Current and Future Use of Artificial Intelligence in Electrocardiography
Abstract
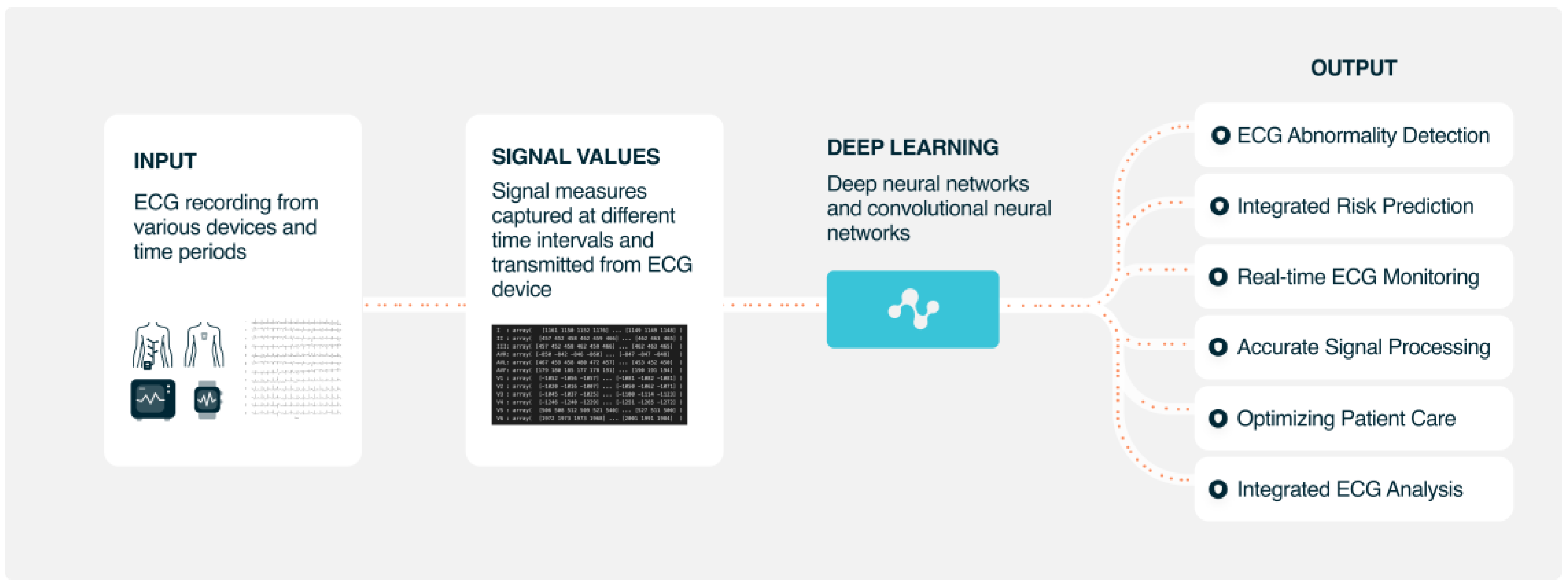
1. Introduction
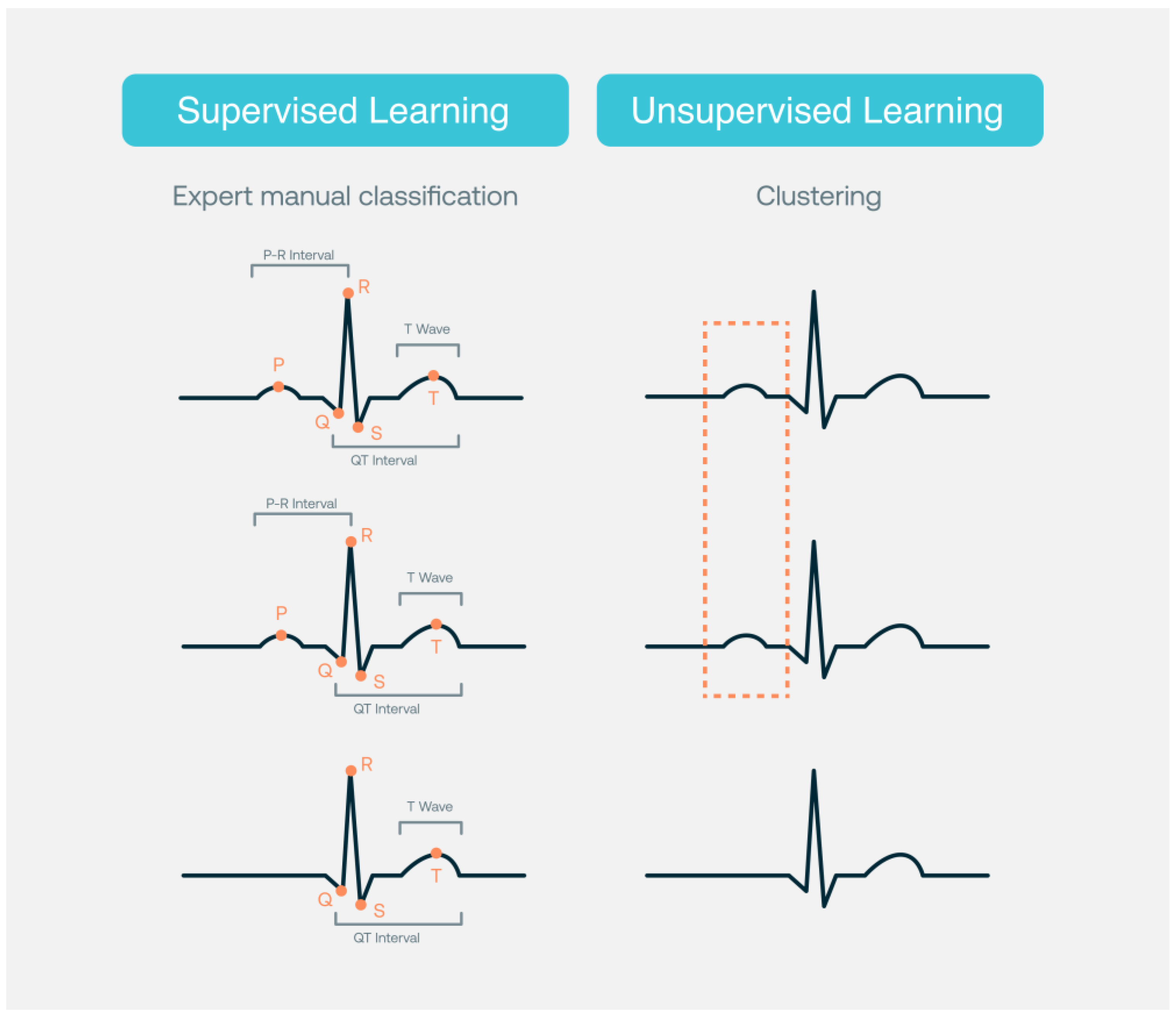
2. Interpretation/Detection of ECG and Cardiac Abnormalities
2.1. Arrhythmias
2.2. Structural Heart Disease
3. Risk Prediction and Integration with Clinical Variables
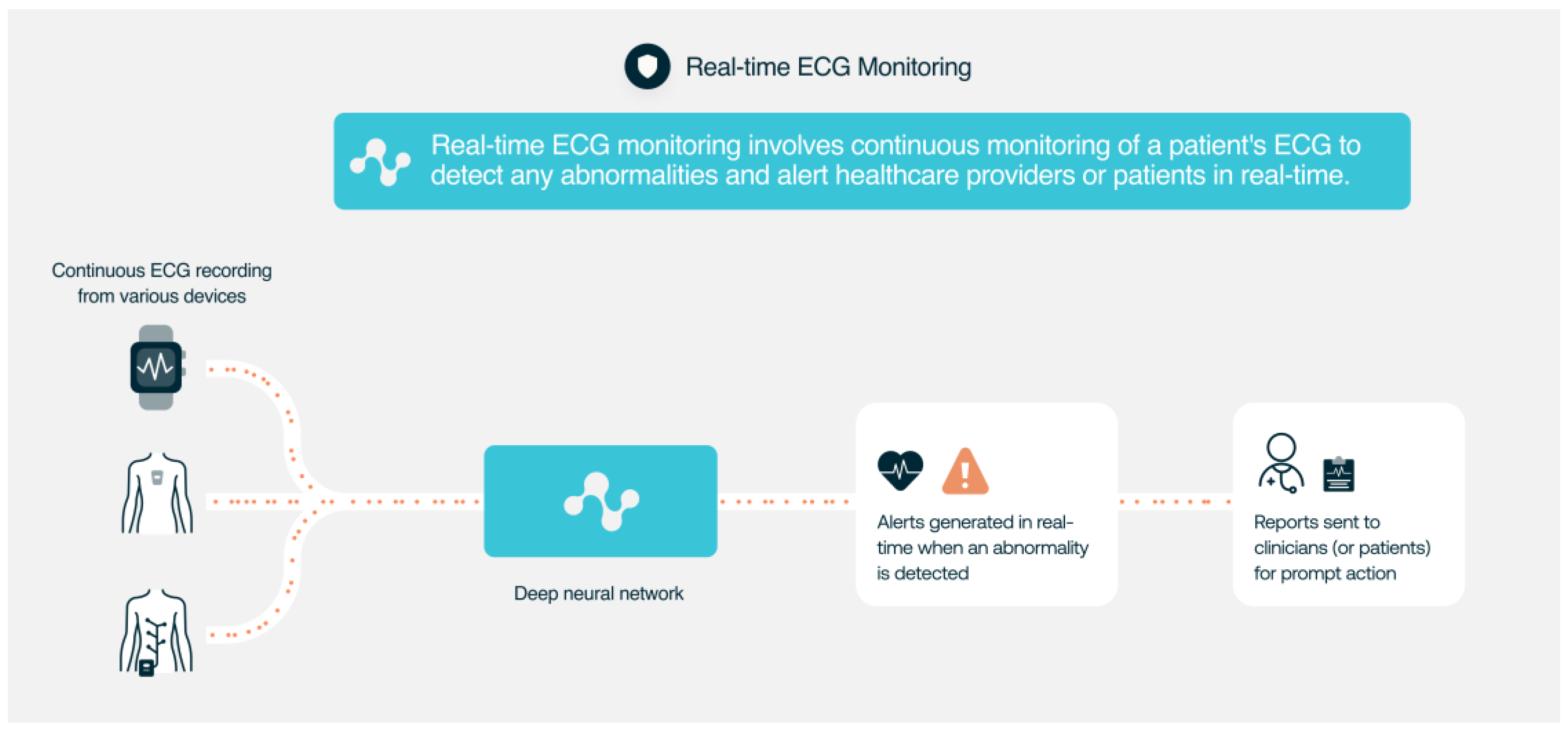
4. Monitoring of ECG Signals
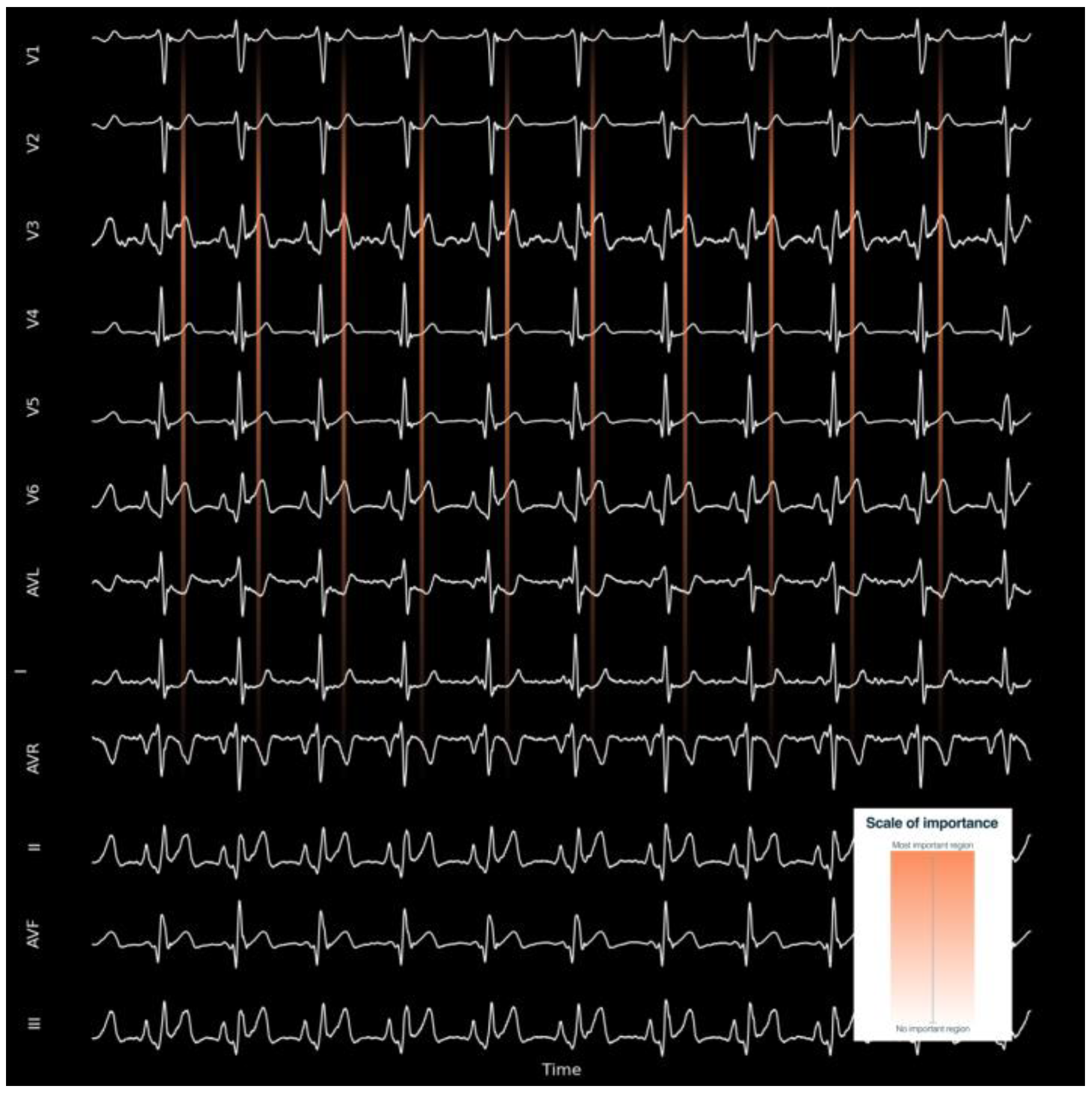
5. AI ECG Signal Processing for Improving Quality and Accuracy
6. Diagnosis of Non-Cardiac Diseases
7. Therapy Guidance and Treatment Optimization
8. Integration of ECG Data with Other Modalities
9. Improvement of Cost-Effectiveness
10. Conclusions
11. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somani, S.; Russak, A.J.; Richter, F.; Zhao, S.; Vaid, A.; Chaudhry, F.; De Freitas, J.K.; Naik, N.; Miotto, R.; Nadkarni, G.N.; et al. Deep learning and the electrocardiogram: Review of the current state-of-the-art. Europace 2021, 23, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, T.; Sullivan, K.; Sauer, A.; A Mamas, M.; A Voors, A.; Gale, C.P.; Metra, M.; Ravindra, N.; Van Spall, H.G.C. Applications of artificial intelligence and machine learning in heart failure. Eur. Heart J. -Digit. Health 2022, 3, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Pratiher, S.; Alam, S.; Hari, A.; Banerjee, N.; Ghosh, N.; Patra, A. A deep residual inception network with channel attention modules for multi-label cardiac abnormality detection from reduced-lead ECG. Physiol. Meas. 2022, 43, 064005. [Google Scholar] [CrossRef]
- Teplitzky, B.A.; McRoberts, M.; Ghanbari, H. Deep learning for comprehensive ECG annotation. Heart Rhythm 2020, 17, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Saeed, H.; Pringle, C.; Eleftheriou, I.; A Bromiley, P.; Brass, A. Artificial intelligence projects in healthcare: 10 practical tips for success in a clinical environment. BMJ Health Care Inform. 2021, 28, e100323. [Google Scholar] [CrossRef]
- Akoum, N.; Zelnick, L.R.; de Boer, I.H.; Hirsch, I.B.; Trence, D.; Henry, C.; Robinson, N.; Bansal, N. Rates of Cardiac Rhythm Abnormalities in Patients with CKD and Diabetes. Clin. J. Am. Soc. Nephrol. 2019, 14, 549–556. [Google Scholar] [CrossRef]
- Tsai, D.-J.; Tsai, S.-H.; Chiang, H.-H.; Lee, C.-C.; Chen, S.-J. Development and Validation of an Artificial Intelligence Electrocardiogram Recommendation System in the Emergency Department. J. Pers. Med. 2022, 12, 700. [Google Scholar] [CrossRef]
- Exarchos, T.P.; Tsipouras, M.G.; Exarchos, C.P.; Papaloukas, C.; Fotiadis, D.I.; Michalis, L.K. A methodology for the automated creation of fuzzy expert systems for ischaemic and arrhythmic beat classification based on a set of rules obtained by a decision tree. Artif. Intell. Med. 2007, 40, 187–200. [Google Scholar] [CrossRef]
- Malik, J.; Devecioglu, O.C.; Kiranyaz, S.; Ince, T.; Gabbouj, M. Real-Time Patient-Specific ECG Classification by 1D Self-Operational Neural Networks. IEEE Trans. Biomed. Eng. 2021, 69, 1788–1801. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; He, J.; Wang, L.; Tian, Y.; Zhou, T.-S.; Li, T.; Li, J.-S. High-Performance Personalized Heartbeat Classification Model for Long-Term ECG Signal. IEEE Trans. Biomed. Eng. 2016, 64, 78–86. [Google Scholar] [CrossRef]
- Quartieri, F.; Marina-Breysse, M.; Pollastrelli, A.; Paini, I.; Lizcano, C.; Lillo-Castellano, J.M.; Grammatico, A. Artificial intelligence augments detection accuracy of cardiac insertable cardiac monitors: Results from a pilot prospective observational study. Cardiovasc. Digit. Health J. 2022, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Frohnert, P.P.; Gluliani, E.R.; Friedberg, M.; Johnson, W.J.; Tauxe, W.N. Statistical Investigation of Correlations Between Serum Potassium Levels and Electrocardiographic Findings in Patients on Intermittent Hemodialysis Therapy. Circulation 1970, 41, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Siddique, Z. Machine learning-based heart disease diagnosis: A systematic literature review. Artif. Intell. Med. 2022, 128, 102289. