Stereotactic Arrhythmia Radioablation Treatment of Ventricular Tachycardia: Current Technology and Evolving Indications
Abstract
1. Introduction
2. Physical Bases and Principles of Radiotherapy in Treatment of Ventricular Arrhythmias
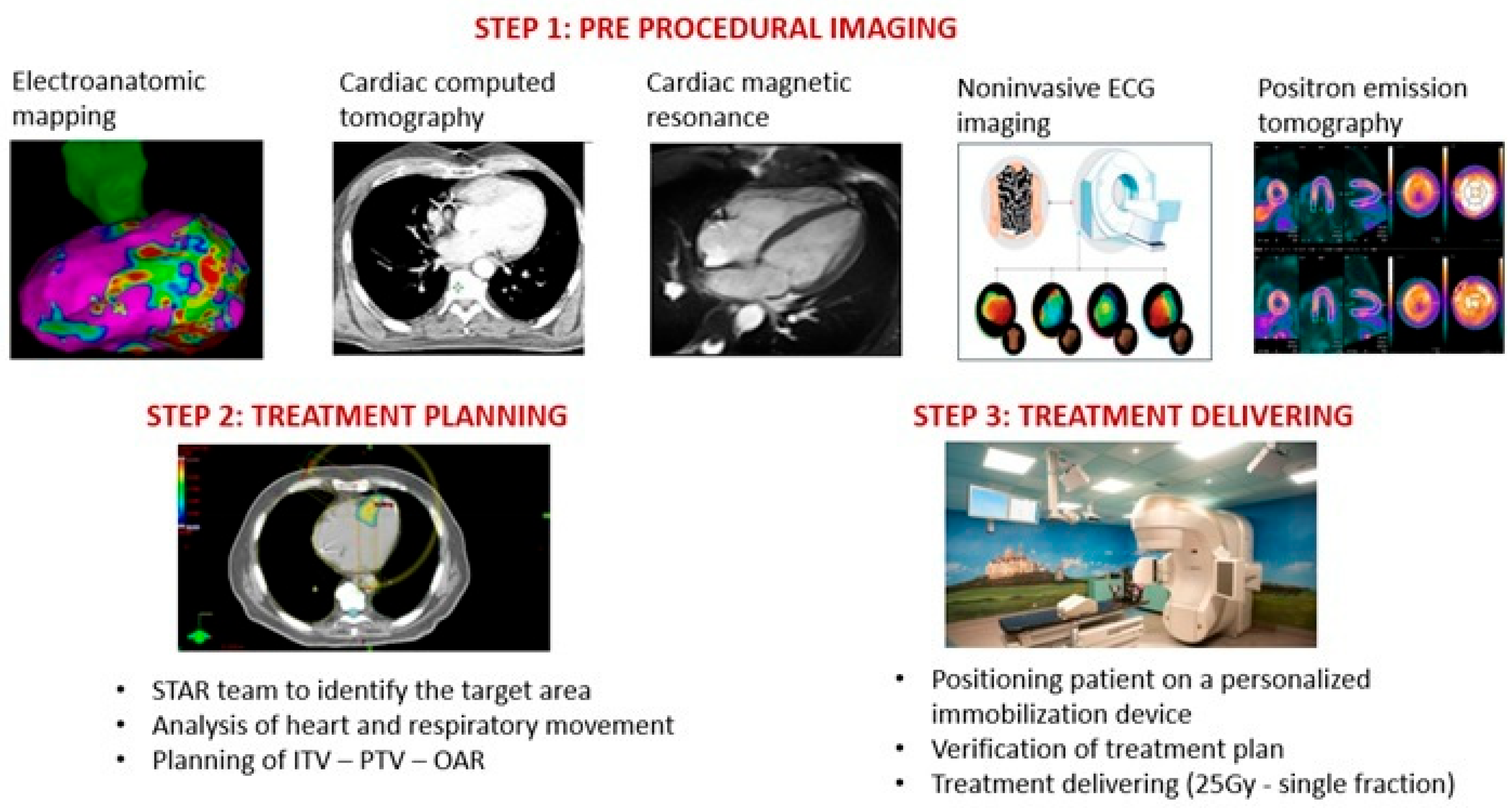
3. Pre-Procedural Imaging and Workflow
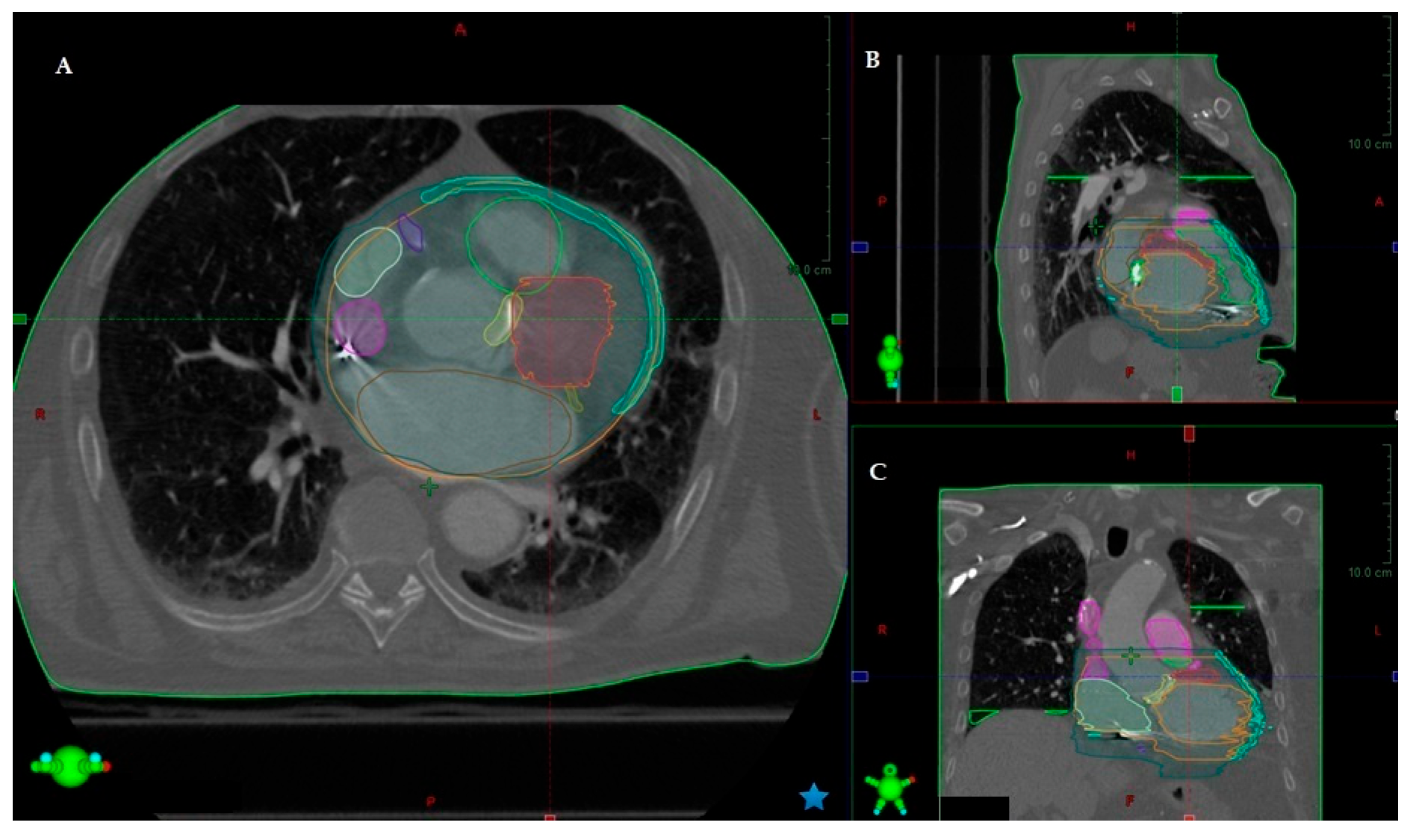
3.1. Identification of Structural Aspects Related to VT
3.2. Mapping the VT
3.3. Further Methods to Improve Imaging Quality
4. Previous Experiences and Ongoing Studies
5. Short- and Long-Term Safety Data
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019, 21, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Prasitlumkum, N.; Navaravong, L.; Desai, A.; Desai, D.; Cheungpasitporn, W.; Rattanawong, P.; Bunch, T.J.; Jongnarangsin, K.; Chokesuwattanaskul, R. Impact of early ventricular tachycardia ablation in patients with an implantable cardioverter-defibrillator: An updated systematic review and meta-analysis of randomized controlled trials. Heart Rhythm 2022, 19, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.K.; Baker, W.L.; Konopka, A.; Giannelli, D.; Coleman, C.I.; Kluger, J.; Cronin, E.M. Systematic review and meta-analysis of catheter ablation of ventricular tachycardia in ischemic heart disease. Heart Rhythm 2020, 17, e206–e219. [Google Scholar] [CrossRef] [PubMed]
- Aryana, A.; Tung, R.; d’Avila, A. Percutaneous Epicardial Approach to Catheter Ablation of Cardiac Arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 1–20. [Google Scholar] [CrossRef]
- Muser, D.; Castro, S.A.; Liang, J.J.; Santangeli, P. Identifying Risk and Management of Acute Haemodynamic Decompensation During Catheter Ablation of Ventricular Tachycardia. Arrhythm. Electrophysiol. Rev. 2018, 7, 282–287. [Google Scholar] [CrossRef]
- Darma, A.; Bertagnolli, L.; Dinov, B.; Torri, F.; Shamloo, A.S.; Lurz, J.A.; Dagres, N.; Husser-Bollmann, D.; Bollmann, A.; Hindricks, G.; et al. Predictors of long-term mortality after catheter ablation of ventricular tachycardia in a contemporary cohort of patients with structural heart disease. Europace 2020, 22, 1672–1679. [Google Scholar] [CrossRef]
- Krug, D.; Blanck, O.; Andratschke, N.; Guckenberger, M.; Jumeau, R.; Mehrhof, F.; Boda-Heggemann, J.; Seidensaal, K.; Dunst, J.; Pruvot, E.; et al. Recommendations regarding cardiac stereotactic body radiotherapy for treatment refractory ventricular tachycardia. Heart Rhythm 2021, 18, 2137–2145. [Google Scholar] [CrossRef]
- Shangguan, W.; Xu, G.; Wang, X.; Zhang, N.; Liu, X.; Li, G.; Tse, G.; Liu, T. Stereotactic Radiotherapy: An Alternative Option for Refractory Ventricular Tachycardia to Drug and Ablation Therapy. J. Clin. Med. 2022, 11, 3549. [Google Scholar] [CrossRef]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef]
- Volpato, G.; Compagnucci, P.; Cipolletta, L.; Parisi, Q.; Valeri, Y.; Carboni, L.; Giovagnoni, A.; Dello Russo, A.; Casella, M. Safety and Efficacy of Stereotactic Arrhythmia Radioablation for the Treatment of Ventricular Tachycardia: A Systematic Review. Front. Cardiovasc. Med. 2022, 9, 870001. [Google Scholar] [CrossRef]
- Carbucicchio, C.; Andreini, D.; Piperno, G.; Catto, V.; Conte, E.; Cattani, F.; Bonomi, A.; Rondi, E.; Piccolo, C.; Vigorito, S.; et al. Stereotactic radioablation for the treatment of ventricular tachycardia: Preliminary data and insights from the STRA-MI-VT phase Ib/II study. J. Interv. Card. Electrophysiol. 2021, 62, 427–439. [Google Scholar] [CrossRef]
- Blanck, O.; Buergy, D.; Vens, M.; Eidinger, L.; Zaman, A.; Krug, D.; Rudic, B.; Boda-Heggemann, J.; Giordano, F.A.; Boldt, L.H.; et al. Radiosurgery for ventricular tachycardia: Preclinical and clinical evidence and study design for a German multi-center multi-platform feasibility trial (RAVENTA). Clin. Res. Cardiol. 2020, 109, 1319–1332. [Google Scholar] [CrossRef]
