Metal-Binding Proteins Cross-Linking with Endoplasmic Reticulum Stress in Cardiovascular Diseases
Abstract
1. Introduction
2. ERS and UPR
3. ERS and Metal-Binding Proteins
3.1. Selenium Protein
3.2. Zinc Transporters
4. Metal-Binding Proteins Cross-Linking with ERS in Cardiovascular Diseases
4.1. Atherosclerosis
4.2. Heart Failure
4.3. I/R Injury
4.4. Hypertension
4.5. Cardiomyopathy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| FAs | fatty acids |
| ER | endoplasmic reticulum |
| ROS | reactive oxygen species |
| ERS | endoplasmic reticulum stress |
| MT | metallothionein |
| UPR | unfolded protein response |
| PERK | protein kinase RNA-like ER kinase |
| eIF2a | eukaryotic initiation factor 2α |
| ATF4 | activating transcription factor 4 |
| ATF6 | activating transcription factor 6 |
| IRE1 | inositol-requiring enzyme type 1 |
| XBP1 | X-box-binding protein 1 |
| GRP78 | 78 kDa glucose-regulated protein |
| SOD | superoxide dismutase |
| CAT | catalase |
| GSH-Px | glutathione peroxidase |
| Sep15 | selenomins 15 |
| SelS | selenomins S |
| SelN | selenomins N |
| SelK | selenomins K |
| SelT | selenomins T |
| ERAD | ER-associated protein degradation |
| Sepp1 | selenoprotein P |
| Txnrd2 | thioredoxin reductase 2 |
| ATP | adenosine triphosphate |
| DCM | diabetic cardiomyopathy |
| I/R | ischemia-reperfusion |
| MDA | malondialdehyde |
| TPEN | Zn2+ chelators N,N,N′,N′-tetrakis (2-pyridylmethyl)-ethylenediamine |
| Nox2 | NADPH oxidase 2 |
| mPTP | mitochondrial permeability transition pore |
| TM | tunicamycin |
| GSK-3β | glycogen synthase kinase-3β |
| ERK | extracellular signal-regulated kinase |
| Mn | manganese |
| ID | iron deficiency |
| ECs | endothelial cells |
| ICAM | intercellular adhesion molecule |
| VCAM | vascular cell adhesion molecule |
| TNFα | tumor necrosis factor α |
| IFN-γ | interferon-γ |
| STIM1 | stromal interaction molecule 1 |
| STIM2 | stromal interaction molecule 2 |
| SOCE | store-operated calcium entry |
| ORAI | calcium channel protein |
| LDL | low-density lipoprotein |
| NLRP3 | nucleotide-binding receptor protein 3 |
| NOD | nucleotide-binding domain |
| STING | stimulator of IFN genes |
| SIRT1 | silent information regulator 1 |
| SKO | seipin gene knockout |
| APJ | angiotensin receptor-like 1 |
| Apelin | APJ endogenous ligand |
| AGEs | advanced glycation end products |
| G-CSF | granulocyte colony-stimulating factor |
References
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Favourable lifestyle reduces cardiovascular disease risks in hypertensive patients. Eur. J. Prev. Cardiol. 2022, 29, 2099–2100. [Google Scholar] [CrossRef]
- Dimmeler, S. Cardiovascular disease review series. EMBO Mol. Med. 2011, 3, 697. [Google Scholar] [CrossRef]
- Lennon, R.P.; Claussen, K.A.; Kuersteiner, K.A. State of the Heart: An Overview of the Disease Burden of Cardiovascular Disease from an Epidemiologic Perspective. Prim. Care 2018, 45, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Chen, C. Energy metabolism homeostasis in cardiovascular diseases. J. Geriatr. Cardiol. 2021, 18, 1044–1057. [Google Scholar]
- Okada, K.; Minamino, T.; Tsukamoto, Y.; Liao, Y.L.; Tsukamoto, O.; Takashima, S.; Hirata, A.; Fujita, M.; Nagamachi, Y.; Nakatani, T.; et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction—Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 2004, 110, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Mikami, Y.; Kanemaru, K.; Iino, M. Role of Endoplasmic Reticulum-Mediated Ca(2+) Signaling in Neuronal Cell Death. Antioxid. Redox Signal. 2018, 29, 1147–1157. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Stevenson, J.; Huang, E.Y.; Olzmann, J.A. Endoplasmic Reticulum-Associated Degradation and Lipid Homeostasis. Annu. Rev. Nutr. 2016, 36, 511–542. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Depaoli, M.R.; Hay, J.C.; Graier, W.F.; Malli, R. The enigmatic ATP supply of the endoplasmic reticulum. Biol. Rev. Camb. Philos. Soc. 2019, 94, 610–628. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.I.; Shiota, S.; Sakakibara, O.; Shimoda, M.; Takafuji, A.; Takabatake, M.; Kadota, Y.; Kawakami, T.; Suzuki, S.; Kawahara, M. Exacerbation of Elastase-Induced Emphysema via Increased Oxidative Stress in Metallothionein-Knockout Mice. Biomolecules 2022, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Menéndez, S.; García, M.; Fernández, B.; Álvarez, L.; Fernández-Vega-Cueto, A.; Coca-Prados, M.; Pereiro, R.; González-Iglesias, H. The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells. Nutrients 2018, 10, 1874. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.V.E.; Gallia, M.C.; Luz, J.; Rezende, A.A.; Bongiovanni, G.A.; Araujo-Silva, G.; Almeida, M.D.G. Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients 2022, 14, 3265. [Google Scholar] [CrossRef]
