Modified Sequential Organ Failure Assessment Score vs. Early Warning Scores in Prehospital Care to Predict Major Adverse Cardiac Events in Acute Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Setting
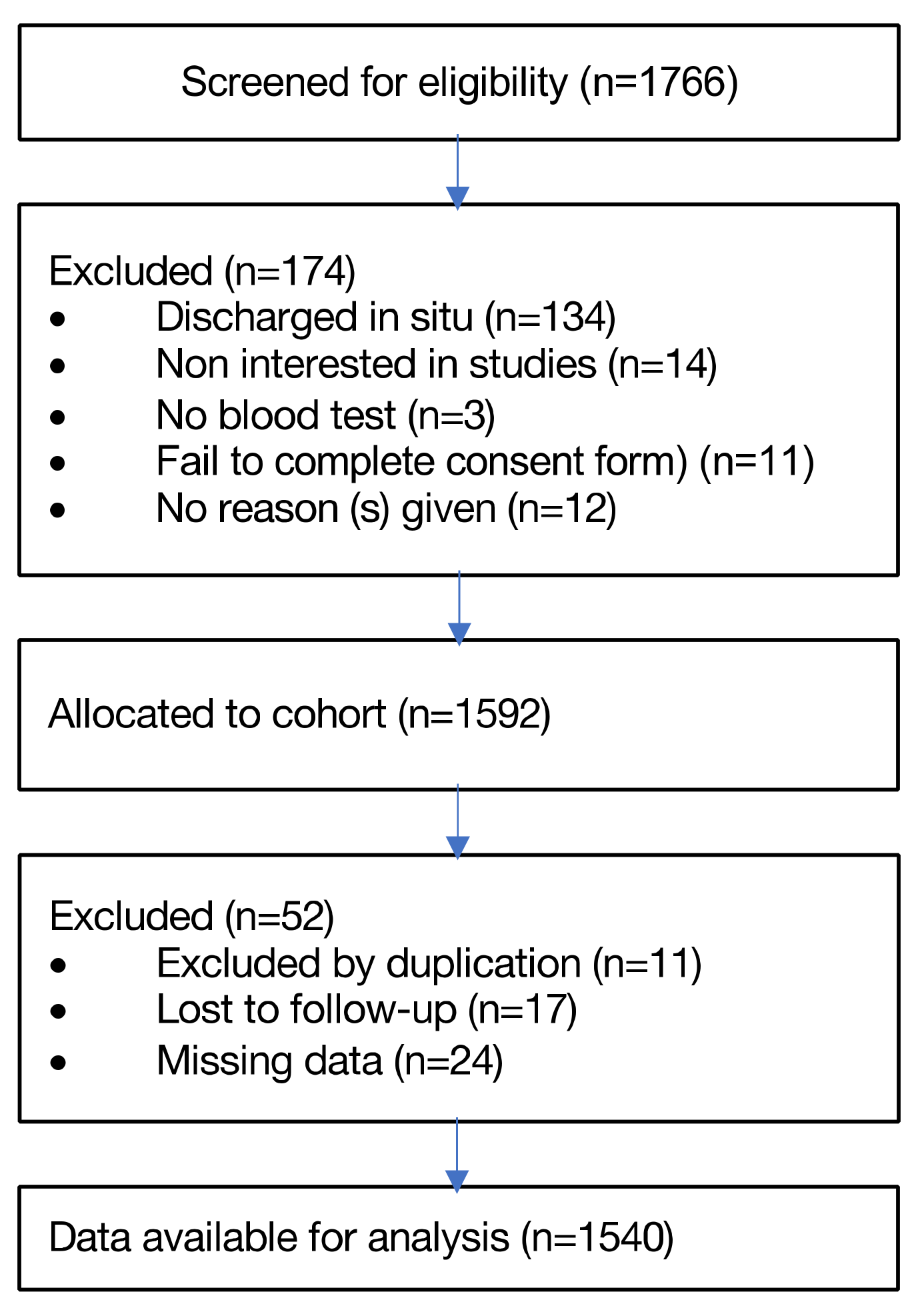
2.2. Participants
2.3. Outcomes
2.4. Data Sources and Predictors
2.5. Statistical Analysis
2.6. Ethic Statements
3. Results
3.1. Baseline Characteristics
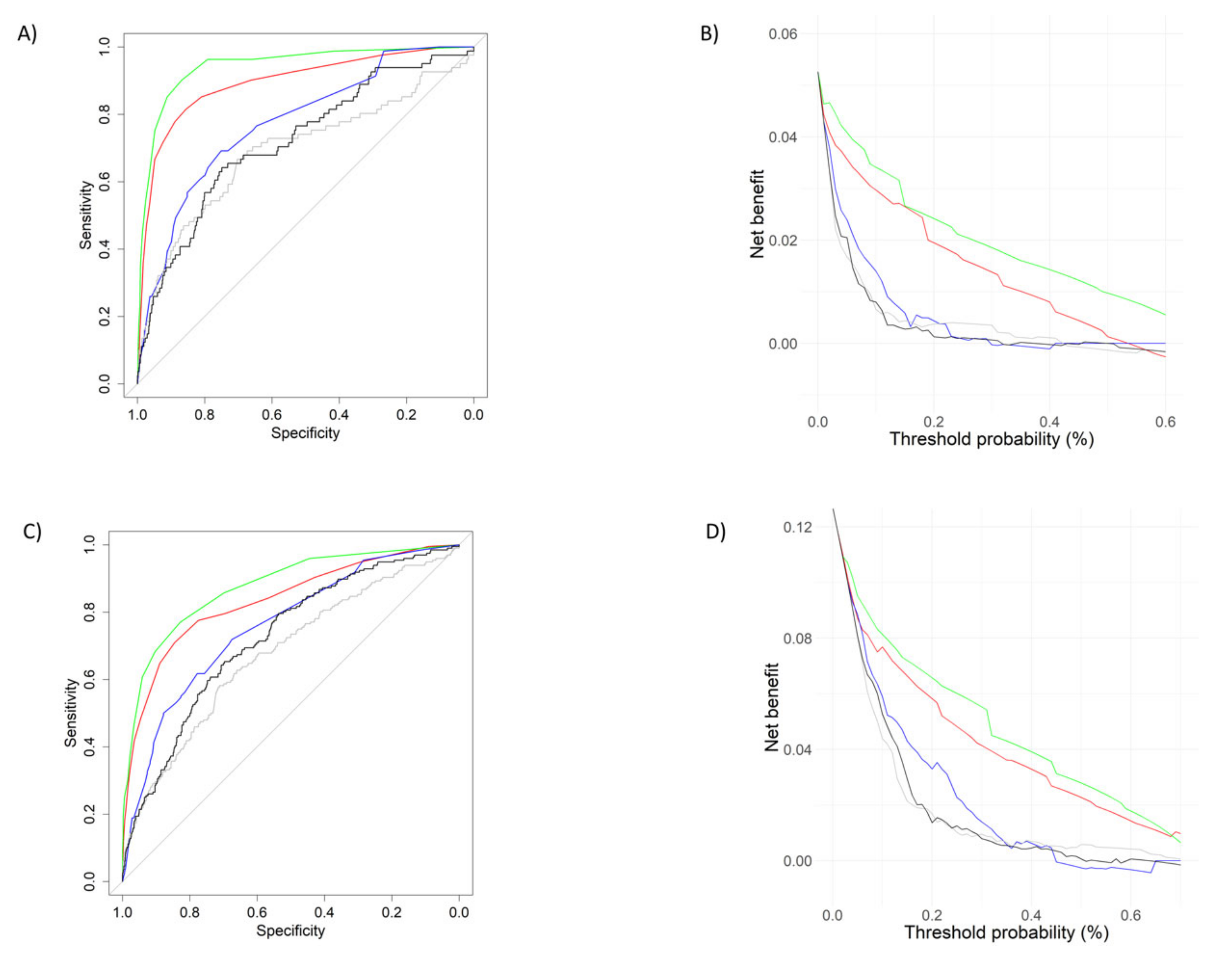
3.2. mSOFA Performance vs. Early Warning Scores
3.3. mSOFA according to Different Confusion Factors
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downey, C.L.; Tahir, W.; Randell, R.; Brown, J.M.; Jayne, D.G. Strengths and Limitations of Early Warning Scores: A Systematic Review and Narrative Synthesis. Int. J. Nurs. Stud. 2017, 76, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Nannan Panday, R.S.; Minderhoud, T.C.; Alam, N.; Nanayakkara, P.W.B. Prognostic Value of Early Warning Scores in the Emergency Department (ED) and Acute Medical Unit (AMU): A Narrative Review. Eur. J. Intern. Med. 2017, 45, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Cho, K.J.; Kwon, O.; Kwon, J.M.; Jeon, K.H.; Park, H.; Lee, Y.; Park, J.; Oh, B.H. Artificial Intelligence Algorithm to Predict the Need for Critical Care in Prehospital Emergency Medical Services. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Von Vopelius-Feldt, J.; Morris, R.W.; Benger, J. The Effect of Prehospital Critical Care on Survival Following Out-of-Hospital Cardiac Arrest: A Prospective Observational Study. Resuscitation 2020, 146, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Miró, Ò.; Hazlitt, M.; Escalada, X.; Llorens, P.; Gil, V.; Martín-Sánchez, F.J.; Harjola, P.; Rico, V.; Herrero-Puente, P.; Jacob, J.; et al. Effects of the Intensity of Prehospital Treatment on Short-Term Outcomes in Patients with Acute Heart Failure: The SEMICA-2 Study. Clin. Res. Cardiol. 2018, 107, 347–361. [Google Scholar] [CrossRef]