- Wight, J.; Bigham, T.; Schwartz, A.; Zahid, A.T.; Bhatia, N.; Kiani, S.; Shah, A.; Westerman, S.; Higgins, K.; Lloyd, M.S. Long Term Follow-Up of Stereotactic Body Radiation Therapy for Refractory Ventricular Tachycardia in Advanced Heart Failure Patients. Front. Cardiovasc. Med. 2022, 9, 849113. [Google Scholar] [CrossRef]
- Chang, W.I.; Jo, H.H.; Cha, M.J.; Chang, J.H.; Choi, C.H.; Kim, H.J.; Oh, S.; Robinson, C.G.; Cuculich, P.S. Short-term and long-term effects of noninvasive cardiac radioablation for ventricular tachycardia: A single-center case series. Heart Rhythm. O2 2023, 4, 119–126. [Google Scholar] [CrossRef]
- Whitaker, J.; Zei, P.C.; Ahmad, S.; Niederer, S.; O’Neill, M.; Rinaldi, C.A. The effect of ionizing radiation through cardiac stereotactic body radiation therapy on myocardial tissue for refractory ventricular arrhythmias: A review. Front. Cardiovasc. Med. 2022, 9, 989886. [Google Scholar] [CrossRef]
- Whitaker, J.; Mak, R.H.; Zei, P.C. Non-invasive ablation of arrhythmias with stereotactic ablative radiotherapy. Trends Cardiovasc. Med. 2022, 32, 287–296. [Google Scholar] [CrossRef]
- Winiecki, J. Principles of radiation therapy. Phys. Sci. Rev. 2022, 7, 1501–1528. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, S.W.; Park, Y.G.; Kim, S.J.; Choi, C.H.; Cha, M.J.; Chang, J.H. Impact of High-Dose Irradiation on Human iPSC-Derived Cardiomyocytes Using Multi-Electrode Arrays: Implications for the Antiarrhythmic Effects of Cardiac Radioablation. Int. J. Mol. Sci. 2021, 23, 351. [Google Scholar] [CrossRef]
- Sacher, F.; Gandjbakhch, E.; Maury, P.; Jenny, C.; Khalifa, J.; Boveda, S.; Defaye, P.; Gras, D.; Klug, D.; Laurent, G.; et al. Focus on stereotactic radiotherapy: A new way to treat severe ventricular arrhythmias? Arch. Cardiovasc. Dis. 2021, 114, 140–149. [Google Scholar] [CrossRef]
- Mohan, R. A Review of Proton Therapy—Current Status and Future Directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Turesson, I.; Carlsson, J.; Brahme, A.; Glimelius, B.; Zackrisson, B.; Stenerlöw, B. Biological response to radiation therapy. Acta Oncol. 2003, 42, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.A.; Giaccia, A.J. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef]
- Benali, K.; Bellec, J.; Jaksic, N.; Caille, P.; Rigal, L.; Simon, A.; Galand, V.; Hammache, N.; Da Costa, A.; De Crevoisier, R.; et al. Cardiac stereotactic ablative radiotherapy for refractory ventricular arrhythmias: A radical alternative? A narrative review of rationale and cardiological aspects. Med. Imaging Radiat. Sci. 2021, 52, 626–635. [Google Scholar] [CrossRef]
- Refaat, M.M.; Ballout, J.A.; Zakka, P.; Hotait, M.; Al Feghali, K.A.; Gheida, I.A.; Saade, C.; Hourani, M.; Geara, F.; Tabbal, M.; et al. Swine Atrioventricular Node Ablation Using Stereotactic Radiosurgery: Methods and In Vivo Feasibility Investigation for Catheter-Free Ablation of Cardiac Arrhythmias. J. Am. Heart Assoc. 2017, 6, e007193. [Google Scholar] [CrossRef]
- Lehmann, H.I.; Deisher, A.J.; Takami, M.; Kruse, J.J.; Song, L.; Anderson, S.E.; Cusma, J.T.; Parker, K.D.; Johnson, S.B.; Asirvatham, S.J.; et al. External Arrhythmia Ablation Using Photon Beams. Circ. Arrhythmia Electrophysiol. 2017, 10, e004304. [Google Scholar] [CrossRef]
- Zei, P.C.; Mak, R. Noninvasive Stereotactic Radioablation for Ventricular Tachycardia. Circulation 2019, 139, 322–324. [Google Scholar] [CrossRef]
- Peichl, P.; Sramko, M.; Cvek, J.; Kautzner, J. A case report of successful elimination of recurrent ventricular tachycardia by repeated stereotactic radiotherapy: The importance of accurate target volume delineation. Eur. Heart J. Case Rep. 2021, 5, ytaa516. [Google Scholar] [CrossRef]