- Vincenz-Donnelly, L.; Holthusen, H.; Körner, R.; Hansen, E.C.; Presto, J.; Johansson, J.; Sawarkar, R.; Hartl, F.U.; Hipp, M.S. High capacity of the endoplasmic reticulum to prevent secretion and aggregation of amyloidogenic proteins. EMBO J. 2018, 37, 337–350. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Luo, C.; Cai, J.; Zhou, X.; Xiao, X.; Liu, S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 6129–6140. [Google Scholar] [CrossRef]
- Turishcheva, E.; Vildanova, M.; Onishchenko, G.; Smirnova, E. The Role of Endoplasmic Reticulum Stress in Differentiation of Cells of Mesenchymal Origin. Biochemistry 2022, 87, 916–931. [Google Scholar] [CrossRef]
- Duan, S.; Chen, X.; Liu, Y.; Guo, W.; Liu, W. Endoplasmic reticulum stress mediates parathyroid hormone-induced apoptosis in vascular smooth muscle cells. Ren. Fail. 2022, 44, 126–136. [Google Scholar] [CrossRef]
- Uchida, T.; Oda, T.; Yamamoto, T.; Inamitsu, M.; Sakai, C.; Uchinoumi, H.; Suetomi, T.; Nakamura, Y.; Okamoto, Y.; Tateda, S.; et al. Endoplasmic reticulum stress promotes nuclear translocation of calmodulin, which activates phenotypic switching of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2022, 628, 155–162. [Google Scholar] [CrossRef]
- Evinova, A.; Hatokova, Z.; Tatarkova, Z.; Brodnanova, M.; Dibdiakova, K.; Racay, P. Endoplasmic reticulum stress induces mitochondrial dysfunction but not mitochondrial unfolded protein response in SH-SY5Y cells. Mol. Cell. Biochem. 2022, 477, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zheng, Y.; Xu, L.; Wang, Z.; Zhou, Y.; Chen, M.; Dong, N.; Cai, Z.; Li, F. Endoplasmic Reticulum Stress and Pathogenesis of Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 918056. [Google Scholar] [CrossRef]
- Ma, X.; Gao, H.J.; Zhang, Q.; Yang, M.G.; Bi, Z.J.; Ji, S.Q.; Li, Y.; Xu, L.; Bu, B.T. Endoplasmic Reticulum Stress Is Involved in Muscular Pathogenesis in Idiopathic Inflammatory Myopathies. Front. Cell Dev. Biol. 2022, 10, 791986. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhang, G.; Wang, X.; Nguyen, C.; May, H.I.; Li, X.; Al-Hashimi, A.A.; Austin, R.C.; Gillette, T.G.; Fu, G.; et al. Endoplasmic Reticulum Chaperone GRP78 Protects Heart from Ischemia/Reperfusion Injury through Akt Activation. Circ. Res. 2018, 122, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Murai, Y.; Jo, U.; Murai, J.; Jenkins, L.M.; Huang, S.N.; Chakka, S.; Chen, L.; Cheng, K.; Fukuda, S.; Takebe, N.; et al. SLFN11 Inactivation Induces Proteotoxic Stress and Sensitizes Cancer Cells to Ubiquitin Activating Enzyme Inhibitor TAK-243. Cancer Res. 2021, 81, 3067–3078. [Google Scholar] [CrossRef]
- Elgendey, F.; Al Wakeel, R.A.; Hemeda, S.A.; Elshwash, A.M.; Fadl, S.E.; Abdelazim, A.M.; Alhujaily, M.; Khalifa, O.A. Selenium and/or vitamin E upregulate the antioxidant gene expression and parameters in broilers. BMC Vet. Res. 2022, 18, 310. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Liu, D.; Li, Z.; Fu, Y.; Tse, G.; Li, G.; Liu, T.; Xu, G. Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation. J. Clin. Med. 2022, 11, 5131. [Google Scholar] [CrossRef]
- Dhar, S.K.; St Clair, D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012, 52, 2209–2222. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Qin, F.; Huang, K. Selenium suppresses oxidative-stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplasmic reticulum stress. J. Biol. Inorg. Chem. 2014, 19, 375–388. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Qiu, F.; Zhou, H.; Peng, Y.; Hao, W.; Xu, J.; Yuan, J.; Wang, S.; Qiang, B.; Xu, C.; et al. Identification and characterization of selenoprotein K: An antioxidant in cardiomyocytes. FEBS Lett. 2006, 580, 5189–5197. [Google Scholar] [CrossRef] [PubMed]
- Meiler, S.; Baumer, Y.; Huang, Z.; Hoffmann, F.W.; Fredericks, G.J.; Rose, A.H.; Norton, R.L.; Hoffmann, P.R.; Boisvert, W.A. Selenoprotein K is required for palmitoylation of CD36 in macrophages: Implications in foam cell formation and atherogenesis. J. Leukoc. Biol. 2013, 93, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.; Stefanovic, N.; Pete, J.; Calkin, A.C.; Giunti, S.; Thallas-Bonke, V.; Jandeleit-Dahm, K.A.; Allen, T.J.; Kola, I.; Cooper, M.E.; et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation 2007, 115, 2178–2187. [Google Scholar] [CrossRef]
- Feng, J.; Yang, F.; Wu, H.; Xing, C.; Xue, H.; Zhang, L.; Zhang, C.; Hu, G.; Cao, H. Selenium protects against cadmium-induced cardiac injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Environ. Toxicol. 2022, 37, 1185–1197. [Google Scholar] [CrossRef]
