Prevalence and Characteristics of Inspiration-Induced Negative Left Atrial Pressure during Pulmonary Vein Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
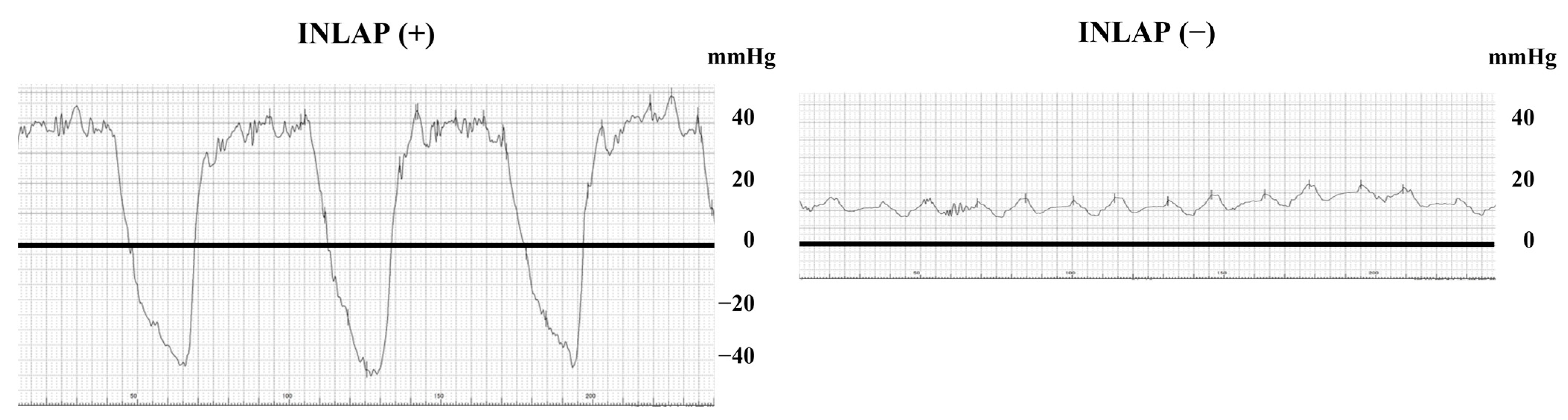
2.2. Measurement of Intracardiac Pressure and the Definition of INLAP and Inspiration-Induced Negative Right Atrial Pressure
2.3. Electrophysiological Study and CA
2.4. Statistical Analysis
3. Results
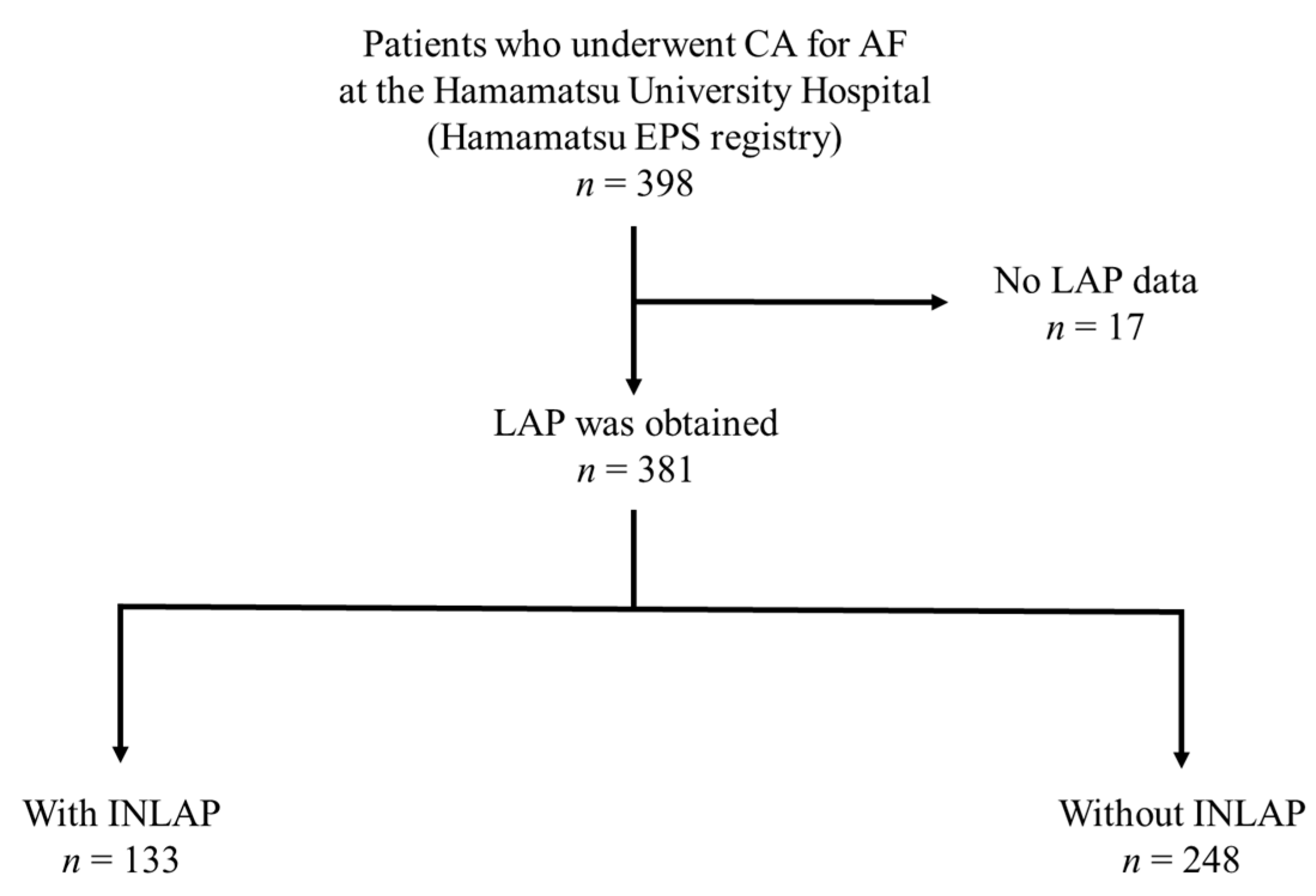
3.1. Subject Characteristics
3.2. Feature of Patients with INLAP
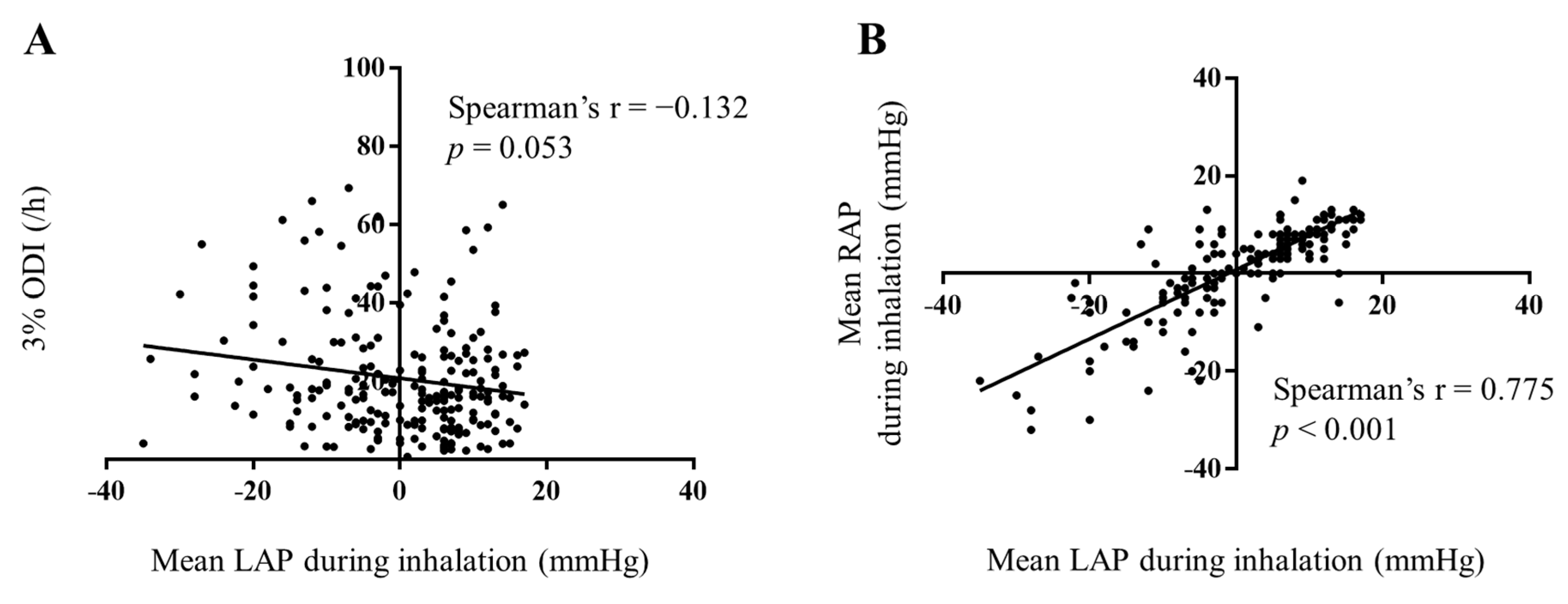
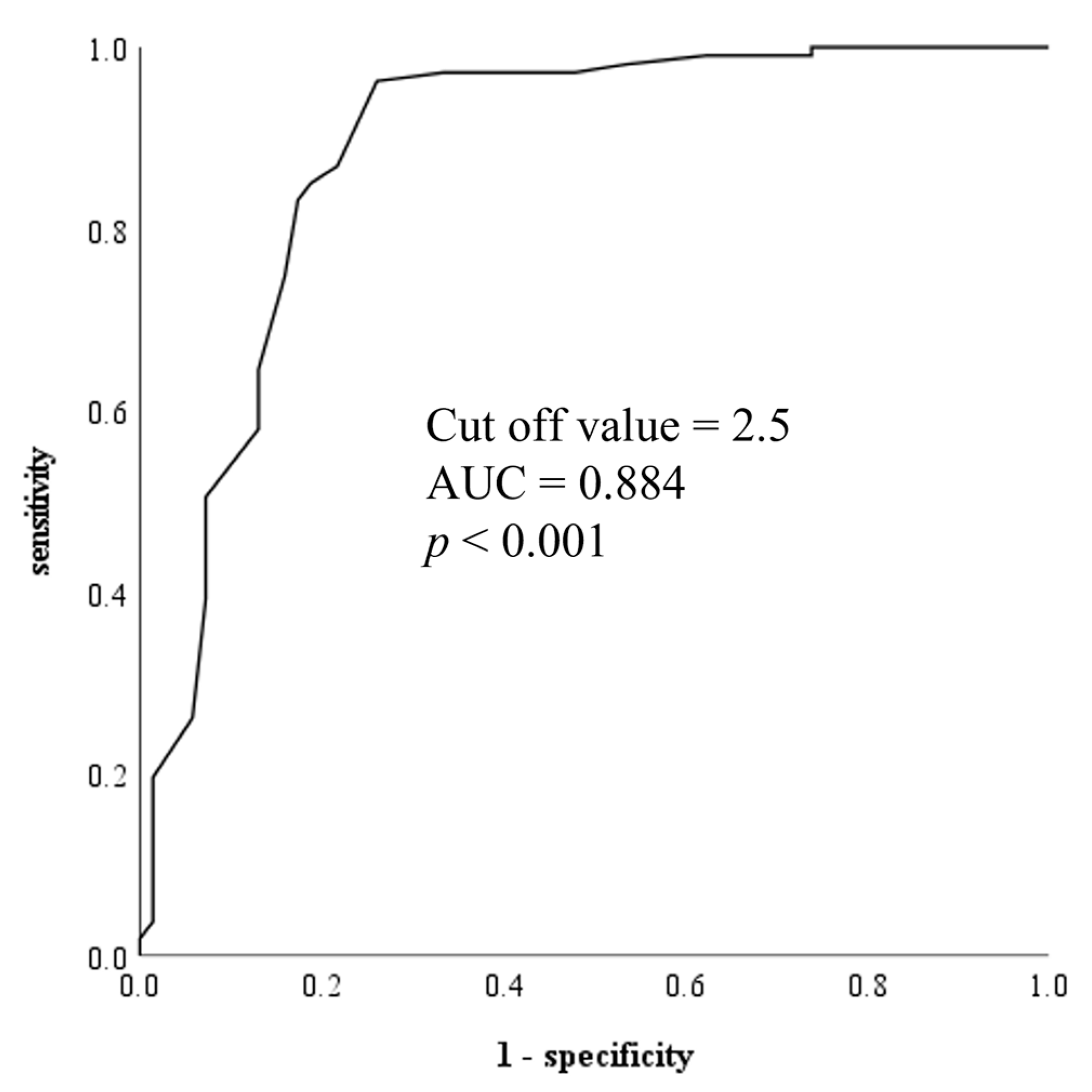
3.3. Prediction of INLAP
4. Discussion
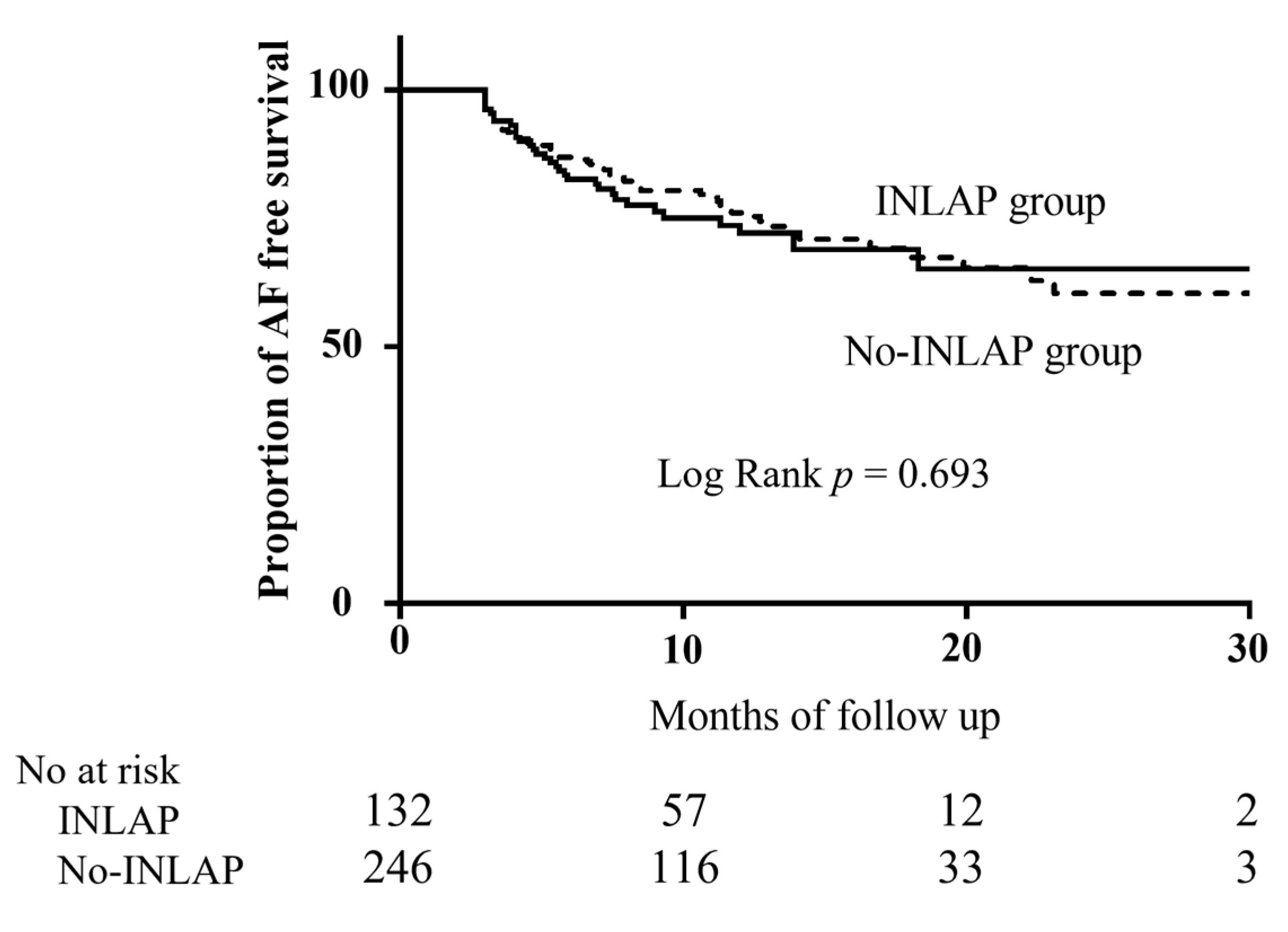
4.1. Main Findings
4.2. Clinical Impact and Importance of Air Embolism
4.3. Possible Mechanisms of INLAP
4.4. Prevention of Complications Due to INLAP
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Anstrom, K.J.; Sheng, S.; Piccini, J.P.; Baloch, K.N.; Monahan, K.H.; Daniels, M.R.; Bahnson, T.D.; Poole, J.E.; Rosenberg, Y.; et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019, 321, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Hakalahti, A.; Biancari, F.; Nielsen, J.C.; Raatikainen, M.J. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: Systematic review and meta-analysis. Europace 2015, 17, 370–378. [Google Scholar] [CrossRef]