- Haskova, J.; Peichl, P.; Pirk, J.; Cvek, J.; Neuwirth, R.; Kautzner, J. Stereotactic radiosurgery as a treatment for recurrent ventricular tachycardia associated with cardiac fibroma. HeartRhythm Case Rep. 2019, 5, 44–47. [Google Scholar] [CrossRef]
- Kautzner, J.; Jedlickova, K.; Sramko, M.; Peichl, P.; Cvek, J.; Ing, L.K.; Neuwirth, R.; Jiravsky, O.; Voska, L.; Kucera, T. Radiation-Induced Changes in Ventricular Myocardium After Stereotactic Body Radiotherapy for Recurrent Ventricular Tachycardia. JACC Clin. Electrophysiol. 2021, 7, 1487–1492. [Google Scholar] [CrossRef]
- Jumeau, R.; Ozsahin, M.; Schwitter, J.; Vallet, V.; Duclos, F.; Zeverino, M.; Moeckli, R.; Pruvot, E.; Bourhis, J. Rescue procedure for an electrical storm using robotic non-invasive cardiac radio-ablation. Radiother. Oncol. 2018, 128, 189–191. [Google Scholar] [CrossRef]
- Loo, B.W., Jr.; Soltys, S.G.; Wang, L.; Lo, A.; Fahimian, B.P.; Iagaru, A.; Norton, L.; Shan, X.; Gardner, E.; Fogarty, T.; et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ. Arrhythm. Electrophysiol. 2015, 8, 748–750. [Google Scholar] [CrossRef]
- Scholz, E.P.; Seidensaal, K.; Naumann, P.; André, F.; Katus, H.A.; Debus, J. Risen from the dead: Cardiac stereotactic ablative radiotherapy as last rescue in a patient with refractory ventricular fibrillation storm. HeartRhythm Case Rep. 2019, 5, 329–332. [Google Scholar] [CrossRef]
- Lloyd, M.S.; Wight, J.; Schneider, F.; Hoskins, M.; Attia, T.; Escott, C.; Lerakis, S.; Higgins, K.A. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm 2020, 17, 415–422. [Google Scholar] [CrossRef]
- Robinson, C.G.; Samson, P.P.; Moore, K.M.S.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Lang, A.; Cooper, D.H.; Faddis, M.; et al. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation 2019, 139, 313–321. [Google Scholar] [CrossRef]
- Kiani, S.; Kutob, L.; Schneider, F.; Higgins, K.A.; Lloyd, M.S. Histopathologic and Ultrastructural Findings in Human Myocardium After Stereotactic Body Radiation Therapy for Recalcitrant Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2020, 13, e008753. [Google Scholar] [CrossRef]
- Cha, M.J.; Seo, J.W.; Kim, H.J.; Kim, M.K.; Yoon, H.S.; Jo, S.W.; Oh, S.; Chang, J.H. Early Changes in Rat Heart After High-Dose Irradiation: Implications for Antiarrhythmic Effects of Cardiac Radioablation. J. Am. Heart Assoc. 2021, 10, e019072. [Google Scholar] [CrossRef]
- Amino, M.; Yoshioka, K.; Tanabe, T.; Tanaka, E.; Mori, H.; Furusawa, Y.; Zareba, W.; Yamazaki, M.; Nakagawa, H.; Honjo, H.; et al. Heavy ion radiation up-regulates Cx43 and ameliorates arrhythmogenic substrates in hearts after myocardial infarction. Cardiovasc. Res. 2006, 72, 412–421. [Google Scholar] [CrossRef]
- Zhang, D.M.; Navara, R.; Yin, T.; Szymanski, J.; Goldsztejn, U.; Kenkel, C.; Lang, A.; Mpoy, C.; Lipovsky, C.E.; Qiao, Y.; et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat. Commun. 2021, 12, 5558. [Google Scholar] [CrossRef]
- Amino, M.; Yoshioka, K.; Fujibayashi, D.; Hashida, T.; Furusawa, Y.; Zareba, W.; Ikari, Y.; Tanaka, E.; Mori, H.; Inokuchi, S.; et al. Year-long upregulation of connexin43 in rabbit hearts by heavy ion irradiation. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1014–H1021. [Google Scholar] [CrossRef] [PubMed]
- Amino, M.; Yoshioka, K.; Furusawa, Y.; Tanaka, S.; Kawabe, N.; Hashida, T.; Tsukada, T.; Izumi, M.; Inokuchi, S.; Tanabe, T.; et al. Inducibility of Ventricular Arrhythmia 1 Year Following Treatment with Heavy Ion Irradiation in Dogs with Myocardial Infarction. Pacing Clin. Electrophysiol. 2017, 40, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kasper, M.; Bierhaus, A.; Langer, S.; Müller, M.; Trott, K.-R. Connexin 43 Expression in Normal and Irradiated Mouse Skin. Radiat. Res. 1997, 147, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Traub, O.; Reimann, T.; Bjermer, L.; Grossmann, H.; Müller, M.; Wenzel, K.W. Upregulation of gap junction protein connexin43 in alveolar epithelial cells of rats with radiation-induced pulmonary fibrosis. Histochem. Cell Biol. 1996, 106, 419–424. [Google Scholar] [CrossRef]
