Low Plasma Levels of Irisin Predict Acutely Decompensated Heart Failure in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure
Abstract
1. Introduction
2. Materials and Methods
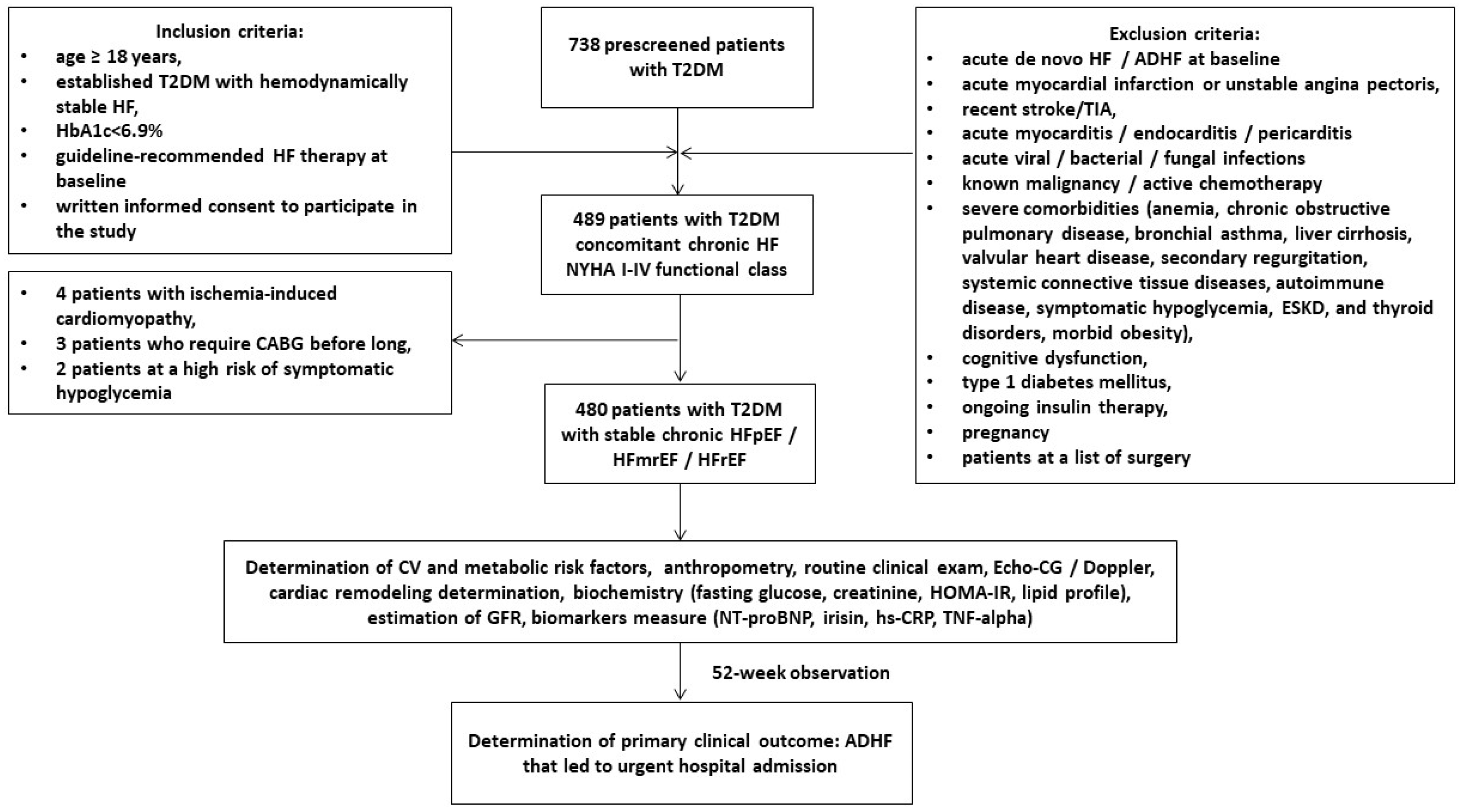
2.1. Study Design and Cohorts of Participants
2.2. Determination of Study End-Points
2.3. Concomitant Medical Information Collection
2.4. Echocardiography Examination
2.5. Blood Sampling and Biomarker Measurements
2.6. Estimation of Glomerular Filtration Rate
2.7. Determination of Insulin Resistance
2.8. Statistics
3. Results
3.1. Basis Characteristic of the Patients Enrolled in the Study
3.2. Determination of Primary Causes for ADHF
3.3. Clinical Features, Echocrdiographic Parameters, and Biomarkers’ Levels during the Follow-Up
3.4. Spearman’s Correlation between Circulating Levels of Irisin and Other Parameters
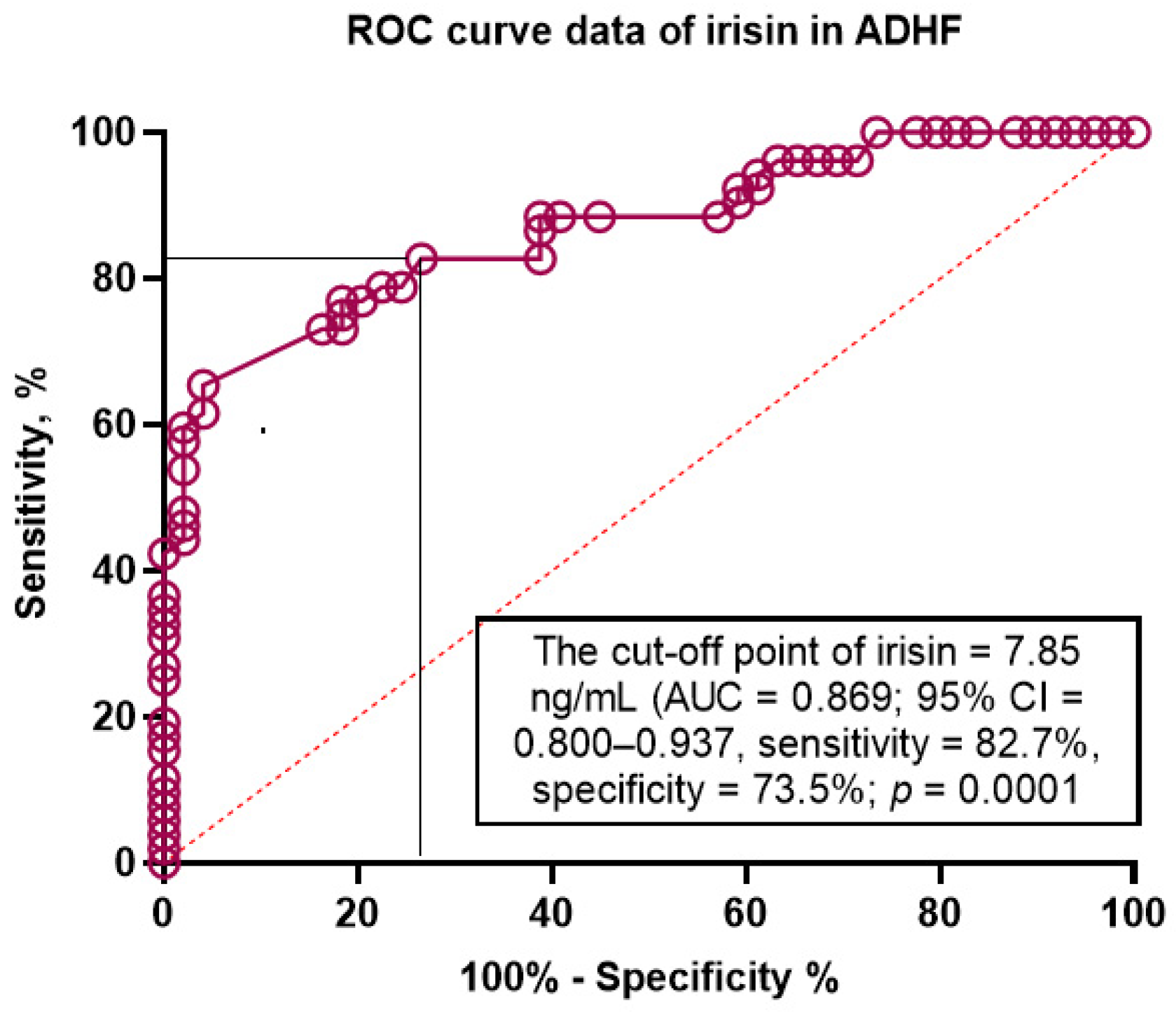
3.5. Discriminative Value of Irisin for ADHF
3.6. The Predictors of HF in T2DM Patients: The Univariate and Multivariate Logistic Regression
3.7. Comparison of the Predictive Models
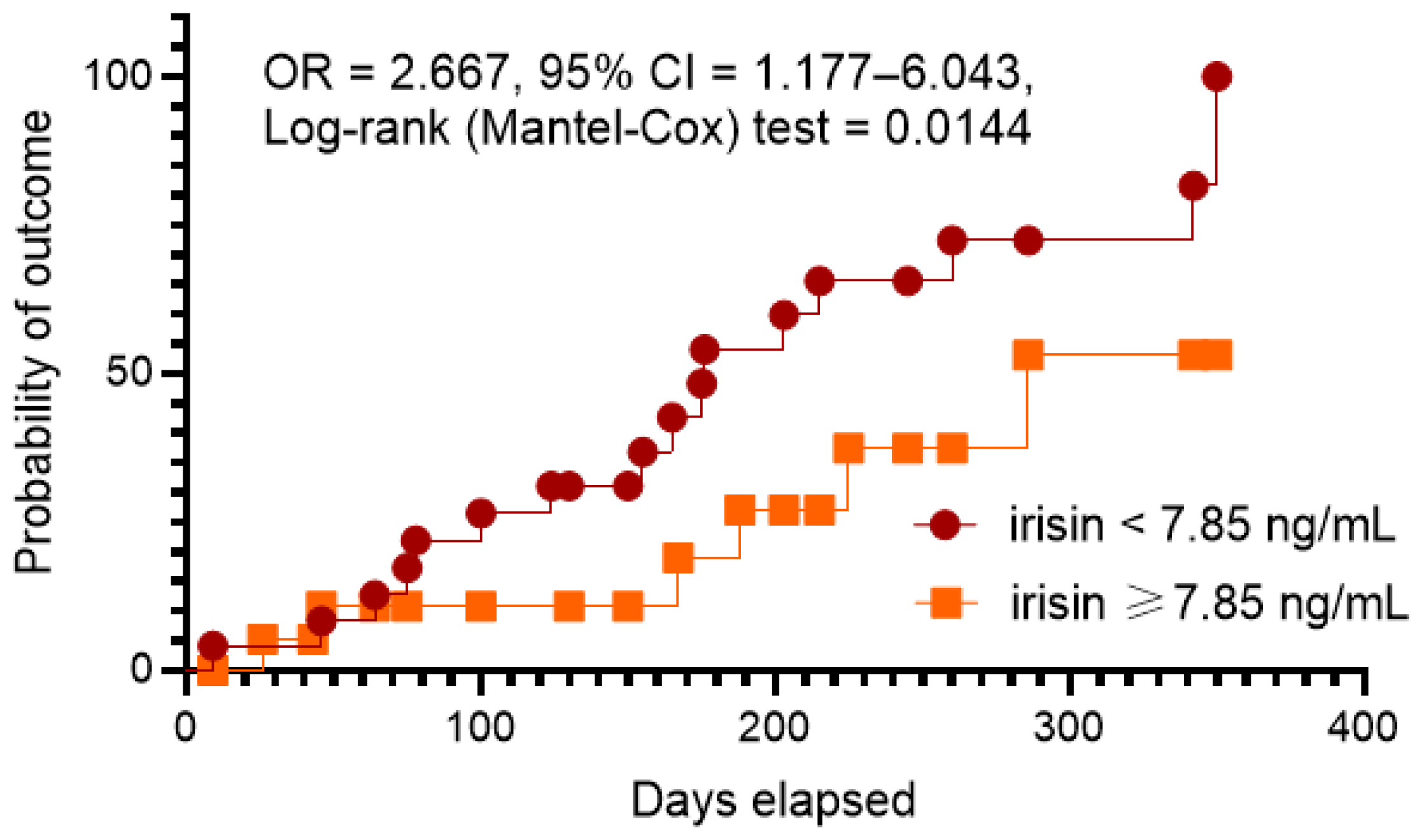
3.8. Kaplan–Meier Curve Analysis
3.9. Reproducibility of Biomarkers
3.10. Reproducibility of Echocardiographic Parameters
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. Acute heart failure congestion and perfusion status—Impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2019, 21, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Sinnenberg, L.; Givertz, M.M. Acute heart failure. Trends Cardiovasc. Med. 2020, 30, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Sato, N.; Takano, T.; Kitai, T.; Yoshikawa, T.; Matsue, Y.; Ross, J.S.; Chen, J.; Lin, Z.; et al. 9-Year Trend in the Management of Acute Heart Failure in Japan: A Report from the National Consortium of Acute Heart Failure Registries. J. Am. Heart Assoc. 2018, 7, e008687. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Stough, W.G.; Gallup, D.S.; Hasselblad, V.; Gheorghiade, M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: Observations from the IMPACT-HF registry. J. Card. Fail. 2005, 11, 200–205. [Google Scholar] [CrossRef]
- Miró, Ò.; Gil, V.Í.; Martín-Sánchez, F.J.; Jacob, J.; Herrero, P.; Alquézar, A.; Llauger, L.; Aguiló, S.; Martínez, G.; Ríos, J.; et al. Short-term outcomes of heart failure patients with reduced and preserved ejection fraction after acute decompensation according to the final destination after emergency department care. Clin. Res. Cardiol. 2018, 107, 698–710. [Google Scholar] [CrossRef]
- Felker, G.M.; Leimberger, J.D.; Califf, R.M.; Cuffe, M.S.; Massie, B.M.; Adams, K.F., Jr.; Gheorghiade, M.; O’Connor, C.M. Risk stratification after hospitalization for decompensated heart failure. J. Card. Fail. 2004, 10, 460–466. [Google Scholar] [CrossRef]
- Bazmpani, M.A.; Papanastasiou, C.A.; Kamperidis, V.; Zebekakis, P.E.; Karvounis, H.; Kalogeropoulos, A.P.; Karamitsos, T.D. Contemporary Data on the Status and Medical Management of Acute Heart Failure. Curr. Cardiol. Rep. 2022, 24, 2009–2022. [Google Scholar] [CrossRef]