- Deshmukh, A.; Patel, N.J.; Pant, S.; Shah, N.; Chothani, A.; Mehta, K.; Grover, P.; Singh, V.; Vallurupalli, S.; Savani, G.T.; et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93 801 procedures. Circulation 2013, 128, 2104–2112. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Hasegawa, K.; Mukai, M.; Ishikawa, E.; Aoyama, D.; Nodera, M.; Kaseno, K.; Ishida, K.; Uzui, H.; Tada, H. Clinically manifesting air embolisms in cryoballoon ablation: Can novel water buckets reduce the risk? JACC Clin. Electrophysiol. 2020, 6, 1067–1072. [Google Scholar] [CrossRef]
- Franzen, O.W.; Klemm, H.; Hamann, F.; Koschyk, D.; Von Kodolitsch, Y.; Weil, J.; Meinertz, T.; Baldus, S. Mechanisms underlying air aspiration in patients undergoing left atrial catheterization. Catheter. Cardiovasc. Interv. 2008, 71, 553–558. [Google Scholar] [CrossRef]
- Aksu, T.; Guler, T.E.; Yalin, K.; Golcuk, S.E.; Ozcan, K.S.; Guler, N. A novel deep inspiration maneuver for difficult transseptal puncture. Am. J. Cardiol. 2017, 119, 428–433. [Google Scholar] [CrossRef]
- Thiyagarajah, A.; Kadhim, K.; Lau, D.H.; Emami, M.; Linz, D.; Khokhar, K.; Munawar, D.A.; Mishima, R.; Malik, V.; O’Shea, C.; et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: A systematic review and meta-analysis. Circ. Arrhythm. Electrophysiol. 2019, 12, e007005. [Google Scholar] [CrossRef]
- Seitz, J.; Bars, C.; Théodore, G.; Beurtheret, S.; Lellouche, N.; Bremondy, M.; Ferracci, A.; Faure, J.; Penaranda, G.; Yamazaki, M.; et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: A wholly patient-tailored approach. J. Am. Coll. Cardiol. 