- Gao, P.C.; Wang, A.Q.; Chen, X.W.; Cui, H.; Li, Y.; Fan, R.F. Selenium alleviates endoplasmic reticulum calcium depletion-induced endoplasmic reticulum stress and apoptosis in chicken myocardium after mercuric chloride exposure. Environ. Sci. Pollut. Res. Int. 2023. [Google Scholar] [CrossRef]
- Wang, S.Q.; Niu, X.L.; Liu, Z.W.; Zhu, Y.H.; Gao, D.F. Selenium deficiency is associated with endoplasmic reticulum stress in a rat model of cardiac malfunction. Biol. Trace Elem. Res. 2013, 156, 196–201. [Google Scholar] [CrossRef]
- Al-Mubarak, A.A.; van der Meer, P.; Bomer, N. Selenium, Selenoproteins, and Heart Failure: Current Knowledge and Future Perspective. Curr. Heart Fail. Rep. 2021, 18, 122–131. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Liu, D.; Sun, Y.; Qian, G.; Xu, S.; Gan, F.; Pan, C.; Huang, K. The aggravating effect of selenium deficiency on T-2 toxin-induced damage on primary cardiomyocyte results from a reduction of protective autophagy. Chem. Biol. Interact. 2019, 300, 27–34. [Google Scholar] [CrossRef]
- Xu, J.; Pan, S.; Gan, F.; Hao, S.; Liu, D.; Xu, H.; Huang, K. Selenium deficiency aggravates T-2 toxin-induced injury of primary neonatal rat cardiomyocytes through ER stress. Chem. Biol. Interact. 2018, 285, 96–105. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Li, S.; Tang, C.; Zhao, Q.; Zhang, J. Selenium supplementation protects against oxidative stress-induced cardiomyocyte cell cycle arrest through activation of PI3K/AKT. Metallomics 2020, 12, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, D.; Yan, Q.; Bian, X.; Yu, J.; Wang, J.; Cheng, X.; Xu, Z. Endoplasmic Reticulum Stress/Ca(2+)-Calmodulin-Dependent Protein Kinase/Signal Transducer and Activator of Transcription 3 Pathway Plays a Role in the Regulation of Cellular Zinc Deficiency in Myocardial Ischemia/Reperfusion Injury. Front. Physiol. 2021, 12, 736920. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Interplay between Zn(2+) Homeostasis and Mitochondrial Functions in Cardiovascular Diseases and Heart Ageing. Int. J. Mol. Sci. 2022, 23, 6890. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.; Wessler, J.D.; Gupta, A.; Maurer, M.S.; Bikdeli, B. Zinc Deficiency and Heart Failure: A Systematic Review of the Current Literature. J. Card. Fail. 2020, 26, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, J.; Xu, Z.; Zhang, Z.; Bai, T.; Xu, J.; Cai, J.; Barnes, G.; Liu, Q.J.; Freedman, J.H.; et al. Zinc rescues obesity-induced cardiac hypertrophy via stimulating metallothionein to suppress oxidative stress-activated BCL10/CARD9/p38 MAPK pathway. J. Cell. Mol. Med. 2017, 21, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Olgar, Y.; Ozdemir, S.; Turan, B. Induction of endoplasmic reticulum stress and changes in expression levels of Zn(2+)-transporters in hypertrophic rat heart. Mol. Cell. Biochem. 2018, 440, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, S.; Olgar, Y.; Durak, A.; Tuncay, E.; Turan, B. Cytosolic increased labile Zn(2+) contributes to arrhythmogenic action potentials in left ventricular cardiomyocytes through protein thiol oxidation and cellular ATP depletion. J. Trace Elem. Med. Biol. 2018, 48, 202–212. [Google Scholar] [CrossRef]
- Bodiga, V.L.; Vemuri, P.K.; Nimmagadda, G.; Bodiga, S. Zinc-dependent changes in oxidative and endoplasmic reticulum stress during cardiomyocyte hypoxia/reoxygenation. Biol. Chem. 2020, 401, 1257–1271. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Li, H.; Wang, X.; Wu, W.; Gao, L. Effect and mechanisms of zinc supplementation in protecting against diabetic cardiomyopathy in a rat model of type 2 diabetes. Bosn. J. Basic Med. Sci. 2015, 15, 14–20. [Google Scholar] [CrossRef]
- Wang, G.; Huang, H.; Zheng, H.; He, Y.; Zhang, Y.; Xu, Z.; Zhang, L.; Xi, J. Zn(2+) and mPTP Mediate Endoplasmic Reticulum Stress Inhibition-Induced Cardioprotection Against Myocardial Ischemia/Reperfusion Injury. Biol. Trace Elem. Res. 2016, 174, 189–197. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Li, X.K.; Cai, L. Zinc and the diabetic heart. Biometals 2005, 18, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Cai, L. Diabetic cardiomyopathy and its prevention by metallothionein: Experimental evidence, possible mechanisms and clinical implications. Curr. Med. Chem. 2007, 14, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, Y.; Zhou, G.; Chen, T.; Song, Y.; Li, X.; Kang, Y.J. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1688–1697. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Tan, Y.; Cai, X.; Cai, L.; Cai, J.; Zheng, Y. Deletion of metallothionein exacerbates intermittent hypoxia-induced oxidative and inflammatory injury in aorta. Oxid. Med. Cell. Longev. 2014, 2014, 141053. [Google Scholar] [CrossRef]