- Rizzi, M.A.; Sarasola, A.G.; Arbé, A.A.; Mateo, S.H.; Gil, V.; Llorens, P.; Jacob, J.; Martín-Sánchez, F.J.; Puente, P.H.; Escoda, R.; et al. Factors associated with in-hospital mortality and adverse outcomes during the vulnerable post-discharge phase after the first episode of acute heart failure: Results of the NOVICA-2 study. Clin. Res. Cardiol. 2021, 110, 993–1005. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.-P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; et al. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur. Heart J. 2006, 27, 2725–2736. [Google Scholar] [CrossRef]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion during and after Hospitalization for Acute Heart Failure: Insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Point-of-care heart failure platform: Where are we now and where are we going to? Expert Rev. Cardiovasc. Ther. 2022, 20, 419–429. [Google Scholar] [CrossRef]
- Baecker, A.; Meyers, M.; Koyama, S.; Taitano, M.; Watson, H.; Machado, M.; Nguyen, H.Q. Evaluation of a Transitional Care Program after Hospitalization for Heart Failure in an Integrated Health Care System. JAMA Netw. Open 2020, 3, e2027410. [Google Scholar] [CrossRef]
- Berezin, A.E. Prognostication of clinical outcomes in diabetes mellitus: Emerging role of cardiac biomarkers. Diabetes Metab. Syndr. 2019, 13, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Wakai, A.; Collins, S.P.; Levy, P.D.; Diercks, D.; Hiestand, B.C.; Fermann, G.J.; de Souza, I.; Sinert, R. Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2016, 23, 223–242. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.; Kozhuharov, N.; Coats, A.J.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Zhang, J.; Song, T.T.; Li, X.; Wang, H.X. Diagnostic accuracy of natriuretic peptides for acute heart failure: A review. Eur Rev. Med. Pharmacol. Sci. 2018, 22, 2415–2420. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Med. 2023, 43, 225–236. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Guo, L.; Jiang, J.; Chen, H. Irisin: Linking metabolism with heart failure. Am. J. Transl. Res. 2020, 12, 6003–6014. [Google Scholar] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, B.; Zhao, C.; Wang, Y.; Zhou, Y.; Lin, J.; Zhao, R. Irisin Regulates Cardiac Responses to Exercise in Health and Diseases: A Narrative Review. J. Cardiovasc. Transl. Res. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gao, R.; Bei, Y.; Li, J.; Zhang, H.; Zhou, Y.; Yao, W.; Xu, D.; Zhou, F.; Jin, M.; et al. Serum Irisin Predicts Mortality Risk in Acute Heart Failure Patients. Cell Physiol. Biochem. 2017, 42, 615–622. [Google Scholar] [CrossRef]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Fushtey, I.M.; Berezin, A.E. Serum Levels of Irisin Predict Cumulative Clinical Outcomes in Heart Failure Patients with Type 2 Diabetes Mellitus. Front. Physiol. 