2017, 69, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Yalin, K.; Guler, T.E.; Bozyel, S.; Heeger, C.H.; Tilz, R.R. Acute procedural complications of cryoballoon ablation: A comprehensive review. J. Atr. Fibrillation 2019, 12, 2208. [Google Scholar] [PubMed]
- Tsukahara, K.; Oginosawa, Y.; Fujino, Y.; Ohe, H.; Yamagishi, Y.; Iwataki, M.; Sonoda, S.; Kohno, R.; Otsuji, Y.; Abe, H. Prevention of serious air embolism during cryoballoon ablation; risk assessment of air intrusion into the sheath by catheter selection and change in intrathoracic pressure: An ex vivo study. J. Cardiovasc. Electrophysiol. 2019, 30, 2944–2949. [Google Scholar] [CrossRef]
- Dinov, B.; Kosiuk, J.; Kircher, S.; Bollmann, A.; Acou, W.-J.; Arya, A.; Hindricks, G.; Rolf, S. Impact of metabolic syndrome on left atrial electroanatomical remodeling and outcomes after radiofrequency ablation of nonvalvular atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2014, 7, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; Mark, A.L.; Zavala, D.C.; Abboud, F.M. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J. Appl. Physiol. 1989, 67, 2095–2100. [Google Scholar] [CrossRef]
- Ng, C.Y.; Liu, T.; Shehata, M.; Stevens, S.; Chugh, S.S.; Wang, X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011, 108, 47–51. [Google Scholar] [CrossRef]
- Eastwood, P.R.; Platt, P.R.; Shepherd, K.; Maddison, K.; Hillman, D.R. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 2005, 103, 470–477. [Google Scholar] [CrossRef]