- Huang, F.; Guo, Y.; Wang, L.; Jing, L.; Chen, Z.; Lu, S.; Fu, R.; Tian, L. High glucose and TGF-β1 reduce expression of endoplasmic reticulum-resident selenoprotein S and selenoprotein N in human mesangial cells. Ren. Fail. 2019, 41, 762–769. [Google Scholar] [CrossRef]
- Addinsall, A.B.; Martin, S.D.; Collier, F.; Conlan, X.A.; Foletta, V.C.; Stupka, N. Differential regulation of cellular stress responses by the endoplasmic reticulum-resident Selenoprotein S (Seps1) in proliferating myoblasts versus myotubes. Physiol. Rep. 2018, 6, e13926. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Dai, J.; Zhang, X.; Zhou, J.; Zeng, Z.; Jia, Y. Selenoprotein K Is Essential for the Migration and Phagocytosis of Immature Dendritic Cells. Antioxidants 2022, 11, 1264. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.; Cao, C.; Xu, S. miR-200a-5p augments cardiomyocyte hypertrophy induced by glucose metabolism disorder via the regulation of selenoproteins. J. Cell. Physiol. 2019, 234, 4095–4103. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, Y.; Zhang, M.; Shi, Y.; Zhang, Y.; Mi, G.; Wang, M.; He, Y.; Chen, Y.; et al. Effects of Selenoprotein S Knockdown on Endoplasmic Reticulum Stress in ATDC5 Cells and Gene Expression Profiles in Hypertrophic Chondrocytes. Biol. Trace Elem. Res. 2022, 201, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Liu, Q.; Wang, Y.; Li, S.; Xu, S. Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. Redox Biol. 2022, 50, 102255. [Google Scholar] [CrossRef]
- You, M.; Wu, F.; Gao, M.; Chen, M.; Zeng, S.; Zhang, Y.; Zhao, W.; Li, D.; Wei, L.; Ruan, X.Z.; et al. Selenoprotein K contributes to CD36 subcellular trafficking in hepatocytes by accelerating nascent COPII vesicle formation and aggravates hepatic steatosis. Redox Biol. 2022, 57, 102500. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Peng, H.; Wang, S.; Xu, X.; Xu, F.; Wang, Q.; Chen, Y.; Barton, L.A.; Chen, Y.; Zhang, Y.; et al. Mitochondrial ALDH2 protects against lipopolysaccharide-induced myocardial contractile dysfunction by suppression of ER stress and autophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1627–1641. [Google Scholar] [CrossRef]
- Eby, G.A.; Halcomb, W.W. High-dose zinc to terminate angina pectoris: A review and hypothesis for action by ICAM inhibition. Med. Hypotheses 2006, 66, 169–172. [Google Scholar] [CrossRef]
- Turan, B. A Brief Overview from the Physiological and Detrimental Roles of Zinc Homeostasis via Zinc Transporters in the Heart. Biol. Trace Elem. Res. 2019, 188, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Hadj Abdallah, N.; Baulies, A.; Bouhlel, A.; Bejaoui, M.; Zaouali, M.A.; Ben Mimouna, S.; Messaoudi, I.; Fernandez-Checa, J.C.; García Ruiz, C.; Ben Abdennebi, H. Zinc mitigates renal ischemia-reperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J. Cell. Physiol. 2018, 233, 8677–8690. [Google Scholar] [CrossRef]
- Olgar, Y.; Durak, A.; Tuncay, E.; Bitirim, C.V.; Ozcinar, E.; Inan, M.B.; Tokcaer-Keskin, Z.; Akcali, K.C.; Akar, A.R.; Turan, B. Increased free Zn(2+) correlates induction of sarco(endo)plasmic reticulum stress via altered expression levels of Zn(2+)-transporters in heart failure. J. Cell. Mol. Med. 2018, 22, 1944–1956. [Google Scholar] [CrossRef]
- Karagulova, G.; Yue, Y.; Moreyra, A.; Boutjdir, M.; Korichneva, I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J. Pharmacol. Exp. Ther. 2007, 321, 517–525. [Google Scholar] [CrossRef]
- Chanoit, G.; Lee, S.; Xi, J.; Zhu, M.; McIntosh, R.A.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. Exogenous zinc protects cardiac cells from reperfusion injury by targeting mitochondrial permeability transition pore through inactivation of glycogen synthase kinase-3beta. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1227–H1233. [Google Scholar] [CrossRef]
- Kusanaga, M.; Oe, S.; Ogino, N.; Minami, S.; Miyagawa, K.; Honma, Y.; Harada, M. Zinc Attenuates the Cytotoxicity of Some Stimuli by Reducing Endoplasmic Reticulum Stress in Hepatocytes. Int. J. Mol. Sci. 2019, 20, 2192. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Liu, T.; Yang, Y.; Guo, J.; He, Y.; Xi, J. Zn(2+) protect cardiac H9c2 cells from endoplasmic reticulum stress by preventing mPTP opening through MCU. Cell. Signal. 2022, 100, 110467. [Google Scholar] [CrossRef]
- He, Y.; Fu, Y.; Xi, M.; Zheng, H.; Zhang, Y.; Liu, Y.; Zhao, Y.; Xi, J.; He, Y. Zn(2+) and mPTP mediate resveratrol-induced myocardial protection from endoplasmic reticulum stress. Metallomics 2020, 12, 290–300. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, R.; Tang, D.; Qin, S.; Guo, Y.; Shi, Z. Manganese Mitigates Heat Stress-Induced Apoptosis by Alleviating Endoplasmic Reticulum Stress and Activating the NRF2/SOD2 Pathway in Primary Chick Embryonic Myocardial Cells. Biol. Trace Elem. Res. 2022, 200, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, X.; Wu, Z.; Tian, M.; Chen, F.; Guan, W.; Zhang, S. Ferroptosis Regulation by Nutrient Signalling. Nutr. Res. Rev. 2022, 35, 282–294. [Google Scholar] [CrossRef]