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tan, Z.; Xu, W.; Xu, F.; Wang, L.; Chen, J.; Wu, K. A particle swarm optimization improved BP neural network intelligent model for electrocardiogram classification. BMC Med. Inform. Decis. Mak. 2021, 21 (Suppl. 2), 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, A.; Gao, M.; Chen, X.; Zhang, X.; Chen, X. ECG-based multi-class arrhythmia detection using spatio-temporal attention-based convolutional recurrent neural network. Artif. Intell. Med. 2020, 106, 101856. [Google Scholar] [CrossRef] [PubMed]
- Haseena, H.H.; Mathew, A.T.; Paul, J.K. Fuzzy Clustered Probabilistic and Multi Layered Feed Forward Neural Networks for Electrocardiogram Arrhythmia Classification. J. Med. Syst. 2009, 35, 179–188. [Google Scholar] [CrossRef]
- Sayantan, G.; Kien, P.T.; Kadambari, K.V. Classification of ECG beats using deep belief network and active learning. Med. Biol. Eng. Comput. 2018, 56, 1887–1898. [Google Scholar] [CrossRef]
- Oh, S.L.; Ng, E.Y.; San Tan, R.; Acharya, U.R. Automated diagnosis of arrhythmia using combination of CNN and LSTM techniques with variable length heart beats. Comput. Biol. Med. 2018, 102, 278–287. [Google Scholar] [CrossRef]
- Taggar, J.S.; Coleman, T.; Lewis, S.; Heneghan, C.; Jones, M. Accuracy of methods for diagnosing atrial fibrillation using 12-lead ECG: A systematic review and meta-analysis. Int. J. Cardiol. 2015, 184, 175–183. [Google Scholar] [CrossRef]
- I Attia, Z.; A Noseworthy, P.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; E Carter, R.; Yao, X.; A Rabinstein, A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kwon, J.-M.; Jeon, K.-H.; Cho, Y.-H.; Shin, J.-H.; Lee, Y.-J.; Jung, M.-S.; Ban, J.-H.; Kim, K.-H.; Lee, S.Y.; et al. Detection and classification of arrhythmia using an explainable deep learning model. J. Electrocardiol. 2021, 67, 124–132. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69, Erratum in 2019, 25, 530. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Hsieh, P.-H.; Wu, M.-Y.; Wang, Y.-C.; Chen, J.-Y.; Tsai, F.-J.; Shih, E.S.; Hwang, M.-J.; Huang, T.-C. Usefulness of Machine Learning-Based Detection and Classification of Cardiac Arrhythmias With 12-Lead Electrocardiograms. Can. J. Cardiol. 2020, 37, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.W.; Olgin, J.E.; Avram, R.; Abreau, S.A.; Sittler, T.; Radia, K.; Hsia, H.; Walters, T.; Lee, B.; Gonzalez, J.E.; et al. Performance of a Convolutional Neural Network and Explainability Technique for 12-Lead Electrocardiogram Interpretation. JAMA Cardiol. 2021, 6, 1285. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.H.; Ribeiro, M.H.; Paixão, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760, Erratum in 2020, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Mak, M.-W.; Cheung, C.-C. Towards End-to-End ECG Classification with Raw Signal Extraction and Deep Neural Networks. IEEE J. Biomed. Health Inform. 2018, 23, 1574–1584. [Google Scholar] [CrossRef]
- Zhu, Z.; Lan, X.; Zhao, T.; Guo, Y.; Kojodjojo, P.; Xu, Z.; Liu, Z.; Liu, S.; Wang, H.; Sun, X.; et al. Identification of 27 abnormalities from multi-lead ECG signals: An ensembled SE_ResNet framework with Sign Loss function. Physiol. Meas. 2021, 42, 065008. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, Y.; Zhao, T.; Zhao, Y.; Liu, Z.; Sun, X.; Xie, G.; Li, Y. Abnormality classification from electrocardiograms with various lead combinations. Physiol. Meas. 2022, 43, 074002. [Google Scholar] [CrossRef]
- Fiorina, L.; Maupain, C.; Gardella, C.; Manenti, V.; Salerno, F.; Socie, P.; Li, J.; Henry, C.; Plesse, A.; Narayanan, K.; et al. Evaluation of an Ambulatory ECG Analysis Platform Using Deep Neural Networks in Routine Clinical Practice. J. Am. Heart Assoc. 2022, 11, e026196. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M.; Gertych, A.; San Tan, R. A deep convolutional neural network model to classify heartbeats. Comput. Biol. Med. 2017, 89, 389–396. [Google Scholar] [CrossRef]