- Norton, J.R.; Ward, D.S.; Karan, S.; Voter, W.A.; Palmer, L.; Varlese, A.; Rackovsky, O.; Bailey, P. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology 2006, 104, 1155–1164. [Google Scholar] [CrossRef]
- Iwasaki, Y.-K.; Fujimoto, Y.; Oka, E.; Hagiwara, K.I.; Takahashi, K.; Tsuboi, I.; Hayashi, H.; Yodogawa, K.; Hayashi, M.; Miyauchi, Y.; et al. Esophageal pressure monitoring for airway management during catheter ablation of atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2021, 33, 100771. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Suzuki, A.; Usui, M.; Yoshikawa, S.; Watanabe, S.; Maeno, R.; Kujiraoka, H.; Sato, K.; Goya, M.; Sasano, T. Clinical effect of adaptive servo-ventilation on left atrial pressure during catheter ablation in sedated patients with atrial fibrillation. Circ. J. 2021, 85, 1321–1328. [Google Scholar] [CrossRef]
- Yokoyama, M.; Tokuda, M.; Tokutake, K.; Sato, H.; Oseto, H.; Yokoyama, K.; Kato, M.; Narui, R.; Tanigawa, S.-I.; Yamashita, S.; et al. Effect of air removal with extracorporeal balloon inflation on incidence of asymptomatic cerebral embolism during cryoballoon ablation of atrial fibrillation: A prospective randomized study. Int. J. Cardiol. Heart Vasc. 2022, 40, 101020. [Google Scholar] [CrossRef] [PubMed]
- Sielski, J.; Grabowska, U.; Kaziród-Wolski, K.; Matejko, M. Correlation analysis of the relationship between B-type natriuretic peptide and selected echocardiographic parameters in patients with permanent pacemakers. Med. Stud./Stud. Med. 2015, 31, 241–248. [Google Scholar] [CrossRef]