- Manceau, H.; Ausseil, J.; Masson, D.; Feugeas, J.P.; Sablonniere, B.; Guieu, R.; Puy, H.; Peoc’h, K. Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency. Nutrients 2022, 14, 3214. [Google Scholar] [CrossRef]
- Toxqui, L.; De Piero, A.; Courtois, V.; Bastida, S.; Sánchez-Muniz, F.J.; Vaquero, M.P. Iron deficiency and overload. Implications in oxidative stress and cardiovascular health. Nutr. Hosp. 2010, 25, 350–365. [Google Scholar]
- Shi, F.; Wang, Z.; Wu, Q.; Zhong, X.; Zhang, M.; Li, B.; Ren, W.; Yuan, S.; Chen, Y. Iron deficiency promotes aortic media degeneration by activating endoplasmic reticulum stress-mediated IRE1 signaling pathway. Pharmacol. Res. 2022, 183, 106366. [Google Scholar] [CrossRef] [PubMed]
- Comín-Colet, J.; Enjuanes, C.; González, G.; Torrens, A.; Cladellas, M.; Meroño, O.; Ribas, N.; Ruiz, S.; Gómez, M.; Verdú, J.M.; et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur. J. Heart Fail. 2013, 15, 1164–1172. [Google Scholar] [CrossRef]
- Klip, I.T.; Jankowska, E.A.; Enjuanes, C.; Voors, A.A.; Banasiak, W.; Bruguera, J.; Rozentryt, P.; Polonski, L.; van Veldhuisen, D.J.; Ponikowski, P.; et al. The additive burden of iron deficiency in the cardiorenal-anaemia axis: Scope of a problem and its consequences. Eur. J. Heart Fail. 2014, 16, 655–662. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Leclercq, C.; Deray, G.; Lasocki, S.; Zambrowski, J.J.; Mebazaa, A.; de Groote, P.; Damy, T.; Galinier, M. Iron deficiency: An emerging therapeutic target in heart failure. Heart 2014, 100, 1414–1420. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Bai, X.; Lin, Y.; Li, Z.; Fu, J.; Li, M.; Zhao, T.; Yang, H.; Xu, R.; et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J. Pineal. Res. 2018, 64, e12449. [Google Scholar] [CrossRef]
- Crane, E.D.; Al-Hashimi, A.A.; Chen, J.; Lynn, E.G.; Won, K.D.; Lhoták, Š.; Naeim, M.; Platko, K.; Lebeau, P.; Byun, J.H.; et al. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI Insight 2018, 3, e99363. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Kwartler, C.S.; Kaw, K.; Li, Y.; Kaw, A.; Chen, J.; LeMaire, S.A.; Shen, Y.H.; Milewicz, D.M. Cholesterol-Induced Phenotypic Modulation of Smooth Muscle Cells to Macrophage/Fibroblast-like Cells Is Driven by an Unfolded Protein Response. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 302–316. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, M.; Guo, Y.S.; Shen, X.Y.; Gao, Z.K.; Bi, X. Mechanism of Endoplasmic Reticulum Stress in Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 704334. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.A.; Doroudgar, S. ER Stress-Induced Secretion of Proteins and Their Extracellular Functions in the Heart. Cells 2020, 9, 2066. [Google Scholar] [CrossRef] [PubMed]
- Vig, S.; Buitinga, M.; Rondas, D.; Crèvecoeur, I.; van Zandvoort, M.; Waelkens, E.; Eizirik, D.L.; Gysemans, C.; Baatsen, P.; Mathieu, C.; et al. Cytokine-induced translocation of GRP78 to the plasma membrane triggers a pro-apoptotic feedback loop in pancreatic beta cells. Cell Death Dis. 2019, 10, 309. [Google Scholar] [CrossRef]
- Lu, G.; Luo, H.; Zhu, X. Targeting the GRP78 Pathway for Cancer Therapy. Front. Med. 2020, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Li, X.; Fan, D.; Xin, H.B.; Fu, M. TRIM14 promotes endothelial activation via activating NF-κB signaling pathway. J. Mol. Cell. Biol. 2020, 12, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Volanti, C.; Gloire, G.; Vanderplasschen, A.; Jacobs, N.; Habraken, Y.; Piette, J. Downregulation of ICAM-1 and VCAM-1 expression in endothelial cells treated by photodynamic therapy. Oncogene 2004, 23, 8649–8658. [Google Scholar] [CrossRef]
- Cheng, W.; Cui, C.; Liu, G.; Ye, C.; Shao, F.; Bagchi, A.K.; Mehta, J.L.; Wang, X. NF-κB, A Potential Therapeutic Target in Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gargalovic, P.S.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.P.; He, A.Q.; Truong, A.; Baruch-Oren, T.; Berliner, J.A.; Kirchgessner, T.G.; et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2490–2496. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Wang, Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed. Pharmacother. 2020, 125, 110022. [Google Scholar] [CrossRef]
- Prola, A.; Pires Da Silva, J.; Guilbert, A.; Lecru, L.; Piquereau, J.; Ribeiro, M.; Mateo, P.; Gressette, M.; Fortin, D.; Boursier, C.; et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017, 24, 343–356. [Google Scholar] [CrossRef]