- Wei, C.; Qian, P.; Tedrow, U.; Mak, R.; Zei, P.C. Non-invasive Stereotactic Radioablation: A New Option for the Treatment of Ventricular Arrhythmias. Arrhythm. Electrophysiol. Rev. 2020, 8, 285–293. [Google Scholar] [CrossRef]
- Carbucicchio, C.; Jereczek-Fossa, B.A.; Andreini, D.; Catto, V.; Piperno, G.; Conte, E.; Cattani, F.; Rondi, E.; Vigorito, S.; Piccolo, C.; et al. STRA-MI-VT (STereotactic RadioAblation by Multimodal Imaging for Ventricular Tachycardia): Rationale and design of an Italian experimental prospective study. J. Interv. Card. Electrophysiol. 2021, 61, 583–593. [Google Scholar] [CrossRef]
- Conte, E.; Mushtaq, S.; Carbucicchio, C.; Piperno, G.; Catto, V.; Mancini, M.E.; Formenti, A.; Annoni, A.; Guglielmo, M.; Baggiano, A.; et al. State of the art paper: Cardiovascular CT for planning ventricular tachycardia ablation procedures. J. Cardiovasc. Comput. Tomogr. 2021, 15, 394–402. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Antunes, S.; Maccabelli, G.; Colantoni, C.; Rancoita, P.M.V.; Baratto, F.; Di Serio, C.; Rizzo, G.; De Cobelli, F.; et al. Cardiac CT With Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate: Relationship Between CT-Segmented Scar and Electro-Anatomic Mapping. JACC Cardiovasc. Imaging 2016, 9, 822–832. [Google Scholar] [CrossRef]
- Ghannam, M.; Cochet, H.; Jais, P.; Sermesant, M.; Patel, S.; Siontis, K.C.; Morady, F.; Bogun, F. Correlation between computer tomography-derived scar topography and critical ablation sites in postinfarction ventricular tachycardia. J. Cardiovasc. Electrophysiol. 2018, 29, 438–445. [Google Scholar] [CrossRef]
- Sramko, M.; Hoogendoorn, J.C.; Glashan, C.A.; Zeppenfeld, K. Advancement in cardiac imaging for treatment of ventricular arrhythmias in structural heart disease. Europace 2019, 21, 383–403. [Google Scholar] [CrossRef]
- Enriquez, A.; Baranchuk, A.; Briceno, D.; Saenz, L.; Garcia, F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm 2019, 16, 1538–1544. [Google Scholar] [CrossRef]
- Dickfeld, T.; Tian, J.; Ahmad, G.; Jimenez, A.; Turgeman, A.; Kuk, R.; Peters, M.; Saliaris, A.; Saba, M.; Shorofsky, S.; et al. MRI-Guided ventricular tachycardia ablation: Integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ. Arrhythm. Electrophysiol. 2011, 4, 172–184. [Google Scholar] [CrossRef]
- Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; Zucchelli, G.; Syrovnev, V.; Zaraket, F.; Terés, C.; et al. Cardiac Magnetic Resonance-Guided Ventricular Tachycardia Substrate Ablation. JACC Clin. Electrophysiol. 2020, 6, 436–447. [Google Scholar] [CrossRef]
- Bianchi, S.; Cauti, F.M. Ablation of ventricular tachycardia in 2021. Eur. Heart J. Suppl. 2021, 23, E25–E27. [Google Scholar] [CrossRef]
- Lilli, A.; Parollo, M.; Mazzocchetti, L.; De Sensi, F.; Rossi, A.; Notarstefano, P.; Santoro, A.; Aquaro, G.D.; Cresti, A.; Lapira, F.; et al. Ventricular tachycardia ablation guided or aided by scar characterization with cardiac magnetic resonance: Rationale and design of VOYAGE study. BMC Cardiovasc. Disord. 2022, 22, 169. [Google Scholar] [CrossRef]
- Benali, K.; Rigal, L.; Simon, A.; Bellec, J.; Jaïs, P.; Kamakura, T.; Robinson, C.G.; Cuculich, P.; De Crevoisier, R.; Martins, R.P. Correlation between the radiation dose and myocardial remodeling after stereotactic radiation therapy for ventricular tachycardia: First assessment of the dose-effect relationship in humans. Heart Rhythm 2022, 19, 1559–1560. [Google Scholar] [CrossRef]