| All (n = 381) | INLAP (n = 133) | No INLAP (n = 248) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 63.9 ± 10.8 | 65.2 ± 9.3 | 63.2 ± 11.5 | 0.077 |
| Female, n (%) | 76 (19.9) | 24 (18.0) | 52 (21.0) | 0.496 |
| Body mass index, kg/m2 | 24.6 ± 4.1 | 25.0 ± 4.4 | 24.4 ± 4.0 | 0.173 |
| Current smoker, n (%) | 41 (12.0) | 17 (13.8) | 24 (11.0) | 0.443 |
| Use of alcohol, n (%) | 194 (56.6) | 72 (58.5) | 122 (55.5) | 0.581 |
| Paroxysmal AF, n (%) | 216 (56.7) | 77 (57.9) | 139 (56.0) | 0.729 |
| History of prior PVI, n (%) | 40 (10.5) | 5 (3.8) | 35 (14.1) | 0.002 |
| Clinical characteristics | ||||
| HT, n (%) | 198 (52.0) | 75 (56.4) | 123 (49.6) | 0.206 |
| Dyslipidemia, n (%) | 98 (25.7) | 36 (27.1) | 62 (25.0) | 0.660 |
| DM, n (%) | 64 (16.8) | 31 (23.3) | 33 (13.3) | 0.013 |
| Stroke, n (%) | 38 (10.0) | 16 (12.0) | 22 (8.9) | 0.327 |
| Heart failure, n (%) | 101 (26.5) | 36 (27.1) | 65 (26.2) | 0.856 |
| 3% ODI | 17.0 (9.5–26.7) | 18.6 (11.2–31.1) | 15.7 (8.1–25.3) | 0.009 |
| CHADS2 score, points | 1.3 ± 1.2 | 1.5 ± 1.2 | 1.3 ± 1.2 | 0.071 |
| CHA2DS2-Vasc score, points | 2.2 ± 1.5 | 2.3 ± 1.5 | 2.1 ± 1.6 | 0.043 |
| Medications | ||||
| ACE-Is / ARBs, n (%) | 137 (36.0) | 54 (40.6) | 83 (33.5) | 0.167 |
| Beta-blockers, n (%) | 225 (59.1) | 83 (62.4) | 142 (57.3) | 0.330 |
| Type I AADs, n (%) | 153 (40.2) | 45 (33.8) | 108 (43.5) | 0.065 |