- Cao, D.J.; Hill, J.A. Copper futures: Ceruloplasmin and heart failure. Circ. Res. 2014, 114, 1678–1680. [Google Scholar] [CrossRef]
- Mohammadi, A.; Balizadeh Karami, A.R.; Dehghan Mashtani, V.; Sahraei, T.; Bandani Tarashoki, Z.; Khattavian, E.; Mobarak, S.; Moradi Kazerouni, H.; Radmanesh, E. Evaluation of Oxidative Stress, Apoptosis, and Expression of MicroRNA-208a and MicroRNA-1 in Cardiovascular Patients. Rep. Biochem. Mol. Biol. 2021, 10, 183–196. [Google Scholar] [CrossRef]
- Groenendyk, J.; Peng, Z.; Dudek, E.; Fan, X.; Mizianty, M.J.; Dufey, E.; Urra, H.; Sepulveda, D.; Rojas-Rivera, D.; Lim, Y.; et al. Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci. Signal. 2014, 7, ra54. [Google Scholar] [CrossRef]
- Jiang, L.; Zang, D.; Yi, S.; Li, X.; Yang, C.; Dong, X.; Zhao, C.; Lan, X.; Chen, X.; Liu, S.; et al. A microRNA-mediated decrease in eukaryotic initiation factor 2α promotes cell survival during PS-341 treatment. Sci. Rep. 2016, 6, 21565. [Google Scholar] [CrossRef] [PubMed]
- Combot, Y.; Salo, V.T.; Chadeuf, G.; Hölttä, M.; Ven, K.; Pulli, I.; Ducheix, S.; Pecqueur, C.; Renoult, O.; Lak, B.; et al. Seipin localizes at endoplasmic-reticulum-mitochondria contact sites to control mitochondrial calcium import and metabolism in adipocytes. Cell Rep. 2022, 38, 110213. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wang, H.; Zhou, Z.; Yang, C.; Li, Z.; Zhang, Y.; Shi, X.; Zhang, L.; Wang, Y.; et al. Seipin Deficiency Accelerates Heart Failure Due to Calcium Handling Abnormalities and Endoplasmic Reticulum Stress in Mice. Front. Cardiovasc. Med. 2021, 8, 644128. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Xu, W.; Shang, Y.; Zhao, C.; Wang, Y.; Yang, R.; Jin, S.; Wu, Y.; Wang, X.; et al. Apelin ameliorated acute heart failure via inhibiting endoplasmic reticulum stress in rabbits. Amino Acids 2021, 53, 417–427. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.; Ma, L.; Zhang, J.; Huang, P.; Zhang, H.; Liu, X.; Wu, Y.; Wang, X.; Teng, X. Feedback Interaction Between Apelin and Endoplasmic Reticulum Stress in the Rat Myocardium. J. Cardiovasc. Pharmacol. 2023, 81, 21–34. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Chen, S.; Xie, J.; Lu, S.; Zhou, Y.; Jiang, H. PERK Overexpression-Mediated Nrf2/HO-1 Pathway Alleviates Hypoxia/Reoxygenation-Induced Injury in Neonatal Murine Cardiomyocytes via Improving Endoplasmic Reticulum Stress. BioMed Res. Int. 2020, 2020, 6458060. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, F.; Li, S.; Wei, R.; Yu, F.; Xu, J. GRP78 effectively protect hypoxia/reperfusion-induced myocardial apoptosis via promotion of the Nrf2/HO-1 signaling pathway. J. Cell. Physiol. 2021, 236, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.K.; Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Fahem, A.G.; Hofmann, C.; Kaufman, R.J.; Doroudgar, S.; Glembotski, C.C. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ. Res. 2017, 120, 862–875. [Google Scholar] [CrossRef]
- Peserico, D.; Stranieri, C.; Garbin, U.; Mozzini, C.C.; Danese, E.; Cominacini, L.; Fratta Pasini, A.M. Ezetimibe Prevents Ischemia/Reperfusion-Induced Oxidative Stress and Up-Regulates Nrf2/ARE and UPR Signaling Pathways. Antioxidants 2020, 9, 349. [Google Scholar] [CrossRef]
- Liu, K.; Lv, M.; Ji, X.; Lou, L.; Nie, B.; Zhao, J.; Wu, A.; Zhao, M. Wenxin Granules Regulate Endoplasmic Reticulum Stress Unfolded Protein Response and Improve Ventricular Remodeling on Rats with Myocardial Infarction. Evid. Based Complement. Altern. Med. 2021, 2021, 7375549. [Google Scholar] [CrossRef]
- Zhang, X.; Gibson, M.E.; Li, Z.L.; Zhu, X.Y.; Jordan, K.L.; Lerman, A.; Lerman, L.O. Autophagy Portends the Level of Cardiac Hypertrophy in Experimental Hypertensive Swine Model. Am. J. Hypertens. 2016, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.M.; Johnson, H.E.; Heo, J. Rac-dependent feedforward autoactivation of NOX2 leads to oxidative burst. J. Biol. Chem. 2021, 297, 100982. [Google Scholar] [CrossRef]
- Hassanain, H.H.; Gregg, D.; Marcelo, M.L.; Zweier, J.L.; Souza, H.P.; Selvakumar, B.; Ma, Q.; Moustafa-Bayoumi, M.; Binkley, P.F.; Flavahan, N.A.; et al. Hypertension caused by transgenic overexpression of Rac1. Antioxid. Redox Signal. 2007, 9, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jie, Q.; Li, G.; Li, Y.; Liu, B.; Li, H.; Luo, J.; Qin, X.; Li, Z.; Wei, Y. Rac1 promotes the survival of H9c2 cells during serum deficiency targeting JNK/c-JUN/Cyclin-D1 and AKT2/MCL1 pathways. Int. J. Med. Sci. 2018, 15, 1062–1071. [Google Scholar] [CrossRef]