2022, 13, 922775. [Google Scholar] [CrossRef]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Stöhr, E.; Berezin, A.E. Discriminative Value of Serum Irisin in Prediction of Heart Failure with Different Phenotypes among Patients with Type 2 Diabetes Mellitus. Cells 2022, 11, 2794. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Yamamoto, J.; Wakami, K.; Muto, K.; Kikuchi, S.; Goto, T.; Fukuta, H.; Seo, Y.; Ohte, N. Verification of Echocardiographic Assessment of Left Ventricular Diastolic Dysfunction in Patients with Preserved Left Ventricular Ejection Fraction Using the American Society of Echocardiography and European Association of Cardiovascular Imaging 2016 Recommendations. Circ. Rep. 2019, 1, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Song, R.; Zhao, X.; Zhang, D.Q.; Wang, R.; Feng, Y. Lower levels of irisin in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2021, 175, 108788. [Google Scholar] [CrossRef]
- Du, X.L.; Jiang, W.X.; Lv, Z.T. Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2016, 48, 644–652. Available online: https://doiorg/101055/s-0042-108730. (accessed on 14 June 2016). [CrossRef]
- Vecchiato, M.; Zanardo, E.; Battista, F.; Quinto, G.; Bergia, C.; Palermi, S.; Duregon, F.; Ermolao, A.; Neunhaeuserer, D. The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review. J. Clin. Med. 2022, 12, 62. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H. Association Between Serum Irisin and Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis. Horm. Metab. Res. 2021, 53, 293–300. [Google Scholar] [CrossRef]
- González, A.; Richards, A.M.; de Boer, R.A.; Thum, T.; Arfsten, H.; Hülsmann, M.; Falcao-Pires, I.; Díez, J.; Foo, R.S.; Chan, M.Y.; et al. Cardiac remodelling—Part 1: From cells and tissues to circulating biomarkers. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 927–943. [Google Scholar] [CrossRef]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Farber-Eger, E.; Arora, P.; Collins, S.; Wells, Q.S.; Wang, T.J. Unexpectedly Low Natriuretic Peptide Levels in Patients With Heart Failure. JACC Heart Fail. 2021, 9, 192–200. [Google Scholar] [CrossRef]
- Silvestrini, A.; Bruno, C.; Vergani, E.; Venuti, A.; Favuzzi, A.M.R.; Guidi, F.; Nicolotti, N.; Meucci, E.; Mordente, A.; Mancini, A. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: A pilot study. PLoS ONE 2019, 14, e0210320. [Google Scholar] [CrossRef]
- Peng, Q.; Ding, R.; Wang, X.; Yang, P.; Jiang, F.; Chen, X. Effect of Irisin on Pressure Overload–Induced Cardiac Remodeling. Arch. Med. Res. 2021, 52, 182–190. [Google Scholar] [CrossRef]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Luna-Ceron, E.; Gonzalez-Gil, A.M.; Casillas-Fikentscher, A.; Villarreal-Calderon, J.R.; Enriquez, C.; de la Peña-Almaguer, E.; Castillo, E.C.; Silva-Platas, C.; et al. Association of irisin levels with cardiac magnetic resonance, inflammatory, and biochemical parameters in patients with chronic heart failure versus controls. Magn. Reson. Imaging 2022, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.-C.; Ho, M.-Y.; Wen, M.-S.; Chen, C.-C.; Hsieh, M.-J.; Lin, C.-P.; Yeh, J.-K.; Tsai, M.-L.; Yang, C.-H.; Wu, V.C.-C.; et al. Serum irisin levels are associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Int. J. Cardiol. 2018, 261, 12–17. [Google Scholar] [CrossRef]