| Type III AADs, n (%) | 31 (8.1) | 9 (6.8) | 22 (8.9) | 0.474 |
| DOAC, n (%) | 350 (88.5) | 126 (94.7) | 224 (90.3) | 0.133 |
| Laboratory data | ||||
| Hb, g/dL | 14.2 ± 1.6 | 14.2 ± 1.6 | 14.2 ± 1.6 | 0.957 |
| eGFR, ml/min/1.73 m2 | 63.7 ± 16.9 | 62.7 ± 13.8 | 64.2 ± 18.3 | 0.462 |
| NT-proBNP, pg/ml | 243.0 (72.0–610.0) | 240.0 (82.0–683.0) | 249.0 (70.0–575.0) | 0.409 |
| Echocardiography | ||||
| LVEF, % | 62.7 ± 10.2 | 62.4 ± 11.0 | 62.8 ± 9.9 | 0.765 |
| LAD, mm | 39.1 ± 7.1 | 39.8 ± 7.3 | 38.7 ± 7.0 | 0.159 |
| LVDd, mm | 47.7 ± 5.9 | 48.0 ± 5.9 | 47.5 ± 5.9 | 0.434 |
| LVDs, mm | 31.3 ± 6.3 | 31.5 ± 6.6 | 31.2 ± 6.2 | 0.683 |
| Computed tomography | ||||
| LA volume index, ml/m2 | 71.5 ± 39.1 | 70.6 ± 24.2 | 72.1 ± 45.1 | 0.727 |
| All (n = 381) | INLAP (n = 133) | No INLAP (n = 248) | p-Value | |
|---|---|---|---|---|
| INRAP, n (%) | 55 (31.2) | 51 (73.9) | 4 (3.7) | < 0.001 |
| RA pressure | ||||
| Inspiration period, mmHg | 1.2 ± 9.3 | −6.2 ± 9.9 | 6.1 ± 4.5 | < 0.001 |
| Expiration period, mmHg | 11.4 ± 5.6 | 12.0 ± 7.0 | 11.1 ± 4.5 | 0.273 |
| LA pressure | ||||
| Inspiration period, mmHg | 1.5 ± 10.4 | −10.1 ± 8.0 | 7.8 ± 4.4 | < 0.001 |
| Expiration period, mmHg | 15.9 ± 6.5 | 17.9 ± 7.3 | 14.9± 5.8 | < 0.001 |
| IVC pressure | ||||
| Inspiration period, mmHg | 5.8 ± 5.8 | 2.8 ± 6.8 | 7.9 ± 3.9 | < 0.001 |
| Expiration period, mmHg | 11.2 ± 6.0 | 11.5 ± 7.7 | 11.0 ± 4.5 | 0.539 |
| Low voltage zones, n (%) | 77 (20.2) | 33 (24.8) | 44 (17.7) | 0.101 |