- Wang, Y.; Cuculich, P.S.; Zhang, J.; Desouza, K.A.; Vijayakumar, R.; Chen, J.; Faddis, M.N.; Lindsay, B.D.; Smith, T.W.; Rudy, Y. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci. Transl. Med. 2011, 3, 98ra84. [Google Scholar] [CrossRef]
- Zhang, J.; Cooper, D.H.; Desouza, K.A.; Cuculich, P.S.; Woodard, P.K.; Smith, T.W.; Rudy, Y. Electrophysiologic Scar Substrate in Relation to VT: Noninvasive High-Resolution Mapping and Risk Assessment with ECGI. Pacing Clin. Electrophysiol. 2016, 39, 781–791. [Google Scholar] [CrossRef]
- Ho, G.; Atwood, T.F.; Bruggeman, A.R.; Moore, K.L.; McVeigh, E.; Villongco, C.T.; Han, F.T.; Hsu, J.C.; Hoffmayer, K.S.; Raissi, F.; et al. Computational ECG mapping and respiratory gating to optimize stereotactic ablative radiotherapy workflow for refractory ventricular tachycardia. Heart Rhythm O2 2021, 2, 511–520. [Google Scholar] [CrossRef]
- Monaco, A.D.; Gregucci, F.; Bonaparte, I.; Troisi, F.; Surgo, A.; Molfetta, D.D.; Vitulano, N.; Quadrini, F.; Carbonara, R.; Ludovico, E.; et al. First Pulmonary Vein Isolation Using LINAC-Based STAR. Circ. Arrhythmia Electrophysiol. 2022, 15, e010880. [Google Scholar] [CrossRef]
- Fiorentino, A.; Di Monaco, A.; Surgo, A.; Vitulano, N.; Gregucci, F.; Ludovico, E.; Carbonara, R.; Quadrini, F.; Rubini, G.; Bonaparte, I.; et al. Linac-based STereotactic Arrhythmia Radioablation (STAR) of ventricular tachycardia: Case report and literature review. Clin. Case Rep. 2021, 9, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Knybel, L.; Cvek, J.; Neuwirth, R.; Jiravsky, O.; Hecko, J.; Penhaker, M.; Sramko, M.; Kautzner, J. Real-time measurement of ICD lead motion during stereotactic body radiotherapy of ventricular tachycardia. Rep. Pract. Oncol. Radiother. 2021, 26, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.L.; Cook, J.A.; Aboud, A.A.; Karim, R.; Shinohara, E.T.; Stevenson, W.G. Novel Workflow for Conversion of Catheter-Based Electroanatomic Mapping to DICOM Imaging for Noninvasive Radioablation of Ventricular Tachycardia. Pract. Radiat. Oncol. 2021, 11, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S.; Henkenberens, C.; Zormpas, C.; Christiansen, H.; Bauersachs, J.; Duncker, D.; Veltmann, C. A novel open-source software-based high-precision workflow for target definition in cardiac radioablation. J. Cardiovasc. Electrophysiol. 2020, 31, 2689–2695. [Google Scholar] [CrossRef]
- Kovacs, B.; Mayinger, M.; Schindler, M.; Steffel, J.; Andratschke, N.; Saguner, A.M. Stereotactic radioablation of ventricular arrhythmias in patients with structural heart disease—A systematic review. Radiother. Oncol. 2021, 162, 132–139. [Google Scholar] [CrossRef]
- Neuwirth, R.; Cvek, J.; Knybel, L.; Jiravsky, O.; Molenda, L.; Kodaj, M.; Fiala, M.; Peichl, P.; Feltl, D.; Januška, J.; et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace 2019, 21, 1088–1095. [Google Scholar] [CrossRef]
- Gianni, C.; Rivera, D.; Burkhardt, J.D.; Pollard, B.; Gardner, E.; Maguire, P.; Zei, P.C.; Natale, A.; Al-Ahmad, A. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm 2020, 17, 1241–1248. [Google Scholar] [CrossRef]
- Yugo, D.; Lo, L.W.; Wu, Y.H.; Chung, F.P.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Chao, T.F.; Liao, J.N.; Chang, T.Y.; et al. Case series on stereotactic body radiation therapy in non-ischemic cardiomyopathy patients with recurrent ventricular tachycardia. Pacing Clin. Electrophysiol. 2021, 44, 1085–1093. [Google Scholar] [CrossRef]
- Chin, R.; Hayase, J.; Hu, P.; Cao, M.; Deng, J.; Ajijola, O.; Do, D.; Vaseghi, M.; Buch, E.; Khakpour, H.; et al. Non-invasive stereotactic body radiation therapy for refractory ventricular arrhythmias: An institutional experience. J. Interv. Card Electrophysiol. 2021, 61, 535–543. [Google Scholar] [CrossRef]
