy children. Arch. Cardiovasc. Dis. Suppl. 2020, 105, 1167–1174. [Google Scholar] [CrossRef]
- Ries, A.L.; Farrow, J.T.; Clausen, J.L. Accuracy of two ear oximeters at rest and during exercise in pulmonary patients. Am. Rev. Respir. Dis. 1985, 132, 685–689. [Google Scholar]
- Eschenbacher, W.L.; Mannina, A. An algorithm for the interpretation of cardiopulmonary exercise tests. Chest 1990, 97, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.R.; Rogers, R.M.; Pennock, B.E.; Levin, D. The diffusing capacity as a predictor of arterial oxygen desaturation during exercise in patients with chronic obstructive pulmonary disease. N. Engl. J. Med. 1984, 310, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Exercise Pathophysiology in Patients with Primary Pulmonary Hypertension. Circulation 2001, 104, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Robertson, D.G.; Kane, J.W. Difference between end-tidal and arterial PCO2 in exercise. J. Appl. Physiol. 1979, 47, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.P.; Alencar, M.C.N.; Treptow, E.; Arbex, F.; Ferreira, E.M.V.; Neder, J.A. Clinical usefulness of response profiles to rapidly incremental cardiopulmonary exercise testing. Pulm. Med. 2013, 2013, 359021. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Itoh, H.; Eto, Y.; Kobayashi, T.; Kato, M.; Omata, M.; Watanabe, H.; Kato, K.; Momomura, S.-I. End-tidal CO2 pressure decreases during exercise in cardiac patients: Association with severity of heart failure and cardiac output reserve. J. Am. Coll. Cardiol. 2000, 36, 242–249. [Google Scholar] [CrossRef]
- Ramos, R.; Ferreira, E.; Valois, F.; Cepeda, A.; Messina, C.; Oliveira, R.; Araújo, A.; Teles, C.; Neder, J.; Nery, L.; et al. Clinical usefulness of end-tidal CO2 profiles during incremental exercise in patients with chronic thromboembolic pulmonary hypertension. Respir. Med. 2016, 120, 70–77. [Google Scholar] [CrossRef]
- Guazzi, M.; Reina, G.; Tumminello, G.; Guazzi, M.D. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: The critical, independent prognostic value of the arterial CO2 partial pressure. Eur. Heart J. 2005, 26, 472–480. [Google Scholar] [CrossRef]
- Urquhart, D.; Vendrusculo, F. Clinical interpretation of cardiopulmonary exercise testing in cystic fibrosis and implications for exercise counseling. Paediatr. Respir. Rev. 2017, 24, 72–78. [Google Scholar]
- Hansen, J.E.; Casaburi, R.; Cooper, D.M.; Wasserman, K. Oxygen uptake as related to work rate increment during cycle ergometer exercise. Eur. J. Appl. Physiol. 1988, 57, 140–145. [Google Scholar] [CrossRef]
- Belardinelli, R.; Lacalaprice, F.; Carle, F.; Minnucci, A.; Cianci, G.; Perna, G.; D’Eusanio, G. Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. Eur. Heart J. 2003, 24, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Tabet, J.; Logeart, D.; Bourgoin, P.; Tokmakova, M.; Dahan, M. A non-invasively determined surrogate of cardiac power (‘circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur. Heart J. 2002, 23, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Castello-Simões, V.; Minatel, V.; Karsten, M.; Simões, R.P.; Perseguini, N.M.; Milan, J.C.; Arena, R.; Neves, L.M.T.; Borghi-Silva, A.; Catai, A.M. Circulatory and ventilatory power: Characterization in patients with coronary artery disease. Arq. Bras. Cardiol. 2015, 104, 476–485. [Google Scholar] [CrossRef]
- Hirashiki, A.; Adachi, S.; Nakano, Y.; Kamimura, Y.; Shimokata, S.; Takeshita, K.; Shimizu, A.; Toba, K.; Murohara, T.; Kondo, T. Circulatory power and ventilatory power over time under goal-oriented sequential combination therapy for pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 448–454. [Google Scholar] [CrossRef]
- Arena, R.; Humphrey, R.; Peberdy, M.A. Measurement of oxygen consumption on-kinetics during exercise: Implications for patients with heart failure. J. Card. Fail. 2001, 7, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.M., Jr.; Higginbotham, M.B. Oxygen deficit during exercise testing in heart failure. Relation to submaximal exercise tolerance. Chest 1995, 107, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Sietsema, K.E.; Ben-Dov, I.; Zhang, Y.Y.; Sullivan, C.; Wasserman, K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest 1994, 105, 1693–1700. [Google Scholar] [CrossRef]