- Stopyra, J.P.; Snavely, A.C.; Ashburn, N.P.; O’Neill, J.; Paradee, B.E.; Hehl, B.; Vorrie, J.; Wells, M.; Nelson, R.D.; Hendley, N.W.; et al. Performance of Prehospital Use of Chest Pain Risk Stratification Tools: The RESCUE Study. Prehospital Emerg. Care 2022, 1–6. [Google Scholar] [CrossRef]
- Wibring, K.; Lingman, M.; Herlitz, J.; Ashfaq, A.; Bång, A. Development of a Prehospital Prediction Model for Risk Stratification of Patients with Chest Pain. American Journal of Emergency Medicine 2022, 51, 26–31. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA Score—Development, Utility and Challenges of Accurate Assessment in Clinical Trials. Crit Care 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Martín-Rodríguez, F.; Sanz-García, A.; del Pozo Vegas, C.; Ortega, G.J.; Castro Villamor, M.A.; López-Izquierdo, R. Time for a Prehospital-Modified Sequential Organ Failure Assessment Score: An Ambulance–Based Cohort Study. Am. J. Emerg. Med. 2021, 49, 331–337. [Google Scholar] [CrossRef]
- Baradari, A.G.; Sharifi, H.; Firouzian, A.; Daneshiyan, M.; Aarabi, M.; Talebiyan Kiakolaye, Y.; Nouraei, S.M.; Kiasari, A.Z.; Habibi, M.R.; Zeydi, A.E.; et al. Comparison of Proposed Modified and Original Sequential Organ Failure Assessment Scores in Predicting ICU Mortality: A Prospective, Observational, Follow-Up Study. Scientifica 2016, 2016, 7379325. [Google Scholar] [CrossRef]
- Rahmatinejad, Z.; Reihani, H.; Tohidinezhad, F.; Rahmatinejad, F.; Peyravi, S.; Pourmand, A.; Abu-Hanna, A.; Eslami, S. Predictive Performance of the SOFA and MSOFA Scoring Systems for Predicting In-Hospital Mortality in the Emergency Department. Am. J. Emerg. Med. 2019, 37, 1237–1241. [Google Scholar] [CrossRef]
- Grissom, C.K.; Brown, S.M.; Kuttler, K.G.; Boltax, J.P.; Jones, J.; Jephson, A.R.; Orme, J.F. A Modified Sequential Organ Failure Assessment Score for Critical Care Triage. Disaster Med. Public Health Prep. 2010, 4, 277–284. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Shahcheragh, S.-M.-T.; Fakhr-Movahedi, A. Comparing the Ability and Accuracy of MSOFA, QSOFA, and QSOFA-65 in Predicting the Status of Nontraumatic Patients Referred to a Hospital Emergency Department: A Prospective Study. Indian J. Crit. Care Med. 2020, 24, 1045. [Google Scholar] [CrossRef]