- Lee, J.; Bates, M.; Shepherd, E.; Riley, S.; Henshaw, M.; Metherall, P.; Daniel, J.; Blower, A.; Scoones, D.; Wilkinson, M.; et al. Cardiac stereotactic ablative radiotherapy for control of refractory ventricular tachycardia: Initial UK multicentre experience. Open Heart 2021, 8, e001770. [Google Scholar] [CrossRef]
- Qian, P.C.; Quadros, K.; Aguilar, M.; Wei, C.; Boeck, M.; Bredfeldt, J.; Cochet, H.; Blankstein, R.; Mak, R.; Sauer, W.H.; et al. Substrate Modification Using Stereotactic Radioablation to Treat Refractory Ventricular Tachycardia in Patients With Ischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2022, 8, 49–58. [Google Scholar] [CrossRef]
- Kurzelowski, R.; Latusek, T.; Miszczyk, M.; Jadczyk, T.; Bednarek, J.; Sajdok, M.; Gołba, K.S.; Wojakowski, W.; Wita, K.; Gardas, R.; et al. Radiosurgery in Treatment of Ventricular Tachycardia—Initial Experience Within the Polish SMART-VT Trial. Front. Cardiovasc. Med. 2022, 9, 874661. [Google Scholar] [CrossRef]
- Bhaskaran, A.; Downar, E.; Chauhan, V.S.; Lindsay, P.; Nair, K.; Ha, A.; Hope, A.; Nanthakumar, K. Electroanatomical mapping-guided stereotactic radiotherapy for right ventricular tachycardia storm. HeartRhythm Case Rep. 2019, 5, 590–592. [Google Scholar] [CrossRef]
- Krug, D.; Blanck, O.; Demming, T.; Dottermusch, M.; Koch, K.; Hirt, M.; Kotzott, L.; Zaman, A.; Eidinger, L.; Siebert, F.A.; et al. Stereotactic body radiotherapy for ventricular tachycardia (cardiac radiosurgery): First-in-patient treatment in Germany. Strahlenther. Onkol. 2020, 196, 23–30. [Google Scholar] [CrossRef]
- Mayinger, M.; Kovacs, B.; Tanadini-Lang, S.; Ehrbar, S.; Wilke, L.; Chamberlain, M.; Moreira, A.; Weitkamp, N.; Brunckhorst, C.; Duru, F.; et al. First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Radiother. Oncol. 2020, 152, 203–207. [Google Scholar] [CrossRef]
- Martí-Almor, J.; Jiménez-López, J.; Rodríguez de Dios, N.; Tizón, H.; Vallés, E.; Algara, M. Noninvasive ablation of ventricular tachycardia with stereotactic radiotherapy in a patient with arrhythmogenic right ventricular cardiomyopathy. Rev. Esp. Cardiol. 2020, 73, 97–99. [Google Scholar] [CrossRef]
- Van der Ree, M.H.; Blanck, O.; Limpens, J.; Lee, C.H.; Balgobind, B.V.; Dieleman, E.M.T.; Wilde, A.A.M.; Zei, P.C.; de Groot, J.R.; Slotman, B.J.; et al. Cardiac radioablation-A systematic review. Heart Rhythm 2020, 17, 1381–1392. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Sami, S.; Daher, I.N. Radiation-induced heart disease: A clinical update. Cardiol. Res. Pract. 2011, 2011, 317659. [Google Scholar] [CrossRef]
- Blanck, O.; Bode, F.; Gebhard, M.; Hunold, P.; Brandt, S.; Bruder, R.; Grossherr, M.; Vonthein, R.; Rades, D.; Dunst, J. Dose-escalation study for cardiac radiosurgery in a porcine model. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 590–598. [Google Scholar] [CrossRef]
- Hohmann, S.; Deisher, A.J.; Suzuki, A.; Konishi, H.; Rettmann, M.E.; Merrell, K.W.; Kruse, J.J.; Newman, L.K.; Parker, K.D.; Monahan, K.H.; et al. Left ventricular function after noninvasive cardiac ablation using proton beam therapy in a porcine model. Heart Rhythm 2019, 16, 1710–1719. [Google Scholar] [CrossRef]
- Sharma, A.; Wong, D.; Weidlich, G.; Fogarty, T.; Jack, A.; Sumanaweera, T.; Maguire, P. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm 2010, 7, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Oermann, E.K.; Murthy, N.; Chen, V.; Baimeedi, A.; Sasaki-Adams, D.; McGrail, K.; Collins, S.P.; Ewend, M.G.; Collins, B.T. A Multicenter Retrospective Study of Frameless Robotic Radiosurgery for Intracranial Arteriovenous Malformation. Front. Oncol. 2014, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Abla, A.A.; Shetter, A.G.; Chang, S.W.; Wait, S.D.; Brachman, D.G.; Ng, Y.T.; Rekate, H.L.; Kerrigan, J.F. Gamma Knife surgery for hypothalamic hamartomas and epilepsy: Patient selection and outcomes. J. Neurosurg. 2010, 113, 207–214. [Google Scholar] [CrossRef] [PubMed]