- Khorasani, Z.M.; Bagheri, R.K.; Yaghoubi, M.A.; Chobkar, S.; Aghaee, M.A.; Abbaszadegan, M.R.; Sahebkar, A. The association between serum irisin levels and cardiovascular disease in diabetic patients. Diabetes Metab. Syndr. 2019, 13, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Parissis, J.T.; Rafouli-Stergiou, P.; Mebazaa, A.; Ikonomidis, I.; Bistola, V.; Nikolaou, M.; Meas, T.; Delgado, J.; Vilas-Boas, F.; Paraskevaidis, I.; et al. Acute heart failure in patients with diabetes mellitus: Clinical characteristics and predictors of in-hospital mortality. Int. J. Cardiol. 2012, 157, 108–113. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Y.; Guo, Y.; Xia, Y.; Li, C.; Du, Y.; Lin, C.; Xu, X.; Qi, T.; Fan, M.; et al. Irisin Promotes Cardiac Homing of Intravenously Delivered MSCs and Protects against Ischemic Heart Injury. Adv. Sci. 2022, 9, 2103697. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association with Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Moore, J.; Dungan, K. Glycemic variability and glycemic control in the acutely ill cardiac patient. Heart Fail. Clin. 2012, 8, 523–538. [Google Scholar] [CrossRef] [PubMed]
- McAlister, F.A.; Ezekowitz, J.; Tonelli, M.; Armstrong, P.W. Renal insufficiency and heart failure: Prognostic and therapeutic implications from a prospective cohort study. Circulation 2004, 109, 1004–1009. [Google Scholar] [CrossRef]
- Valente, M.A.E.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, K.; Hsieh, A.F.; Kulkarni, V.T.; Lin, Z.; Ross, J.; Horwitz, L.; Kim, N.; Suter, L.G.; Lin, H.; Normand, S.-L.; et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: Retrospective cohort study. BMJ 2015, 350, h411. [Google Scholar] [CrossRef]
- Akyuz, A.; Mert, B.; Gur, D.O.; Efe, M.M.; Aykac, H.; Alpsoy, S.; Guzel, S. Association of Lower Serum Irisin Levels with Diabetes Mellitus: Irrespective of Coronary Collateral Circulation, And SYNTAX Score. North. Clin. Istanb. 2021, 8, 607–614. [Google Scholar] [CrossRef]
- El-Mottaleb, N.A.A.; Galal, H.M.; El Maghraby, K.M.; Gadallah, A.I. Serum irisin level in myocardial infarction patients with or without heart failure. Can. J. Physiol. Pharmacol. 2019, 97, 932–938. [Google Scholar] [CrossRef]
- Kawada, T. Serum Irisin and Diabetic Nephropathy in Patients with Diabetes Mellitus. Horm. Metab. Res. 2021, 53, 825. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, J.; Zhou, X.; Li, R. Irisin Pretreatment Protects Kidneys against Acute Kidney Injury Induced by Ischemia/Reperfusion via Upregulating the Expression of Uncoupling Protein 2. Biomed. Res. Int. 2020, 2020, 6537371. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, Q.; Zhong, J.; Xia, F.; Zheng, S.; Lu, J.; Deng, Y.; Hu, Y. Combination of melatonin and irisin ameliorates lipopolysaccharide-induced cardiac dysfunction through suppressing the Mst1-JNK pathways. J. Cell Physiol. 2020, 235, 6647–6659. [Google Scholar] [CrossRef]