- Puszkarski, B.; Hryniów, K.; Sarwas, G. Comparison of neural basis expansion analysis for interpretable time series (N-BEATS) and recurrent neural networks for heart dysfunction classification. Physiol. Meas. 2022, 43, 064006. [Google Scholar] [CrossRef]
- Badertscher, P.; Lischer, M.; Mannhart, D.; Knecht, S.; Isenegger, C.; Lavallaz, J.D.F.D.; Schaer, B.; Osswald, S.; Kühne, M.; Sticherling, C. Clinical validation of a novel smartwatch for automated detection of atrial fibrillation. Heart Rhythm. O2 2022, 3, 208–210. [Google Scholar] [CrossRef]
- Shah, A.P.; Rubin, S.A. Errors in the computerized electrocardiogram interpretation of cardiac rhythm. J. Electrocardiol. 2007, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Sabut, S.; Pandey, O.; Mishra, B.S.P.; Mohanty, M. Detection of ventricular arrhythmia using hybrid time–frequency-based features and deep neural network. Phys. Eng. Sci. Med. 2021, 44, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-Y.; Chen, K.-W.; Liu, C.-M.; Chang, S.-L.; Lin, Y.-J.; Lo, L.-W.; Hu, Y.-F.; Chung, F.-P.; Lin, C.-Y.; Kuo, L.; et al. A High-Precision Deep Learning Algorithm to Localize Idiopathic Ventricular Arrhythmias. J. Pers. Med. 2022, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.P.; Freed, B.C.; Walter, D.P.; Perry, J.C.; Barakat, A.F.; Elashery, A.R.A.; Shah, K.S.; Kutty, S.; McGillion, M.; Ng, F.S.; et al. Convolution Neural Network Algorithm for Shockable Arrhythmia Classification Within a Digitally Connected Automated External Defibrillator. J. Am. Heart Assoc. 2023, 21, e026974. [Google Scholar] [CrossRef] [PubMed]
- Hajeb-M, S.; Cascella, A.; Valentine, M.; Chon, K.H. Deep Neural Network Approach for Continuous ECG-Based Automated External Defibrillator Shock Advisory System During Cardiopulmonary Resuscitation. J. Am. Heart Assoc. 2021, 10, e019065. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, V.; Ménétré, S.; Didon, J.-P.; Jekova, I. Fully Convolutional Deep Neural Networks with Optimized Hyperparameters for Detection of Shockable and Non-Shockable Rhythms. Sensors 2020, 20, 2875. [Google Scholar] [CrossRef]
- Irusta, U.; Aramendi, E.; Chicote, B.; Alonso, D.; Corcuera, C.; Veintemillas, J.; Larrea, A.; Olabarria, M. Deep learning approach for a shock advise algorithm using short electrocardiogram analysis intervals. Resuscitation 2019, 142, e85. [Google Scholar] [CrossRef]
- Picon, A.; Irusta, U.; Álvarez-Gila, A.; Aramendi, E.; Alonso-Atienza, F.; Figuera, C.; Ayala, U.; Garrote, E.; Wik, L.; Kramer-Johansen, J.; et al. Mixed convolutional and long short-term memory network for the detection of lethal ventricular arrhythmia. PLoS ONE 2019, 14, e0216756. [Google Scholar] [CrossRef]
- Jekova, I.; Krasteva, V. Optimization of end-to-end convolutional neural networks for analysis of out-of-hospital cardiac arrest rhythms during cardiopulmonary resuscitation. Sensors 2021, 21, 4105. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wei, L.; Yan, S.; Zuo, F.; Zhang, H.; Li, Y. Transfer learning based deep network for signal restoration and rhythm analysis during cardiopulmonary resuscitation using only the ECG waveform. Inf. Sci. 2023, 626, 754–772. [Google Scholar] [CrossRef]
- Isasi, I.; Irusta, U.; Aramendi, E.; Eftestøl, T.; Kramer-Johansen, J.; Wik, L. Rhythm Analysis during Cardiopulmonary Resuscitation Using Convolutional Neural Networks. Entropy 2020, 22, 595. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Liu, C.; Moody, B.; Lehman, L.H.; Silva, I.; Li, Q.; Johnson, A.E.; Mark, R.G. AF classification from a short single lead ECG recording: The PhysioNet/Computing in Cardiology Challenge 2017. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Perez Alday, E.A.; Gu, A.; Shah, A.; Liu, C.; Sharma, A.; Seyedi, S.; Bahrami Rad, A.; Reyna, M.; Clifford, G. Classi-Fication of 12-Lead ECGs: The PhysioNet/Computing in Cardiology Challenge 2020 (Version 1.0.2); PhysioNet, 2022. [Google Scholar] [CrossRef]