- Margaritis, M.; Sanna, F.; Antoniades, C. Statins and oxidative stress in the cardiovascular system. Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Hypoxia and the integrated stress response promote pulmonary hypertension and preeclampsia: Implications in drug development. Drug Discov. Today 2021, 26, 2754–2773. [Google Scholar] [CrossRef]
- Wu, Y.; Adi, D.; Long, M.; Wang, J.; Liu, F.; Gai, M.T.; Aierken, A.; Li, M.Y.; Li, Q.; Wu, L.Q.; et al. 4-Phenylbutyric Acid Induces Protection against Pulmonary Arterial Hypertension in Rats. PLoS ONE 2016, 11, e0157538. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Q.; Xu, W.; Cai, L. Endoplasmic reticulum stress and diabetic cardiomyopathy. Exp. Diabetes Res. 2012, 2012, 827971. [Google Scholar] [CrossRef]
- Wu, M.X.; Wang, S.H.; Xie, Y.; Chen, Z.T.; Guo, Q.; Yuan, W.L.; Guan, C.; Xu, C.Z.; Huang, Y.N.; Wang, J.F.; et al. Interleukin-33 alleviates diabetic cardiomyopathy through regulation of endoplasmic reticulum stress and autophagy via insulin-like growth factor-binding protein 3. J. Cell. Physiol. 2021, 236, 4403–4419. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhu, H.T.; Chen, K.L.; Dong, X.; Wei, J.; Qiu, C.; Xue, J.H. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2013, 12, 158. [Google Scholar] [CrossRef]
- Belali, O.M.; Ahmed, M.M.; Mohany, M.; Belali, T.M.; Alotaibi, M.M.; Al-Hoshani, A.; Al-Rejaie, S.S. LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 1288. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Wang, Y.; Liu, Q.; Xu, W.; Tan, Y.; Cai, L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: Metallothionein protection. J. Cell. Mol. Med. 2009, 13, 1499–1512. [Google Scholar] [CrossRef]
- Park, I.H.; Shen, G.Y.; Song, Y.S.; Jong Cho, Y.; Kim, B.S.; Lee, Y.; Lim, Y.H.; Shin, J.H.; Kim, K.S. Granulocyte colony-stimulating factor reduces the endoplasmic reticulum stress in a rat model of diabetic cardiomyopathy. Endocr. J. 2021, 68, 1293–1301. [Google Scholar] [CrossRef]
- Sun, S.; Yang, S.; An, N.; Wang, G.; Xu, Q.; Liu, J.; Mao, Y. Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J. Ethnopharmacol. 2019, 238, 111857. [Google Scholar] [CrossRef]
- Tao, S.; Chen, L.; Song, J.; Zhu, N.; Song, X.; Shi, R.; Ge, G.; Zhang, Y. Tanshinone IIA ameliorates diabetic cardiomyopathy by inhibiting Grp78 and CHOP expression in STZ-induced diabetes rats. Exp. Ther. Med. 2019, 18, 729–734. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Li, J.; Yao, Z. Rutin alleviates cardiomyocyte injury induced by high glucose through inhibiting apoptosis and endoplasmic reticulum stress. Exp. Ther. Med. 2021, 22, 944. [Google Scholar] [CrossRef]
- Chengji, W.; Xianjin, F. Exercise protects against diabetic cardiomyopathy by the inhibition of the endoplasmic reticulum stress pathway in rats. J. Cell. Physiol. 2019, 234, 1682–1688. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, W.; Liu, Y.; Jiang, B.; Yin, L.; Chen, X. LncRNA AC061961.2 overexpression inhibited endoplasmic reticulum stress induced apoptosis in dilated cardiomyopathy rats and cardiomyocytes via activating wnt/β-catenin pathway. J. Recept. Signal Transduct. Res. 2021, 41, 494–503. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Mangas, A.; Belmonte, T.; Quezada-Feijoo, M.; Ramos, M.; Toro, R. Ischemic dilated cardiomyopathy pathophysiology through microRNA-16-5p. Rev. Esp. Cardiol. 2021, 74, 740–749. [Google Scholar] [CrossRef]
- Al-Yacoub, N.; Colak, D.; Mahmoud, S.A.; Hammonds, M.; Muhammed, K.; Al-Harazi, O.; Assiri, A.M.; Al-Buraiki, J.; Al-Habeeb, W.; Poizat, C. Mutation in FBXO32 causes dilated cardiomyopathy through up-regulation of ER-stress mediated apoptosis. Commun. Biol. 2021, 4, 884. [Google Scholar] [CrossRef] [PubMed]
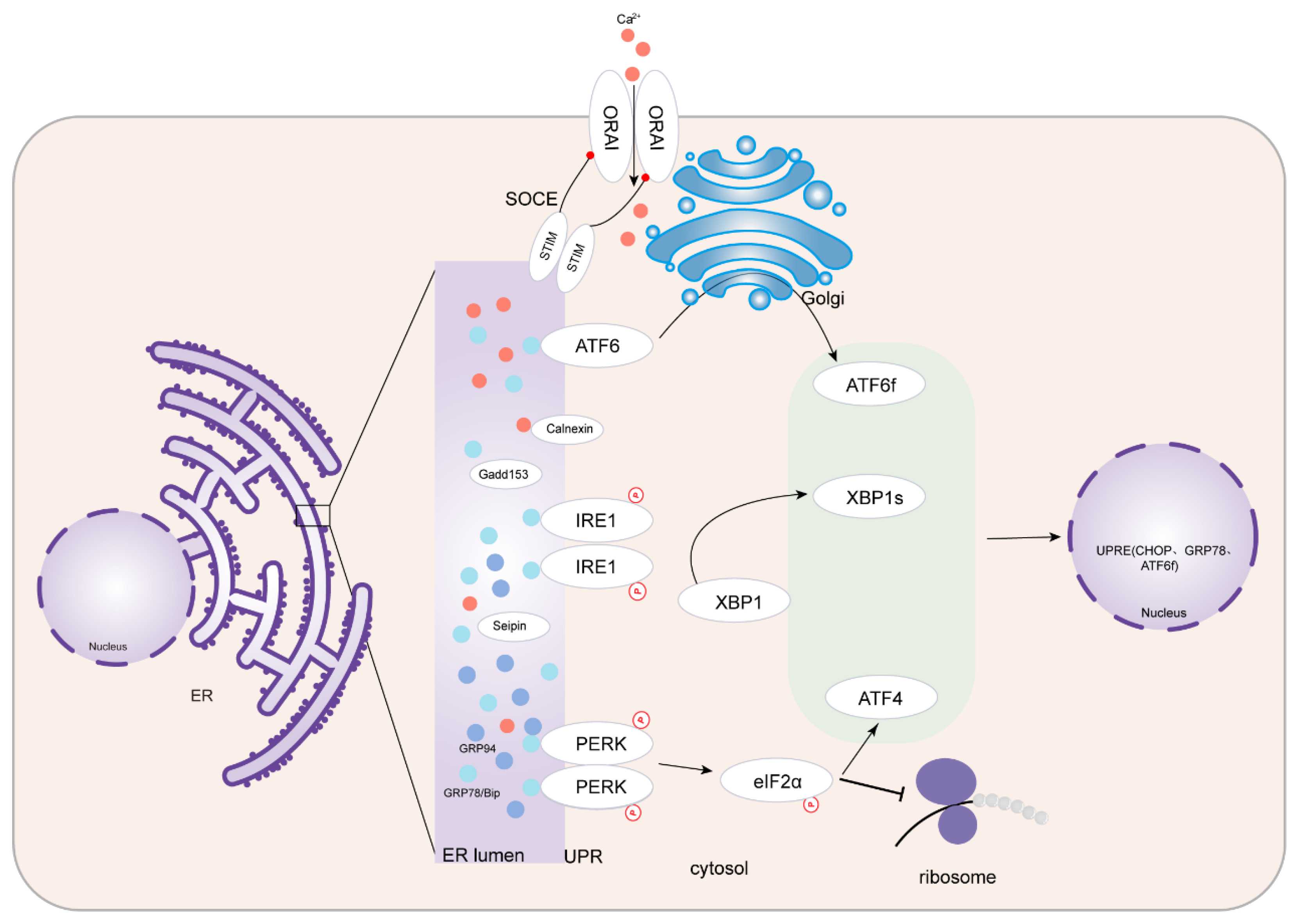
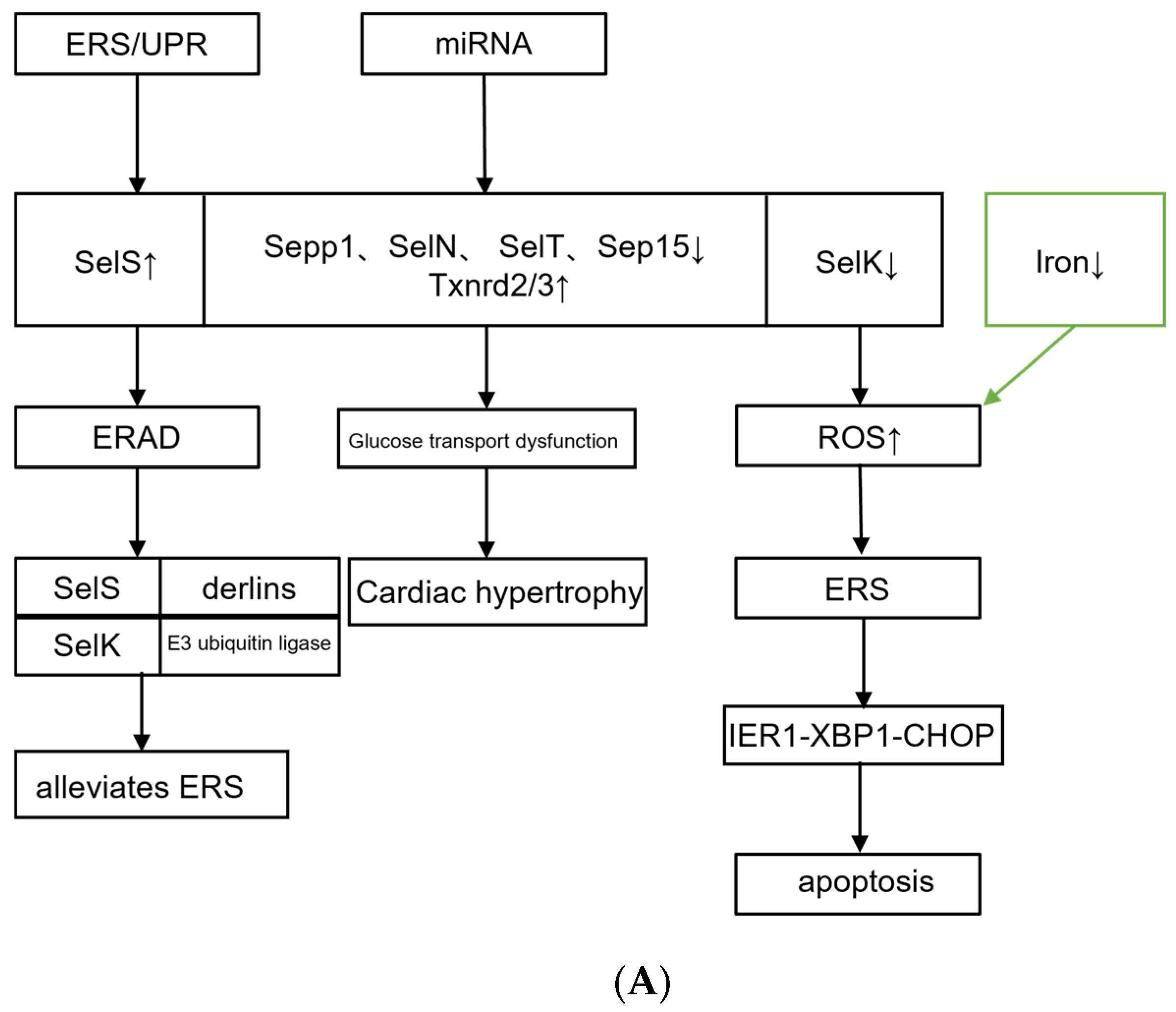
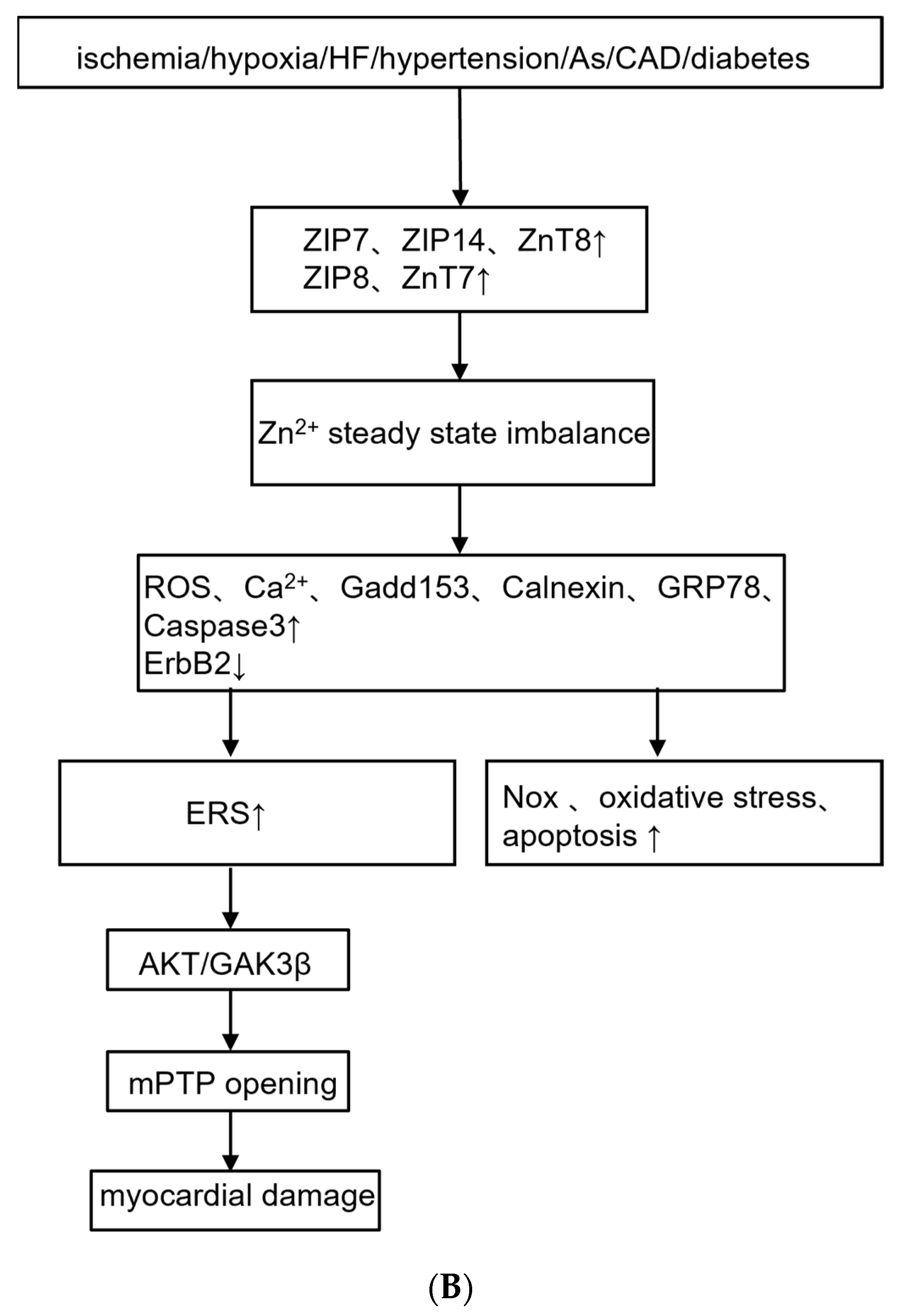
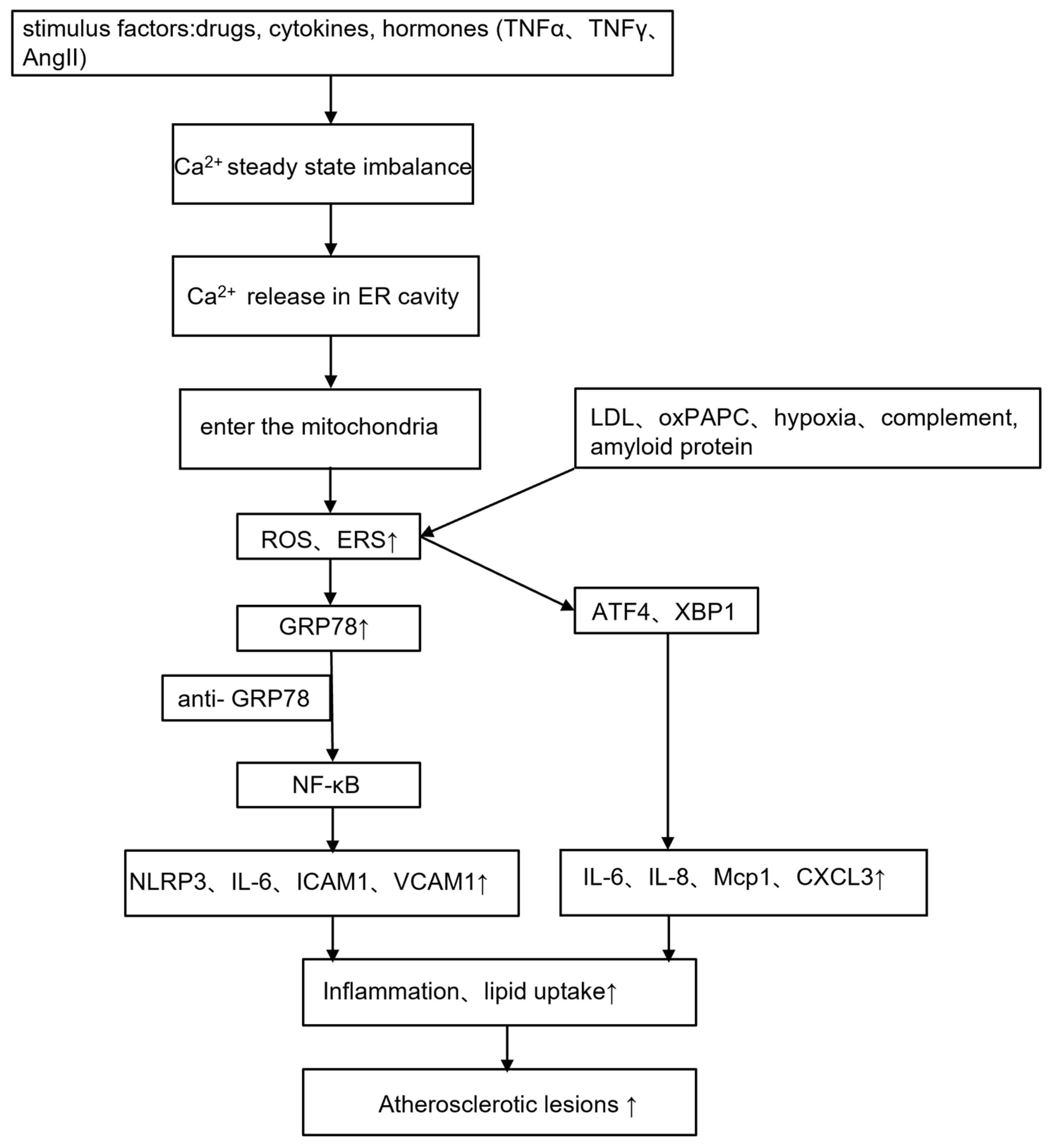

| Reference | Trace Element | Cardiovascular Changes | Mechanism Mediated by Trace Element/Metal-Binding Proteins |
|---|---|---|---|
| Liu et al. [30] | selenium | atherosclerosis | Selenium inhibits endoplasmic reticulum stress and activation of PI3K/AKT and ERK signaling pathways, regulates inflammation, and then inhibits vascular calcification and apoptosis |
| Liu et al. [31] | |||
| Lu et al. [32] | SelK overexpression attenuates intracellular reactive oxygen species levels and protects cells from oxidative stress-induced toxicity in cardiomyocytes | ||
| Meiler et al. [33] | SelK enhances the expression and distribution of CD36 on the plasma membrane. SelK deficiency can reduce LDL uptake and foam cell formation, promote foam cell formation, and promote the occurrence of atherosclerosis | ||