- Yu, Q.; Li, G.; Li, J.; Sun, L.; Yang, Y.; Tao, L. Irisin Protects Cerebral Neurons from Hypoxia/Reoxygenation via Suppression of Apoptosis and Expression of Pro-Inflammatory Cytokines. Neuroimmunomodulation 2022, 29, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Hu, M.; Teng, T.; Yuan, Y.P.; Song, P.; Kong, C.Y.; Xu, S.C.; Ma, Z.G.; Tang, Q.Z. Fibronectin type III domain-containing 5 improves aging-related cardiac dysfunction in mice. Aging Cell 2022, 21, e13556. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; He, S.; Wang, J.; Gong, Q.; An, Y.; Li, H.; Chen, Y.; Li, G. Prediction of 10-year mortality using hs-CRP in Chinese people with hyperglycemia: Findings from the Da Qing diabetes prevention outcomes study. Diabetes Res. Clin. Pract. 2021, 173, 108668. [Google Scholar] [CrossRef]
- Packer, M. Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 873–878. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
| Variables | Entire Chronic HF Patient Cohort (n = 480) | Patients with ADHF (n = 106) | Patients without ADHF (n = 374) | p Value |
|---|---|---|---|---|
| Demographics and anthropomorphic parameters | ||||
| Age, year | 53 (40–67) | 55 (46–67) | 51 (41–63) | 0.062 |
| Male/female n (%) | 272 (56.7)/208 (43.3) | 62 (58.4)/44 (41.6) | 210 (56.2)/164 (43.8) | 0.124 |
| BMI, kg/m2 | 25.6 ± 2.78 | 25.8 ± 2.60 | 25.3 ± 2.52 | 0.782 |
| Waist circumference, cm | 96.7 ± 3.90 | 97.7 ± 3.70 | 95.3 ± 3.36 | 0.420 |
| WHR, units | 0.88 ± 0.07 | 0.89 ± 0.08 | 0.85 ± 0.04 | 0.823 |
| Comorbidities and CV risk factors | ||||
| Dyslipidemia, n (%) | 385 (80.2) | 84 (79.2) | 301 (80.5) | 0.781 |
| Hypertension, n (%) | 307(64.0) | 71 (66.9) | 236 (63.1) | 0.643 |
| Stable CAD, n (%) | 166 (34.6) | 42 (42.5) | 124 (33.2) | 0.024 |
| DCM, n (%) | 19 (4.0) | 6 (5.7) | 13 (3.5) | 0.042 |
| AF, n (%) | 105 (21.9) | 47 (44.3) | 58 (15.5) | 0.001 |
| Paroxysmal/persistent AF, n (%) | 56 (11.7) | 29 (27.3) | 27 (7.2) | 0.001 |
| Permanent AF, n (%) | 49 (10.2) | 18 (17.0) | 31 (8.3) | 0.012 |
| Smoking, n (%) | 196 (40.8) | 42 (39.6) | 154 (41.2) | 0.860 |
| Abdominal obesity, n (%) | 215 (44.8) | 48 (45.3) | 167 (44.7) | 0.823 |
| LV hypertrophy, n (%) | 382 (79.5) | 85 (80.2) | 297 (79.4) | 0.837 |
| CKD 1–3 grades, n (%) | 118 (24.6) | 39 (36.8) | 79 (21.1) | 0.012 |
| HF phenotypes | ||||
| HFpEF, n (%) | 212 (44.2) | 40 (37.7) | 172 (46.0) | 0.056 |
| HFmrEF, n (%) | 156 (32.5) | 35 (33.0) | 121 (32.4) | 0.523 |
| HFrEF, n (%) | 112 (23.3) | 31 (29.2) | 81 (21.7) | 0.073 |
| I/II HF NYHA class, n (%) | 281 (58.6) | 54 (50.9) | 227 (60.7) | 0.024 |
| III HF NYHA class, n (%) | 144 (30.0) | 35 (33.1) | 109 (29.1) | 0.468 |