- Alday, E.A.P.; Gu, A.; Shah, A.J.; Robichaux, C.; Wong, A.-K.I.; Liu, C.; Liu, F.; Rad, A.B.; Elola, A.; Seyedi, S.; et al. Classification of 12-lead ECGs: The PhysioNet/Computing in Cardiology Challenge 2020. Physiol. Meas. 2020, 41, 124003. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, F.; Xia, S.; Shi, S.; Chen, L.; Wang, Z. Dynamic ECG signal quality evaluation based on persistent homology and GoogLeNet method. Front Neurosci. 2023, 17, 1153386. [Google Scholar] [CrossRef]
- Reyna, M.; Sadr, N.; Alday, E.A.P.; Gu, A.; Shah, A.J.; Robichaux, C.; Rad, A.B.; Elola, A.; Seyedi, S.; Ansari, S.; et al. Will Two Do? Varying Dimensions in Electrocardiography: The PhysioNet/Computing in Cardiology Challenge 2021. Comput. Cardiol. 2021, 48, 1–4. [Google Scholar] [CrossRef]
- Reyna, M.; Sadr, N.; Alday, E.A.P.; Gu, A.; Shah, A.J.; Robichaux, C.; Rad, A.B.; Elola, A.; Seyedi, S.; Ansari, S.; et al. Issues in the automated classification of multilead ecgs using heterogeneous labels and populations. Physiol. Meas. 2022, 43, 084001. [Google Scholar] [CrossRef]
- Liu, C.-W.; Wu, F.-H.; Hu, Y.-L.; Pan, R.-H.; Lin, C.-H.; Chen, Y.-F.; Tseng, G.-S.; Chan, Y.-K.; Wang, C.-L. Left ventricular hypertrophy detection using electrocardiographic signal. Sci. Rep. 2023, 13, 2556. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Cho, J.; Lee, B.; Kwon, J.-M.; Lee, Y.; Park, H.; Oh, B.-H.; Jeon, K.-H.; Park, J.; Kim, K.-H. Artificial Intelligence Algorithm for Screening Heart Failure with Reduced Ejection Fraction Using Electrocardiography. ASAIO J. 2020, 67, 314–321. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Dugan, J.; Manka, L.; Lopez-Jimenez, F.; Lerman, A.; Siontis, K.C.; Noseworthy, P.A.; Yao, X.; Klavetter, E.W.; et al. Prospective evaluation of smartwatch-enabled detection of left ventricular dysfunction. Nat. Med. 2022, 28, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-M.; Jo, Y.-Y.; Lee, S.Y.; Kang, S.; Lim, S.-Y.; Lee, M.S.; Kim, K.-H. Artificial Intelligence-Enhanced Smartwatch ECG for Heart Failure-Reduced Ejection Fraction Detection by Generating 12-Lead ECG. Diagnostics 2022, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Grün, D.; Rudolph, F.; Gumpfer, N.; Hannig, J.; Elsner, L.K.; von Jeinsen, B.; Hamm, C.W.; Rieth, A.; Guckert, M.; Keller, T. Identifying Heart Failure in ECG Data with Artificial Intelligence—A Meta-Analysis. Front. Digit. Health 2021, 2, 584555. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Ezzedine, O.F.A.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence–Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778, Erratum in 2023, 98, 211. [Google Scholar] [CrossRef]
- Tison, G.; Zhang, J.; Delling, F.N.; Deo, R.C. Automated and Interpretable Patient ECG Profiles for Disease Detection, Tracking, and Discovery. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005289. [Google Scholar] [CrossRef]
- Ulloa-Cerna, A.E.; Jing, L.; Pfeifer, J.M.; Raghunath, S.; Ruhl, J.A.; Rocha, D.B.; Leader, J.B.; Zimmerman, N.; Lee, G.; Steinhubl, S.R.; et al. rECHOmmend: An ECG-Based Machine Learning Approach for Identifying Patients at Increased Risk of Undiagnosed Structural Heart Disease Detectable by Echocardiography. Circulation 2022, 146, 36–47. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, S.Y.; Jeon, K.; Lee, Y.; Kim, K.; Park, J.; Oh, B.; Lee, M. Deep Learning–Based Algorithm for Detecting Aortic Stenosis Using Electrocardiography. J. Am. Heart Assoc. 2020, 9, e014717. [Google Scholar] [CrossRef]
- Kwon, J.-M.; Kim, K.-H.; Akkus, Z.; Jeon, K.-H.; Park, J.; Oh, B.-H. Artificial intelligence for detecting mitral regurgitation using electrocardiography. J. Electrocardiol. 2020, 59, 151–157. [Google Scholar] [CrossRef]