| Lewis et al. [34] | Macrophages, α-smooth muscle actin, RAGE receptors, VCAM-1, and CTGF are increased and atherosclerotic lesions are increased in diabetic mice lacking GPx1 and ApoE | ||
| Feng et al. [35] | heavy metal toxicity (cadmium) | Selenium inhibits oxidative stress and programmed cell death via PI3K/AKT/PTEN signaling pathway | |
| Gao et al. [36] | heavy metal toxicity (mercury) | Selenium attenuates HgCl2-induced ERS and apoptosis, restores Ca2+ homeostasis, and attenuates oxidative stress in cardiomyocytes | |
| Wang et al. [37] | cardiomyopathy | Selenium deficiency causes Ca2+ homeostasis imbalance in the endoplasmic reticulum, significantly reduces GPx activity, increases ROS production, triggers endoplasmic reticulum stress, and increases CHOP and GRP18 | |
| Al-Mubarak et al. [38] | ischemia and reperfusion | Inhibits oxidative stress and apoptosis, senses luminal calcium levels, and thereby regulates SERCA-mediated replenishment of ER calcium stores | |
| Chen et al. [39] | Keshan disease | Selenium deficiency reduces cytoprotective autophagy in primary cardiomyocytes | |
| Xu et al. [40] | Selenium deficiency reduces myocardial Gpx1 activity, causes ERS, and promotes GRP78, CHOP, and p-eIF2α expression | ||
| Sun et al. [41] | heart failure | Selenium inhibits oxidative stress-induced cell cycle arrest by activating the PI3K/AKT signaling pathway | |
| Zhao et al. [42] | zinc | ischemia and reperfusion | Inhibition of the “endoplasmic reticulum stress/CaMKII/STAT3 pathway” to protect myocardium |
| Dabravolski et al. [43] | Zinc protects the heart from H/R injury by preventing mitochondrial superoxide production and dissipation of mitochondrial membrane potential by inducing autophagy and mitochondrial autophagy by increasing ERK activity and Beclin1 expression and stabilizing PINK1 | ||
| Rosenblum et al. [44] | heart failure | Zinc deficiency causes oxidative stress, cardiac hypertrophy, and significant degeneration and massive fibrosis of cardiomyocytes | |
| Wang et al. [45] | myocardial hypertrophy | Zinc deficiency leads to activation of BCL10/CARD9/p38MAPK signaling pathway and induces obstruction-related cardiac hypertrophy | |
| Olgar et al. [46] | Significantly increased expression of ZIP7, ZIP14, and ZnT8, and decreased levels of ZIP8 and ZnT7 in myocardial hypertrophy | ||
| Degirmenci et al. [47] | arrhythmia | High-[Zn2+]i induces significant activation of ATP-sensitive K+-channel currents and zinc plays an important role in cardioprotection through changes in cellular ATP and sulfur oxygenation levels | |
| Bodiga et al. [48] | hypoxia–reoxygenation | Zinc supplementation inhibits NOX2 mRNA, reduces oxidative stress and endoplasmic reticulum stress, and reduces cell death | |
| Lu et al. [49] | diabetic cardiomyopathy | Zinc supplementation inhibits autophagy and endoplasmic reticulum stress: myocardial content of LC3 and GRP78 proteins is significantly reduced | |
| Wang et al. [50] | ischemia/reperfusion | Inhibition of mPTP opening, inhibition of ERS, GRP 78 and GRP 94 expression decline, and protect the heart | |
| Song et al. [51] | diabetic cardiomyopathy | Involvement of SOD and thioredoxin in enzyme activity and chelator activity, stabilization of cell membranes, and inhibition of lipid peroxidation thioredoxin | |
| Cai et al. [52] | metallothionein | diabetic cardiomyopathy | MT binds Zn2+ under physiological conditions and releases Zn2+ under diabetes-induced oxidative stress |
| Cai et al. [53] | MT inhibits mitochondrial GSH depletion, cardiac protein nitration, lipid peroxidation, and cardiac cell apoptosis | ||
| Zhou et al. [54] | aortic lesions caused by IH | MT inhibits oxidative damage, inflammatory response, and apoptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, Y.; Ding, H.; Chen, J.; Zhang, X. Metal-Binding Proteins Cross-Linking with Endoplasmic Reticulum Stress in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 171. https://doi.org/10.3390/jcdd10040171
Li K, Li Y, Ding H, Chen J, Zhang X. Metal-Binding Proteins Cross-Linking with Endoplasmic Reticulum Stress in Cardiovascular Diseases. Journal of Cardiovascular Development and Disease. 2023; 10(4):171. https://doi.org/10.3390/jcdd10040171
Chicago/Turabian StyleLi, Kejuan, Yongnan Li, Hong Ding, Jianshu Chen, and Xiaowei Zhang. 2023. "Metal-Binding Proteins Cross-Linking with Endoplasmic Reticulum Stress in Cardiovascular Diseases" Journal of Cardiovascular Development and Disease 10, no. 4: 171. https://doi.org/10.3390/jcdd10040171
APA StyleLi, K., Li, Y., Ding, H., Chen, J., & Zhang, X. (2023). Metal-Binding Proteins Cross-Linking with Endoplasmic Reticulum Stress in Cardiovascular Diseases. Journal of Cardiovascular Development and Disease, 10(4), 171. https://doi.org/10.3390/jcdd10040171