| IV HF NYHA class, n (%) | 55 (11.4) | 17 (16.0) | 38 (10.2) | 0.001 |
| Hemodynamics performances | ||||
| SBP, mm Hg | 133 ± 8 | 132 ± 6 | 136 ± 7 | 0.621 |
| DBP, mm Hg | 77 ± 7 | 74 ± 5 | 79 ± 6 | 0.634 |
| LVEDV, mL | 162 (139–178) | 168 (158–179) | 160 (136–177) | 0.442 |
| LVESV, mL | 88 (59–97) | 93 (82–103) | 84 (57–94) | 0.012 |
| LVEF, % | 45 (34–57) | 44 (32–55) | 47 (36–67) | 0.050 |
| LVMMI, g/m2 | 138 ± 11 | 158 ± 13 | 129 ± 15 | 0.014 |
| LAVI, mL/m2 | 41 (33–52) | 45 (38–53) | 39 (32–47) | 0.010 |
| E/e`, unit | 11 ± 2 | 14 ± 3 | 13 ± 2 | 0.764 |
| Biochemistry parameters | ||||
| eGFR, mL/min/1.73 m2 | 74 ± 9 | 63 ± 8 | 81 ± 9 | 0.026 |
| HOMA-IR | 6.95 ± 1.9 | 7.76 ± 2.9 | 6.12± 1.9 | 0.524 |
| Fasting glucose, mmol/L | 6.20 ± 1.2 | 6.81 ± 1.5 | 6.03 ± 1.3 | 0.642 |
| HbA1c, % | 6.40 ± 0.14 | 6.52 ± 0.12 | 6.25 ± 0.15 | 0.758 |
| Creatinine, µmol/L | 98.7 ± 9.8 | 108.6 ± 11.5 | 77.4 ± 8.9 | 0.042 |
| TC, mmol/L | 5.90 ± 0.91 | 6.22 ± 0.80 | 5.74 ± 0.70 | 0.186 |
| HDL-C, mmol/L | 0.96 ± 0.15 | 0.97 ± 0.17 | 1.01 ± 0.15 | 0.48 |
| LDL-C, mmol/L | 3.10± 0.20 | 3.38 ± 0.10 | 2.80 ± 0.14 | 0.016 |
| TG, mmol/L | 1.52 ± 0.19 | 1.61 ± 0.12 | 1.39 ± 0.15 | 0.044 |
| Biomarkers | ||||
| hs-CRP, mg/L | 4.20 (2.51–7.10) | 4.35 (2.92–7.17) | 4.06 (2.41–6.37) | 0.641 |
| TNF-alpha, pg/mL | 2.95 (1.66–3.82) | 3.41 (2.79–4.02) | 2.39 (1.51–3.03) | 0.042 |
| NT-proBNP, pmol/mL | 1215 (562–2155) | 1719 (980–2457) | 1057 (570–2607) | 0.044 |
| Irisin, ng/mL | 5.64 (3.80–7.53) | 4.96 (3.14–6.85) | 7.95 (5.73–9.16) | 0.001 |
| Concomitant medications | ||||
| ACEIs, n (%) | 296 (61.7) | 61 (57.5) | 208 (55.6) | 0.541 |
| ARBs, n (%) | 72 (15.0) | 19 (18.0) | 53 (14.2) | 0.226 |
| ARNI, n (%) | 112 (23.3) | 26 (27.4) | 86 (23.0) | 0.211 |
| Beta-blockers, n (%) | 427 (89.0) | 89 (83.9) | 338 (90.4) | 0.052 |
| Ivabradine, n (%) | 93 (19.4) | 13 (12.3) | 80 (21.4) | 0.001 |
| Calcium channel blockers, n (%) | 131 (27.3) | 24 (22.6) | 107 (28.6) | 0.064 |
| MRA, n (%) | 147 (30.6) | 35 (33.0) | 112 (29.9) | 0.052 |
| Loop diuretics, n (%) | 383 (79.8) | 76 (71.7) | 307 (82.1) | 0.014 |
| Antiplatelet, n (%) | 166 (34.6) | 39 (36.8) | 127 (34.0) | 0.348 |
| Anticoagulants, n (%) | 105 (21.9) | 47 (44.3) | 58 (15.5) | 0.001 |
| Metformin, n (%) | 480 (100.0) | 106 (100.0) | 374 (100.0) | 1.000 |
| SGLT2 inhibitors, n (%) | 429 (89.4) | 92 (86.8) | 337 (90.1) | 0.218 |
| Statins, n (%) | 453 (94.4) | 93 (87.7) | 360 (96.3) | 0.048 |
| Variables | Patient Groups | Baseline | 52 weeks | Δ% | p Value |
|---|---|---|---|---|---|
| BMI, kg/m2 | Entire group | 25.6 ± 2.78 | 24.3 ± 1.92 | −5.10 | 0.52 |
| ADHF | 25.8 ± 2.60 | 25.6 ± 2.90 | −0.80 | 0.76 | |
| Non-ADHF | 25.3 ± 2.52 | 23.8 ± 2.47 | −5.90 | 0.13 | |
| SBP, mm Hg | Entire group | 133 ± 8 | 129 ± 6 | −3.00 | 0.46 |
| ADHF | 132 ± 6 | 130 ± 5 | −1.52 | 0.52 | |
| Non-ADHF | 136 ± 7 | 131 ± 6 | −3.68 | 0.42 | |
| DBP, mm Hg | Entire group | 77 ± 7 | 75 ± 5 | −2.60 | 0.42 |