- John, R.M.; Shinohara, E.T.; Price, M.; Stevenson, W.G. Radiotherapy for ablation of ventricular tachycardia: Assessing collateral dosing. Comput. Biol. Med. 2018, 102, 376–380. [Google Scholar] [CrossRef]
- Hayase, J.; Chin, R.; Cao, M.; Hu, P.; Shivkumar, K.; Bradfield, J. Non-invasive Stereotactic Body Radiation Therapy for Refractory Ventricular Arrhythmias: Venturing into the Unknown. J. Innov. Card Rhythm Manag. 2022, 13, 4894–4899. [Google Scholar] [CrossRef]
| Indication | Comment |
|---|---|
| Challenging location of the tachycardia | Patients with VT that originates from a specific, localized area of the heart, which is not possible or very challenging to achieve with catheter ablation. |
| Contraindications to catheter ablation | Inaccessible anatomical sites, inability to access the heart itself, or inability for a patient to tolerate a catheter ablation procedure |
| General health and medical history | Patients who are in generally poor health, with significant comorbidities, which would be considered at high risk for a transcatheter approach. |
| Method | Strengths | Limitations |
|---|---|---|
| Cardiac CT | Identification of coronary artery disease assessing left ventricular anatomy and scar burden | Radiation exposure Risk of contrast-induced nephropathy and allergic reaction Low accuracy in detecting small fibrous areas of scar tissue |
| Cardiac MRI | Highly spatial resolution and detailed 3D images of the heart Comprehensive assessment of myocardial tissue by use of multiparametric approach (T1 and T2 mapping, late gadolinium enhancement, and diffusion-weighted imaging) Quantitative assessment of myocardial scar | Significant time commitment required Contraindicated in patients with certain types of implanted devices Interpretation challenges |
| Nuclear imaging | Detection of areas of hypoperfusion or scar that may not visible on other imaging modalities Study of myocardial viability | Expensive Low spatial resolution and sensitivity Radiation exposure |
| Electroanatomical mapping | Real-time mapping of cardiac electrical activity in both endocardium and epicardium Detailed 3D images of the heart Accurately identification of VT circuit | Invasive catheterization Technical expertise |
| Reference | Cuculich et al. [10] | Robinson et al. [36] | Neuwirth et al. [66] | Gianni et al. [67] | Lloyd et al. [35] | Yugo et al. [68] | Chin et al. [69] | Carbucicchio et al. [12] | Lee et al. [70] | Qian et al. [71] | Kurzelowski R et al. [72] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication year | 2017 | 2019 | 2019 | 2020 | 2020 | 2021 | 2021 | 2021 | 2021 | 2022 | 2022 |
| Study design | Case series | Prospective | Case series | Prospective | Retrospective | Case series | Retrospective | Prospective | Prospective | Prospective | Case series |
| Single center | Single center | Single center | Dual center | Single center | Single center | Single center | Single center | Three centers | Single center | Single center | |
| No. of enrolled patients | 5 | 19 | 10 | 5 | 10 | 3 | 8 | 7 | 7 | 6 | 2 |
| Male—n (%) | 4 (80) | 17 (89.5) | 9 (90) | 5 (100) | 7 (70) | 2 (69) | 8 (100) | 7 (100) | 4 (57) | 6 (100) | 2 (100) |
| Age range | 66 (60–83) | 66 (49–81) | 66 (61–78) | 62 | 61 (51–78) | 72 (65–83) | 75 ± 7.3 | 70 ± 7 | 60s–70s | 72 (70–73) | 69–72 |
| Ischemic heart disease—n (%) | 2 (40) | 11 (57.9) | 8 (80) | 4 (80) | 4 (40) | 0 | 4 (50) | 3 (43) | 5 (71.4) | 6 (100) | 2 (100) |