- Makimoto, H.; Höckmann, M.; Lin, T.; Glöckner, D.; Gerguri, S.; Clasen, L.; Schmidt, J.; Assadi-Schmidt, A.; Bejinariu, A.; Müller, P.; et al. Performance of a convolutional neural network derived from an ECG database in recognizing myocardial infarction. Sci. Rep. 2020, 10, 8445. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Yuan, X.; Zhang, P. Interpretable deep learning for automatic diagnosis of 12-lead electrocardiogram. iScience 2021, 24, 102373. [Google Scholar] [CrossRef]
- Krasteva, V.; Christov, I.; Naydenov, S.; Stoyanov, T.; Jekova, I. Application of Dense Neural Networks for Detection of Atrial Fibrillation and Ranking of Augmented ECG Feature Set. Sensors 2021, 21, 6848. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Castellano, J.M.; González-Ferrer, J.J.; Marina-Breysse, M.; Martínez-Ferrer, J.B.; Pérez-Álvarez, L.; Alzueta, J.; Martínez, J.G.; Rodríguez, A.; Rodríguez-Pérez, J.C.; Anguera, I.; et al. Personalized monitoring of electrical remodelling during atrial fibrillation progression via remote transmissions from implantable devices. Europace 2019, 22, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, S.; Pfeifer, J.M.; Ulloa-Cerna, A.E.; Nemani, A.; Carbonati, T.; Jing, L.; Vanmaanen, D.P.; Hartzel, D.N.; Ruhl, J.A.; Lagerman, B.F.; et al. Deep Neural Networks Can Predict New-Onset Atrial Fibrillation From the 12-Lead ECG and Help Identify Those at Risk of Atrial Fibrillation–Related Stroke. Circulation 2021, 143, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Tran, G.; Genaidy, A.; Marroquin, P.; Estes, C.; Landsheftl, J. Improving dynamic stroke risk prediction in non-anticoagulated patients with and without atrial fibrillation: Comparing common clinical risk scores and machine learning algorithms. Eur. Heart J. -Qual. Care Clin. Outcomes 2021, 8, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Anh, D.; Krishnan, S.; Bogun, F. Accuracy of electrocardiogram interpretation by cardiologists in the setting of incorrect computer analysis. J. Electrocardiol. 2006, 39, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras-Rama, D.; Calvo, C.J.; Salvador-Montañés, Ó.; Cádenas, R.; Ruiz-Cantador, J.; Armada, E.; Rey, J.R.; Merino, J.; Peinado, R.; Pérez-Castellano, N.; et al. Spectral analysis-based risk score enables early prediction of mortality and cerebral performance in patients undergoing therapeutic hypothermia for ventricular fibrillation and comatose status. Int. J. Cardiol. 2015, 186, 250–258. [Google Scholar] [CrossRef]
- Palacios-Rubio, J.; Marina-Breysse, M.; Quintanilla, J.G.; Gil-Perdomo, J.M.; Juárez-Fernández, M.; Garcia-Gonzalez, I.; Rial-Bastón, V.; Corcobado, M.C.; Espinosa, M.C.; Ruiz, F.; et al. Early prognostic value of an Algorithm based on spectral Variables of Ventricular fibrillAtion from the EKG of patients with suddEn cardiac death: A multicentre observational study (AWAKE). Arch. Cardiol. Mex. 2018, 88, 460–467. [Google Scholar] [CrossRef]
- Akbilgic, O.; Butler, L.; Karabayir, I.; Chang, P.P.; Kitzman, D.W.; Alonso, A.; Chen, L.Y.; Soliman, E.Z. ECG-AI: Electrocardiographic artificial intelligence model for prediction of heart failure. Eur. Heart J. -Digit. Health 2021, 2, 626–634. [Google Scholar] [CrossRef]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef]
- Lin, C.; Chau, T.; Shang, H.-S.; Fang, W.-H.; Lee, D.-J.; Lee, C.-C.; Tsai, S.-H.; Wang, C.-H.; Lin, S.-H. Point-of-care artificial intelligence-enabled ECG for dyskalemia: A retrospective cohort analysis for accuracy and outcome prediction. NPJ Digit. Med. 2022, 5, 8. [Google Scholar] [CrossRef]