| ADHF | 74 ± 5 | 73 ± 6 | −1.40 | 0.48 | |
| Non-ADHF | 79 ± 6 | 76 ± 5 | −3.80 | 0.44 | |
| LVEDV, mL | Entire group | 162 (139–178) | 160 (150–167) | −1.20 | 0.54 |
| ADHF | 168 (158–179) | 170 (156–182) | +1.20 | 0.18 | |
| Non-ADHF | 160 (136–177) | 156 (135–171) | −2.50 | 0.14 | |
| LVESV, mL | Entire group | 88 (59–97) | 82 (78–86) | −6.90 | 0.04 |
| ADHF | 93 (82–103) | 92 (80–101) | −1.12 | 0.56 | |
| Non-ADHF | 84 (57–94) | 78 (55–92) | −7.10 | 0.04 | |
| LVEF, % | Entire group | 45 (34–57) | 49 (44–55) | +8.80 | 0.05 |
| ADHF | 44 (32–55) | 45 (31–57) | +2.27 | 0.38 | |
| Non-ADHF | 47 (36–67) | 52 (38–69) | 10.60 | 0.05 | |
| LVMMI, g/m2 | Entire group | 138 ± 11 | 141 ± 5 | +2.20 | 0.64 |
| ADHF | 158 ± 13 | 162 ± 11 | +2.50 | 0.36 | |
| Non-ADHF | 129 ± 15 | 130 ± 13 | +0.80 | 0.82 | |
| LAVI, mL/m2 | Entire group | 41 (33–52) | 40 (34–47) | −2.40 | 0.61 |
| ADHF | 45 (38–53) | 46 (40–52) | +2.20 | 0.43 | |
| Non-ADHF | 39 (32–47) | 37 (33–42) | −5.12 | 0.14 | |
| E/e’, unit | Entire group | 11 ± 2.0 | 10 ± 1.5 | −9.00 | 0.52 |
| ADHF | 14 ± 3 | 15 ± 4 | +7.10 | 0.62 | |
| Non-ADHF | 13 ± 2 | 11 ± 3 | −15.40 | 0.16 | |
| eGFR, mL/min/1.73 m2 | Entire group | 74 ± 9.0 | 76 ± 3.0 | +2.70 | 0.46 |
| ADHF | 63 ± 8 | 59 ± 5 | −6.30 | 0.05 | |
| Non-ADHF | 81 ± 9 | 89 ± 6 | +8.60 | 0.24 | |
| Fasting glucose, mmol/L | Entire group | 6.20 ± 1.2 | 5.72 ± 1.1 | −7.74 | 0.28 |
| ADHF | 6.81 ± 1.5 | 6.89 ± 1.4 | +1.00 | 0.64 | |
| Non-ADHF | 6.03 ± 1.3 | 5.43 ± 1.5 | −10.0 | 0.24 | |
| HbA1c, % | Entire group | 6.40 ± 0.14 | 6.47 ± 0.03 | −1.74 | 0.31 |
| ADHF | 6.52 ± 0.12 | 6.64 ± 0.15 | +1.90 | 0.12 | |
| Non-ADHF | 6.25 ± 0.15 | 6.09 ± 0.13 | −2.24 | 0.76 | |
| Creatinine, µmol/L | Entire group | 98.7 ± 9.8 | 114.7 ± 7.5 | +13.90 | 0.20 |
| ADHF | 108.6 ± 11.5 | 138.2 ± 14.1 | +21.70 | 0.04 | |
| Non-ADHF | 77.4 ± 8.9 | 80.5 ± 7.5 | +3.90 | 0.72 | |
| hs-CRP, mg/L | Entire group | 4.20 (2.51–7.10) | 4.82 (2.39–7.31) | +13.1 | 0.026 |
| ADHF | 4.35 (2.92–7.17) | 5.70 (3.44–8.20) | +23.60 | 0.042 | |
| Non-ADHF | 4.06 (2.41–6.37) | 3.85 (2.27–5.17) | −5.17 | 0.052 | |
| TNF-alpha, pg/mL | Entire group | 2.95 (1.66–3.82) | 2.97 (1.70–3.90) | +0.70 | 0.72 |
| ADHF | 3.41 (2.79–4.02) | 3.67 (2.90–4.22) | +7.60 | 0.18 | |
| Non-ADHF | 2.39 (1.51–3.03) | 2.21 (1.38–3.01) | −7.50 | 0.042 | |
| NT-proBNP, pmol/mL | Entire group | 1215 (562–2155) | 1296 (672–1935) | +6.60 | 0.10 |
| ADHF | 1719 (980–2457) | 2142 (1170–3275) | +24.60 | 0.02 | |
| Non-ADHF | 1057 (570–2607) | 887 (460–1215) | −16.1 | 0.04 | |
| Irisin, ng/mL | Entire group | 5.64 (3.80–7.53) | 5.81 (4.20–7.22) | +3.00 | 0.05 |
| ADHF | 4.96 (3.14–6.85) | 4.15 (2.83–5.58) | −0.81 | 0.26 | |
| Non-ADHF | 7.95 (5.73–9.16) | 8.22 (6.40–10.12) | +3.30 | 0.04 |
| Variables | Dependent Variable: ADHF | |||||
|---|---|---|---|---|---|---|
| Univariate Log Regression | Multivariate Log Regression | |||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Irisin (<7.85 ng/mL vs. ≥7.85 ng/mL) | 1.24 | 1.08–1.46 | 0.001 | 1.20 | 1.08–1.45 | 0.001 |