| Non-ischemic Cardiomyopathy—n (%) | 3 (60) | 8 (42.1) | 2 (20) | 1 (20) | 6 (60) | 3 (100) | 4 (50) | 4 (57) | 2 (28.6) | 0 | 0 |
| LVEF (%) | 23 (15–37) | 25 (15–58) | 26.5 ± 3.2 | 34 | / | 20–59 | 21 ± 7 | 27 ± 11 | 27 | 20 (16–25) | 20–22 |
| NYHA class- % | |||||||||||
| I | 5.3 | 20 | 69 | 29 | / | 0 | |||||
| II | 21.1 | 60 | 80 | / | 33 | 71 | 43 | / | 0 | ||
| III | 20 | 52.6 | 40 | 62.5 | 43 | / | 100 | ||||
| IV | 80 | 21.1 | 37.5 | 14 | / | 0 | |||||
| Radiation type | Linear accelerator | Linear accelerator | Cyberknife | Cyberknife | Linear accelerator | Linear accelerator | Linear accelerator | Linear accelerator | Linear accelerator | Linear accelerator | Linear accelerator |
| Dose (Gy) | 25 | 25 | 25 | 25 | 25 | 25 | 22.2 ± 3.6 | 25 | 25 | 25 | 25 |
| Treatment time (min) | 14 | 15 (5–32) | 68 (45–80) | 82 (66–92) | / | / | 18 ± 6 | 31 ± 6 | 38 | 14 (11–15) | 13 |
| Average follow up | 12 months | 6 months | 28 (16–54) months | 12 ± 2 months | 174 (118–273) days | 2–54 weeks | 234 (145–299) days | 4 pt complete 6 months FU | 6 months | 231 (212–311) days | 6 months |
| VT burden reduction | 99.9% | 94% | 87.6% | No reduction | 69% | 61% | 80% | 93% | 85% | 31% | Sustained VT abolition after blanking |
| Complications related to STAR | 1 stroke (not crearly related) | 1 pericarditis; 1 heart failure (possible) | 1 nausea 1 progression of mitral regurgitation | none | 2 pneumonitis | none | none | 1 nausea/vomiting 1 pulmonary fibrosis | none | 1 pneumonitis 1 heart failure 1 moderate pericardial effusion | 1 heart failure exacerbation And concomitant pulmonary embolism |
| Irradiated Organ | Possible Complication | Symptoms |
|---|---|---|
| Heart | Arrhythmias, pericarditis, myocarditis, myocardial and valvular fibrosis, coronary atherosclerosis | Chest pain, palpitations, symptoms of heart failure |
| Lung | Pneumonitis and fibrosis leading | Cough, dyspnea, chest pain |
| Esophagus | Esophagitis and perforation | Dysphagia, chest pain, cough |
| Spinal cord | Inflammation and fibrosis | Sensory and motor dysfunction, paralysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarracini, F.; Tritto, M.; Di Monaco, A.; Mariani, M.V.; Gasperetti, A.; Compagnucci, P.; Muser, D.; Preda, A.; Mazzone, P.; Themistoclakis, S.; et al. Stereotactic Arrhythmia Radioablation Treatment of Ventricular Tachycardia: Current Technology and Evolving Indications. J. Cardiovasc. Dev. Dis. 2023, 10, 172. https://doi.org/10.3390/jcdd10040172
Guarracini F, Tritto M, Di Monaco A, Mariani MV, Gasperetti A, Compagnucci P, Muser D, Preda A, Mazzone P, Themistoclakis S, et al. Stereotactic Arrhythmia Radioablation Treatment of Ventricular Tachycardia: Current Technology and Evolving Indications. Journal of Cardiovascular Development and Disease. 2023; 10(4):172. https://doi.org/10.3390/jcdd10040172
Chicago/Turabian StyleGuarracini, Fabrizio, Massimo Tritto, Antonio Di Monaco, Marco Valerio Mariani, Alessio Gasperetti, Paolo Compagnucci, Daniele Muser, Alberto Preda, Patrizio Mazzone, Sakis Themistoclakis, and et al. 2023. "Stereotactic Arrhythmia Radioablation Treatment of Ventricular Tachycardia: Current Technology and Evolving Indications" Journal of Cardiovascular Development and Disease 10, no. 4: 172. https://doi.org/10.3390/jcdd10040172
APA StyleGuarracini, F., Tritto, M., Di Monaco, A., Mariani, M. V., Gasperetti, A., Compagnucci, P., Muser, D., Preda, A., Mazzone, P., Themistoclakis, S., & Carbucicchio, C. (2023). Stereotactic Arrhythmia Radioablation Treatment of Ventricular Tachycardia: Current Technology and Evolving Indications. Journal of Cardiovascular Development and Disease, 10(4), 172. https://doi.org/10.3390/jcdd10040172