| Type of ablation technology | ||||
| Radiofrequency catheters, n (%) | 106 (27.8) | 43 (32.3) | 63 (25.4) | 0.150 |
| Cryoballoons and laser balloons, n (%) | 275 (72.2) | 90 (67.7) | 185 (74.6) | 0.150 |
| Additional ablation, n (%) | ||||
| CTI, n (%) | 179 (47.0) | 50 (37.6) | 129 (52.0) | 0.007 |
| Bottom line, n (%) | 123 (32.3) | 39 (29.3) | 84 (33.9) | 0.365 |
| Mitral isthmus, n (%) | 25 (6.6) | 4 (3.0) | 21 (8.5) | 0.040 |
| Complications | ||||
| Air embolism, n (%) | 4 (1.0) | 4 (3.0) | 0 (0.0) | 0.014 |
| Diaphragmatic paralysis, n (%) | 9 (2.4) | 4 (3.1) | 5 (2.0) | 0.725 |
| Bleeding from puncture site, n (%) | 6 (1.6) | 2 (1.5) | 4 (1.6) | 1.000 |
| Cardiac tamponade, n (%) | 7 (1.8) | 5 (3.8) | 2 (0.8) | 0.053 |
| Sedation level | ||||
| RASS | −4.0 (−4.0–−5.0) | −4.0 (−4.0–−5.0) | −4.0 (−4.0–−4.0) | 0.131 |
| BIS | 57.0 (48.0–70.0) | 58.5 (50.8–73.0) | 56.0 (47.0–68.0) | 0.098 |
| Procedure time, minute | 207.3 ± 83.8 | 208.5 ± 81.2 | 206.6 ± 85.3 | 0.834 |
| Fluoroscopic time, minute | 53.4 ± 34.8 | 50.5 ± 32.4 | 54.9 ± 35.9 | 0.234 |
| Variable | Beta | Standard Error | T Value | p-Value |
|---|---|---|---|---|
| (Constant) | 1.785 | 6.358 | 0.281 | 0.779 |
| Age | 0.002 | 0.062 | 0.034 | 0.973 |
| BMI | 0.201 | 0.158 | 1.267 | 0.207 |
| DM | 0.954 | 1.524 | 0.626 | 0.532 |
| INRAP | −16.795 | 1.238 | −13.571 | < 0.001 |
| 3% ODI | −0.780 | 0.876 | −0.890 | 0.375 |
| History of prior PVI | 0.063 | 2.225 | 0.028 | 0.978 |