- Perkins, G.D.; Graesner, J.-T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; van de Voorde, P.; Madar, J.; Zideman, D.; Mentzelopoulos, S.; et al. European Resuscitation Council Guidelines 2021: Executive Summary. Resuscitation 2021, 161, 1–60. [Google Scholar] [CrossRef]
- Martín-Rodríguez, F.; Castro-Villamor, M.Á.; del Pozo Vegas, C.; Martín-Conty, J.L.; Mayo-Iscar, A.; Delgado Benito, J.F.; del Brio Ibañez, P.; Arnillas-Gómez, P.; Escudero-Cuadrillero, C.; López-Izquierdo, R. Analysis of the Early Warning Score to Detect Critical or High-Risk Patients in the Prehospital Setting. Intern. Emerg. Med. 2019, 14, 581–589. [Google Scholar] [CrossRef]
- Antman, E.M.; Cohen, M.; Bernink, P.J.L.M.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI Risk Score for Unstable Angina/Non–ST Elevation MI. JAMA 2000, 284, 835. [Google Scholar] [CrossRef]
- Çınar, T.; Karabağ, Y.; Burak, C.; Tanık, V.O.; Yesin, M.; Çağdaş, M.; Rencüzoğulları, İ. A Simple Score for the Prediction of Stent Thrombosis in Patients with ST Elevation Myocardial Infarction: TIMI Risk Index. J. Cardiovasc. Thorac. Res. 2019, 11, 182–188. [Google Scholar] [CrossRef]
- Hall, J.B.; Edelson, D.P. Derivation of a Cardiac Arrest Prediction Model Using Ward Vital Signs. Crit. Care Med. 2012, 40, 2102–2108. [Google Scholar] [CrossRef]
- Guan, G.; Lee, C.M.Y.; Begg, S.; Crombie, A.; Mnatzaganian, G. The Use of Early Warning System Scores in Prehospital and Emergency Department Settings to Predict Clinical Deterioration: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0265559. [Google Scholar] [CrossRef]
- Lane, D.J.; Wunsch, H.; Saskin, R.; Cheskes, S.; Lin, S.; Morrison, L.J.; Scales, D.C. Assessing Severity of Illness in Patients Transported to Hospital by Paramedics: External Validation of 3 Prognostic Scores. Prehospital Emerg. Care 2020, 24, 273–281. [Google Scholar] [CrossRef]