- Hayashida, W.; Kumuda, T.; Kohono, F.; Noda, M.; Ishikawa, N.; Kambayashi, M.; Kawai, C. Post-exercise oxygen uptake kinetics in patients with left ventricular dysfunction. Int. J. Cardiol. 1993, 38, 63–72. [Google Scholar] [CrossRef]
- Kremser, C.B.; O’Toole, M.F.; Leff, A.R. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am. J. Cardiol. 1987, 59, 900–905. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Knutzen, A.; Rocco, M.B.; Hartley, L.H.; Colucci, W.S. Periodic breathing during exercise in severe heart failure. Chest 1987, 92, 555–556. [Google Scholar] [CrossRef]
- Ben-Dov, I.; Sietsema, K.E.; Casaburi, R.; Wasserman, K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am. Rev. Respir. Dis. 1992, 145, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Koike, A.; Shimizu, N.; Tajima, A.; Aizawa, T.; Fu, L.T.; Watanabe, H.; Itoh, H. Relation between oscillatory ventilation at rest before cardiopulmonary exercise testing and prognosis in patients with left ventricular dysfunction. Chest 2003, 123, 372–379. [Google Scholar] [CrossRef] [PubMed]
| Physiological Variables | Rest | Exercise |
|---|---|---|
| Heart Rate/min | 75 | Increase 2 to 2.5 times |
| Cardiac cycle in seconds | 0.8 | 0.35 |
| LV end-diastolic volume (mL) | 60–145 | 150–180 |
| Systolic blood pressure (mean) mmHg | 120 | Increases by 30–40% |
| Cardiac Output (L/min) | 3–5 | Increases by 3–5 times |
| Arteriovenous oxygen difference (mL/dL) | 3.5–5 | 7–10 |
| Oxygen Uptake (mL/kg/min) | 3.5 | Increases by 3–5 times |
| Myocardial oxygen usage | Increases by 3–5 times | |
| Breathing rate/min | 12–16 | 40–50 |
| Tidal volume (mL) | 500 | 2300 to 3000 |
| Minute ventilation (L/min) | 5–6 | 100 |
| Pulmonary capillary blood transit time (s) | 0.75 | Decrease (0.38) |
| Alveolar-arterial oxygen difference (mmHg) | 10 | 20–30 |
| Maximum voluntary ventilation (MVV) |
| Heart rate (HR) |
| Blood pressure (BP) |
| Respiratory exchange ratio (RER) |
| Peak oxygen consumption (Peak VO2) |
| Anaerobic threshold (AT) |
| Ventilatory equivalents: VE/VO2, VE/VCO2 Slopes |
| Oxygen pulse |
| Heart rate reserve (HRR) |
| Ventilatory reserve (VR) |
| Oxygen uptake efficiency slope (OUES) Oxygen saturation (SpO2) |
| End-tidal CO2 partial pressure (PETCO2) |
| Dead space ventilation/Tidal volume ventilation (VD/VT) |
| Work efficiency (VO2/WR) |
| Circulatory Power |
| Ventilatory power |
| Oxygen kinetics |
| Exercise oscillatory ventilation (EOV) |
| A. No pulmonary or circulatory limitations (Normal CPET) |
| B. Mild circulatory limitation |
| C. Moderate gas exchange abnormality |
| D. Moderate mechanical ventilation abnormality |
| E. Mild gas exchange abnormality (exercise-induced bronchospasm) |
| F. Moderate circulatory impairment |
| G. Physical deconditioning |
| H. Cardiac limitation due to “pump” dysfunction |
| I. Moderate ventilation-perfusion abnormality |
| J. Poor effort |
| K. Modretae circulatory limitation |
| L. Moderate gas exchange abnormality |
| M. Severe mechanical ventilation abnormality |
| N. Mixed cardiac and pulmonary limitation. |
| O. Physical deconditioning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.B. A Systematic Approach for the Interpretation of Cardiopulmonary Exercise Testing in Children with Focus on Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 178. https://doi.org/10.3390/jcdd10040178
Das BB. A Systematic Approach for the Interpretation of Cardiopulmonary Exercise Testing in Children with Focus on Cardiovascular Diseases. Journal of Cardiovascular Development and Disease. 2023; 10(4):178. https://doi.org/10.3390/jcdd10040178
Chicago/Turabian StyleDas, Bibhuti B. 2023. "A Systematic Approach for the Interpretation of Cardiopulmonary Exercise Testing in Children with Focus on Cardiovascular Diseases" Journal of Cardiovascular Development and Disease 10, no. 4: 178. https://doi.org/10.3390/jcdd10040178
APA StyleDas, B. B. (2023). A Systematic Approach for the Interpretation of Cardiopulmonary Exercise Testing in Children with Focus on Cardiovascular Diseases. Journal of Cardiovascular Development and Disease, 10(4), 178. https://doi.org/10.3390/jcdd10040178