- Raghunath, S.; Cerna, A.E.U.; Jing, L.; Vanmaanen, D.P.; Stough, J.; Hartzel, D.N.; Leader, J.B.; Kirchner, H.L.; Stumpe, M.C.; Hafez, A.; et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat. Med. 2020, 26, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Rogovoy, N.M.; Howell, S.J.; Lee, T.L.; Hamilton, C.; Alday, E.A.P.; Kabir, M.M.; Zhang, Y.; Kim, E.D.; Fitzpatrick, J.; Monroy-Trujillo, J.M.; et al. Hemodialysis Procedure–Associated Autonomic Imbalance and Cardiac Arrhythmias: Insights From Continuous 14-Day ECG Monitoring. J. Am. Heart Assoc. 2019, 8, e013748. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Wilkin, M.; Million, M.; Rességuier, N.; Franceschi, F.; Koutbi-Franceschi, L.; Hourdain, J.; Martinez, E.; Zabern, M.; Gardella, C.; et al. Smartwatch Electrocardiogram and Artificial Intelligence for Assessing Cardiac-Rhythm Safety of Drug Therapy in the COVID-19 Pandemic. The QT-logs study. Int. J. Cardiol. 2021, 331, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.H.; Eshghi, M.; Raoufy, M.R. Premature Ventricular Contraction (PVC) Detection System Based on Tunable Q-Factor Wavelet Transform. J. Biomed. Phys. Eng. 2022, 12, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.; Krishnan, S. Horizons in Single-Lead ECG Analysis from Devices to Data. Front. Signal Process. 2022, 2, 866047. [Google Scholar] [CrossRef]
- Barrett, P.M.; Komatireddy, R.; Haaser, S.; Topol, S.; Sheard, J.; Encinas, J.; Fought, A.J.; Topol, E.J. Comparison of 24-hour Holter Monitoring with 14-day Novel Adhesive Patch Electrocardiographic Monitoring. Am. J. Med. 2014, 127, 95.e11–95.e17. [Google Scholar] [CrossRef]
- Mannhart, D.; Lischer, M.; Knecht, S.; Lavallaz, J.D.F.D.; Strebel, I.; Serban, T.; Vögeli, D.; Schaer, B.; Osswald, S.; Mueller, C.; et al. Clinical Validation of 5 Direct-to-Consumer Wearable Smart Devices to Detect Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 232–242. [Google Scholar] [CrossRef]
- Kim, J.; Shin, H. Simple and Robust Realtime QRS Detection Algorithm Based on Spatiotemporal Characteristic of the QRS Complex. PLoS ONE 2016, 11, e0150144. [Google Scholar] [CrossRef]
- Herraiz, H.; Martínez-Rodrigo, A.; Bertomeu-González, V.; Quesada, A.; Rieta, J.J.; Alcaraz, R. A Deep Learning Approach for Featureless Robust Quality Assessment of Intermittent Atrial Fibrillation Recordings from Portable and Wearable Devices. Entropy 2020, 22, 733. [Google Scholar] [CrossRef]
- Tison, G.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive Detection of Atrial Fibrillation Using a Commercially Available Smartwatch. JAMA Cardiol. 2018, 3, 409–416. [Google Scholar] [CrossRef]
- Deevi, S.A.; Kaniraja, C.P.; Mani, V.D.; Mishra, D.; Ummar, S.; Satheesh, C. HeartNetEC: A deep representation learning approach for ECG beat classification. Biomed. Eng. Lett. 2021, 11, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Bollepalli, S.C.; Sevakula, R.K.; Au-Yeung, W.M.; Kassab, M.B.; Merchant, F.M.; Bazoukis, G.; Boyer, R.; Isselbacher, E.M.; Armoundas, A.A. Real-Time Arrhythmia Detection Using Hybrid Convolutional Neural Networks. J. Am. Heart Assoc. 2021, 10, e023222. [Google Scholar] [CrossRef] [PubMed]
- Nurmaini, S.; Darmawahyuni, A.; Mukti, A.N.S.; Rachmatullah, M.N.; Firdaus, F.; Tutuko, B. Deep Learning-Based Stacked Denoising and Autoencoder for ECG Heartbeat Classification. Electronics 2020, 9, 135. [Google Scholar] [CrossRef]
- Pravin, C.; Ojha, V. A Novel ECG Signal Denoising Filter Selection Algorithm Based on Conventional Neural Networks. In Proceedings of the 2020 19th IEEE International Conference on Machine Learning and Applications (ICMLA), Miami, FL, USA, 7–8 June 2020; pp. 1094–1100. [Google Scholar] [CrossRef]
- Liu, C.; Lehman, L.; Moody, B.; Li, Q.; Clifford, G. Focus on Detection of Arrhythmia and Noise from Cardiovascular Data. Physiol. Meas. 2018. Available online: https://iopscience.iop.org/journal/0967-3334/page/Focus_on_detection_of_arrhythmia_and_noise_from_cardiovascular_data (accessed on 3 March 2023).
- Lou, Y.-S.; Lin, C.-S.; Fang, W.-H.; Lee, C.-C.; Wang, C.-H. Development and validation of a dynamic deep learning algorithm using electrocardiogram to predict dyskalaemias in patients with multiple visits. Eur. Heart J. -Digit. Health 2022, 4, 22–32. [Google Scholar] [CrossRef]