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Illness Severity in the NHS; RCP Press: London, UK, 2017. [Google Scholar]
- Burgos-Esteban, A.; Gea-Caballero, V.; Marín-Maicas, P.; Santillán-García, A.; Cordón-Hurtado, M.d.V.; Marqués-Sule, E.; Giménez-Luzuriaga, M.; Juárez-Vela, R.; Sanchez-Gonzalez, J.L.; García-Criado, J.; et al. Effectiveness of Early Warning Scores for Early Severity Assessment in Outpatient Emergency Care: A Systematic Review. Front. Public Health 2022, 10, 894906. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Endo, T.; Yoshida, T.; Shinozaki, T.; Motohashi, T.; Hsu, H.C.; Fukuda, S.; Tsukuda, J.; Naito, T.; Morisawa, K.; et al. Efficacy of Prehospital National Early Warning Score to Predict Outpatient Disposition at an Emergency Department of a Japanese Tertiary Hospital: A Retrospective Study. BMJ Open 2020, 10, e034602. [Google Scholar] [CrossRef]
- Vihonen, H.; Lääperi, M.; Kuisma, M.; Pirneskoski, J.; Nurmi, J. Glucose as an Additional Parameter to National Early Warning Score (NEWS) in Prehospital Setting Enhances Identification of Patients at Risk of Death: An Observational Cohort Study. Emerg. Med. J. 2020, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMJ. 2015, 162, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An Open-source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinformatics 2011, 12, 77. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. rms: Regression Modeling Strategies. Available online: https://hbiostat.org/doc/rms.pdf (accessed on 10 December 2022).
- Alba, A.C.; Agoritsas, T.; Walsh, M.; Hanna, S.; Iorio, A.; Devereaux, P.J.; McGinn, T.; Guyatt, G. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA 2017, 318, 1377–1384. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Medical Decision Making. Med. Decis. Mak. 2006, 26, 565-74. [Google Scholar] [CrossRef]
- A Simple Method for Evaluating Prediction Models, Diagnostic Tests, and Molecular Markers. Available online: https://www.mskcc.org/departments/epidemiology-biostatistics/biostatistics/decision-curve-analysis (accessed on 24 December 2022).