| NT-proBNP (>1215 pmol/mL vs. ≤1215 pmol/mL) | 1.16 | 1.03–1.37 | 0.001 | 1.18 | 1.02–1.35 | 0.001 |
| TNF-alpha (>2.95 pg/mL vs. ≤2.95 pg/mL) | 1.06 | 1.02–1.11 | 0.012 | 1.05 | 1.00–1.08 | 0.112 |
| hs-CRP (> 4.2 mg/L vs. ≤4.2 mg/L) | 1.04 | 1.00–1.10 | 0.064 | - | ||
| eGFR (<74 mL/min/1.73 m2 vs. ≥74 mL/min/1.73 m2). | 0.94 | 0.86–1.10 | 0.924 | - | ||
| LV hypertrophy (presence vs. absent) | 1.10 | 1.01–1.18 | 0.044 | 1.05 | 1.00–1.12 | 0.124 |
| AF (presence vs. absent) | 1.12 | 1.00–1.25 | 0.066 | - | ||
| E/e’ (>11 units vs. ≤11 units) | 1.03 | 0.99–1.06 | 0.682 | - | ||
| LVEF (<45% vs. ≥45%) | 1.09 | 1.02–1.17 | 0.042 | 1.09 | 1.00–1.20 | 0.144 |
| LAVI (>41 mL/m2 vs. ≤41 mL/m2) | 1.12 | 1.03–1.21 | 0.026 | 1.07 | 1.00–1.13 | 0.148 |
| ARBs (presence vs. absent) | 0.97 | 0.93–1.05 | 0.645 | - | ||
| ARNI (presence vs. absent) | 0.95 | 0.91–1.01 | 0.144 | - | ||
| SGLT2i (presence vs. absent) | 0.94 | 0.90–1.02 | 0.268 | - | ||
| Predictive Models | Dependent Variable: ADHF | |||||
|---|---|---|---|---|---|---|
| AUC | NRI | IDI | ||||
| M (95% CI) | p Value | M (95% CI) | p Value | M (95% CI) | p Value | |
| Model 1 (NT-proBNP > 1215 pg/mL) | 0.840 (0.791–0.865) | - | Reference | - | Reference | - |
| Model 2 (irisin < 7.85 ng/mL) | 0.869 (0.800–0.937) | 0.001 | 0.68 (0.63–0.72) | 0.012 | 0.57 (0.53–0.62) | 0.014 |
| Model 3 (NT-proBNP > 1215 pg/mL + irisin < 7.85 ng/mL) | 0.872 (0.808–0.942) | 0.001 | 0.69 (0.65–0.74) | 0.001 | 0.63 (0.59–0.68) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezin, A.A.; Obradovic, A.B.; Fushtey, I.M.; Berezina, T.A.; Lichtenauer, M.; Berezin, A.E. Low Plasma Levels of Irisin Predict Acutely Decompensated Heart Failure in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. J. Cardiovasc. Dev. Dis. 2023, 10, 136. https://doi.org/10.3390/jcdd10040136
Berezin AA, Obradovic AB, Fushtey IM, Berezina TA, Lichtenauer M, Berezin AE. Low Plasma Levels of Irisin Predict Acutely Decompensated Heart Failure in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. Journal of Cardiovascular Development and Disease. 2023; 10(4):136. https://doi.org/10.3390/jcdd10040136
Chicago/Turabian StyleBerezin, Alexander A., Anica Babic Obradovic, Ivan M. Fushtey, Tetiana A Berezina, Michael Lichtenauer, and Alexander E Berezin. 2023. "Low Plasma Levels of Irisin Predict Acutely Decompensated Heart Failure in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure" Journal of Cardiovascular Development and Disease 10, no. 4: 136. https://doi.org/10.3390/jcdd10040136
APA StyleBerezin, A. A., Obradovic, A. B., Fushtey, I. M., Berezina, T. A., Lichtenauer, M., & Berezin, A. E. (2023). Low Plasma Levels of Irisin Predict Acutely Decompensated Heart Failure in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. Journal of Cardiovascular Development and Disease, 10(4), 136. https://doi.org/10.3390/jcdd10040136