- Choi, B.; Jang, J.H.; Son, M.; Lee, M.S.; Jo, Y.Y.; Jeon, J.Y.; Jin, U.; Soh, M.; Park, R.W.; Kwon, J.M. Electrocardiographic biomarker based on machine learning for detecting overt hyperthyroidism. Eur. Heart J. -Digit. Health 2022, 3, 255–264. [Google Scholar] [CrossRef]
- Kwon, J.-M.; Cho, Y.; Jeon, K.-H.; Cho, S.; Kim, K.-H.; Baek, S.D.; Jeung, S.; Park, J.; Oh, B.-H. A deep learning algorithm to detect anaemia with ECGs: A retrospective, multicentre study. Lancet Digit. Health 2020, 2, e358–e367. [Google Scholar] [CrossRef]
- Ahn, J.C.; Attia, Z.I.; Rattan, P.; Mullan, A.F.; Buryska, S.; Allen, A.M.; Kamath, P.S.; Friedman, P.A.; Shah, V.H.; Noseworthy, P.A.; et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am. J. Gastroenterol. 2021, 117, 424–432. [Google Scholar] [CrossRef]
- Shrivastava, S.; Cohen-Shelly, M.; Attia, Z.I.; Rosenbaum, A.N.; Wang, L.; Giudicessi, J.R.; Redfield, M.; Bailey, K.; Lopez-Jimenez, F.; Lin, G.; et al. Artificial Intelligence-Enabled Electrocardiography to Screen Patients with Dilated Cardiomyopathy. Am. J. Cardiol. 2021, 155, 121–127. [Google Scholar] [CrossRef]
- Tison, G.H.; Siontis, K.C.; Abreau, S.; Attia, Z.; Agarwal, P.; Balasubramanyam, A.; Li, Y.; Sehnert, A.J.; Edelberg, J.M.; Friedman, P.A.; et al. Assessment of Disease Status and Treatment Response with Artificial Intelligence−Enhanced Electrocardiography in Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 1032–1034. [Google Scholar] [CrossRef]
- Hatem, S.N.; Cohen, A. Atrial fibrillation and stroke: Are we looking in the right direction? Cardiovasc. Res. 2021, 118, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Dugan, J.; Pereira, N.; Noseworthy, P.A.; Jimenez, F.L.; Cruz, J.; Carter, R.E.; DeSimone, D.C.; Signorino, J.; et al. Rapid Exclusion of COVID Infection With the Artificial Intelligence Electrocardiogram. Mayo Clin. Proc. 2021, 96, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Prifti, E.; Fall, A.; Davogustto, G.; Pulini, A.; Denjoy, I.; Funck-Brentano, C.; Khan, Y.; Durand-Salmon, A.; Badilini, F.; Wells, Q.S.; et al. Deep learning analysis of electrocardiogram for risk prediction of drug-induced arrhythmias and diagnosis of long QT syndrome. Eur. Heart J. 2021, 42, 3948–3961. [Google Scholar] [CrossRef]
- McLaren, J.T.; Meyers, H.P.; Smith, S.W. Kenichi Harumi Plenary Address at Annual Meeting of the International Society of Computers in Electrocardiology: “What Should ECG Deep Learning Focus on? The diagnosis of acute coronary occlusion!”. J. Electrocardiol. 2023, 76, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Juárez, M.; Marina-Breysse, M.; Lillo-Castellano, J.M.; Ariza, A. Rational and design of ST-segment elevation not associated with acute cardiac necrosis (LESTONNAC). A prospective registry for validation of a deep learning system assisted by artificial intelligence. J. Electrocardiol. 2021, 69, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-W.; Wang, Y.-C.; Liu, M.-H.; Tsai, B.-Y.; Wu, M.-Y.; Hsieh, P.-H.; Wei, J.-T.; Shih, E.S.C.; Shiao, Y.-T.; Hwang, M.-J.; et al. Artificial intelligence-assisted remote detection of ST-elevation myocardial infarction using a mini-12-lead electrocardiogram device in prehospital ambulance care. Front. Cardiovasc. Med. 2022, 9, 1001982, Erratum in 2022, 9, 1078223. [Google Scholar] [CrossRef]
- Cho, Y.; Kwon, J.-M.; Kim, K.-H.; Medina-Inojosa, J.R.; Jeon, K.-H.; Cho, S.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial intelligence algorithm for detecting myocardial infarction using six-lead electrocardiography. Sci. Rep. 2020, 10, 20495. [Google Scholar] [CrossRef]
- Gustafsson, S.; Gedon, D.; Lampa, E.; Ribeiro, A.H.; Holzmann, M.J.; Schön, T.B.; Sundström, J. Development and validation of deep learning ECG-based prediction of myocardial infarction in emergency department patients. Sci. Rep. 2022, 12, 19615. [Google Scholar] [CrossRef]