| R2 = 0.532 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikoma, T.; Naruse, Y.; Kaneko, Y.; Sakakibara, T.; Narumi, T.; Sano, M.; Mogi, S.; Suwa, K.; Ohtani, H.; Saotome, M.; et al. Prevalence and Characteristics of Inspiration-Induced Negative Left Atrial Pressure during Pulmonary Vein Isolation. J. Cardiovasc. Dev. Dis. 2023, 10, 101. https://doi.org/10.3390/jcdd10030101
Ikoma T, Naruse Y, Kaneko Y, Sakakibara T, Narumi T, Sano M, Mogi S, Suwa K, Ohtani H, Saotome M, et al. Prevalence and Characteristics of Inspiration-Induced Negative Left Atrial Pressure during Pulmonary Vein Isolation. Journal of Cardiovascular Development and Disease. 2023; 10(3):101. https://doi.org/10.3390/jcdd10030101
Chicago/Turabian StyleIkoma, Takenori, Yoshihisa Naruse, Yutaro Kaneko, Tomoaki Sakakibara, Taro Narumi, Makoto Sano, Satoshi Mogi, Kenichiro Suwa, Hayato Ohtani, Masao Saotome, and et al. 2023. "Prevalence and Characteristics of Inspiration-Induced Negative Left Atrial Pressure during Pulmonary Vein Isolation" Journal of Cardiovascular Development and Disease 10, no. 3: 101. https://doi.org/10.3390/jcdd10030101
APA StyleIkoma, T., Naruse, Y., Kaneko, Y., Sakakibara, T., Narumi, T., Sano, M., Mogi, S., Suwa, K., Ohtani, H., Saotome, M., Urushida, T., & Maekawa, Y. (2023). Prevalence and Characteristics of Inspiration-Induced Negative Left Atrial Pressure during Pulmonary Vein Isolation. Journal of Cardiovascular Development and Disease, 10(3), 101. https://doi.org/10.3390/jcdd10030101