- Su, H.-Y.; Tsai, J.-L.; Hsu, Y.-C.; Lee, K.-H.; Chang, C.-S.; Sun, C.-K.; Wang, Y.-H.; Chi, S.-C.; Hsu, C.-W. A modified cardiac triage strategy reduces door to ECG time in patients with ST elevation myocardial infarction. Sci. Rep. 2021, 11, 6358. [Google Scholar] [CrossRef]
- Antoniades, C.; Asselbergs, F.W.; Vardas, P. The year in cardiovascular medicine 2020: Digital health and innovation. Eur. Heart J. 2021, 42, 732–739. [Google Scholar] [CrossRef]
- Bachtiger, P.; Plymen, C.M.; A Pabari, P.; Howard, J.P.; I Whinnett, Z.; Opoku, F.; Janering, S.; A Faisal, A.; Francis, D.P.; Peters, N.S. Artificial Intelligence, Data Sensors and Interconnectivity: Future Opportunities for Heart Failure. Card. Fail. Rev. 2020, 6, e11. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Harfiya, L.N.; Purwandari, K.; Lin, Y.-D. Real-Time Cuffless Continuous Blood Pressure Estimation Using Deep Learning Model. Sensors 2020, 20, 5606. [Google Scholar] [CrossRef] [PubMed]
- Karpiel, I.; Richter-Laskowska, M.; Feige, D.; Gacek, A.; Sobotnicki, A. An Effective Method of Detecting Characteristic Points of Impedance Cardiogram Verified in the Clinical Pilot Study. Sensors 2022, 22, 9872. [Google Scholar] [CrossRef] [PubMed]
- Ganti, V.G.; Gazi, A.H.; An, S.; Srivatsa, A.V.; Nevius, B.N.; Nichols, C.J.; Carek, A.M.; Fares, M.; Abdulkarim, M.; Hussain, T.; et al. Wearable Seismocardiography-Based Assessment of Stroke Volume in Congenital Heart Disease. J. Am. Heart Assoc. 2022, 11, e026067. [Google Scholar] [CrossRef]
- Sivanandarajah, P.; Wu, H.; Bajaj, N.; Khan, S.; Ng, F.S. Is machine learning the future for atrial fibrillation screening? Cardiovasc. Digit. Health J. 2022, 3, 136–145. [Google Scholar] [CrossRef]
- Harmon, D.M.; Witt, D.R.; Friedman, P.A.; Attia, Z.I. Diagnosis and treatment of new heart failure with reduced ejection fraction by the artificial intelligence–enhanced electrocardiogram. Cardiovasc. Digit. Health J. 2021, 2, 282–284, Erratum in 2021, 2, 336. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hamano, T.; Oka, T.; Doi, Y.; Kajimoto, S.; Yasuda, S.; Shimada, K.; Matsumoto, A.; Sakaguchi, Y.; Inoue, K.; et al. Electrocardiogram findings at the initiation of hemodialysis and types of subsequent cardiovascular events. Hypertens. Res. 2021, 44, 571–580. [Google Scholar] [CrossRef]
- Chen, T.-M.; Huang, C.-H.; Shih, E.S.; Hu, Y.-F.; Hwang, M.-J. Detection and Classification of Cardiac Arrhythmias by a Challenge-Best Deep Learning Neural Network Model. iScience 2020, 23, 100886. [Google Scholar] [CrossRef]
- Goldberger, A.; Amaral, L.; Glass, L.; Hausdorff, J.; Ivanov, P.C.; Mark, R.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, Phys-ioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
| Artificial Intelligence Use in Electrocardiography |
|---|
| Interpretation and detection of ECG abnormalities |
| Risk prediction integrated with or without clinical variables |
| Monitoring of ECG signals |
| ECG signal processing for improving quality and accuracy |
| Diagnosis of non-cardiac diseases |
| Therapy guidance and treatment optimization |
| Integration of ECG data with other modalities |
| Improvement of cost effectiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. https://doi.org/10.3390/jcdd10040175
Martínez-Sellés M, Marina-Breysse M. Current and Future Use of Artificial Intelligence in Electrocardiography. Journal of Cardiovascular Development and Disease. 2023; 10(4):175. https://doi.org/10.3390/jcdd10040175
Chicago/Turabian StyleMartínez-Sellés, Manuel, and Manuel Marina-Breysse. 2023. "Current and Future Use of Artificial Intelligence in Electrocardiography" Journal of Cardiovascular Development and Disease 10, no. 4: 175. https://doi.org/10.3390/jcdd10040175
APA StyleMartínez-Sellés, M., & Marina-Breysse, M. (2023). Current and Future Use of Artificial Intelligence in Electrocardiography. Journal of Cardiovascular Development and Disease, 10(4), 175. https://doi.org/10.3390/jcdd10040175