| 2-Day Mortality | ||||
|---|---|---|---|---|
| Variable | Total | Survivors | Non-Survivors | p Value 2 |
| No. (%) with data 1 | 1540 | 1459 (94.7) | 81 (5.3) | N.A. |
| Epidemiological variables | ||||
| Sex, female | 640 (41.6) | 610 (41.8) | 30 (37) | 0.369 |
| Age, year | 74 (62–83) | 74 (53–95) | 79 (65–86.5) | 0.139 |
| On-scene vital signs | ||||
| Respiratory rate, bpm | 17 (14–21) | 17 (14–20) | 26 (14.5–35) | <0.001 |
| Oxygen saturation, % | 96 (94–98) | 97 (94–98) | 86 (74.5–94) | <0.001 |
| FiO2, % | 0.21 (0.21–0.21) | 0.21 (0.21–0.21) | 0.21 (0.21–0.28) | <0.001 |
| SaFi | 457 (442–466) | 457 (447–466) | 366 (255–423) | <0.001 |
| SBP, mmHg | 134 (122–155) | 136 (115–156) | 100 (76–141) | <0.001 |
| DBP, mmHg | 78 (64–91) | 78 (65–91) | 60 (44–87) | <0.001 |
| MBP, mmHg | 97 (82–111) | 97 (83–112) | 73 (57–104) | <0.001 |
| Heart rate, beats/min | 79 (64–91) | 79 (64–100) | 97 (68–132) | <0.001 |
| Temperature, °C | 36 (35.8–36.5) | 36 (35.8–36.5) | 36.1 (35.15–36.5) | 0.355 |
| GCS, points | 15 (15–15) | 15 (15–15) | 12 (5–15) | <0.001 |
| Creatinine, mg/dL | 0.98 (0.81–1.31) | 0.97 (0.79–1.24) | 1.99 (1.145–2.80) | <0.001 |
| Lactate, mmol/L | 1.86 (1.15–2.94) | 1.83 (1.14–2.83) | 7.21 (4.91–12.05) | <0.001 |
| Prehospital syndromic | ||||
| Ischemic heart disease | 637 (41.4) | 608 (41.7) | 29 (35.8) | <0.001 |
| Acute heart failure | 281 (18.2) | 245 (16.8) | 36 (44.4) | <0.001 |
| Arrhythmia | 187 (12.1) | 182 (12.5) | 5 (6.2) | <0.001 |
| Syncope | 435 (28.2) | 424 (29.1) | 11 (13.6) | <0.001 |
| Hospital outcomes | ||||
| ACCI, points | 5 (3–7) | 5 (3–7) | 7 (5–9) | <0.001 |
| Inpatient | 830 (53.9) | 751 (51.5) | 79 (97.5%) | <0.001 |
| Fibrinolysis | 35 (2.3) | 23 (1.6) | 12 (14.8) | <0.001 |
| PIVS | 361 (23.4) | 339 (23.1) | 22 (27.1) | 0.417 |
| Emergent surgery | 38 (2.5) | 27 (2.5) | 1 (1.2) | 0.463 |
| ACCU-admission | 370 (24.0) | 328 (22.5) | 42 (51.9) | <0.001 |
| Scores calculation, points | ||||
| mSOFA | 1 (0–2) | 1 (0–2) | 8 (5–10) | <0.001 |
| TIMI risk index | 8 (6–12) | 8 (6–11) | 13 (8–19) | <0.001 |
| Modified shock index | 0.82 (0.65–1.08) | 0.81 (0.65–1.05) | 1.21 (0.77–1.74) | <0.001 |
| Cardiac Arrest Risk Triage | 9 (4–17) | 9 (4–16) | 25 (12–35) | <0.001 |
| NEWS2 | 3 (1–6) | 3 (1–6) | 12 (9–15) | <0.001 |
| (a) | mSOFA | NEWS2 | CART | MSI | TIMI |
|---|---|---|---|---|---|
| mSOFA | 0.94 (0.91–0.96) | ||||
| NEWS2 | 0.011 | 0.89 (0.85–0.94) | |||
| CART | <0.001 | <0.001 | 0.77 (0.72–0.82) | ||
| MSI | <0.001 | <0.001 | 0.032 | 0.69 (0.62–0.76) | |
| TIMI | <0.001 | <0.001 | 0.095 | 0.047 | 0.71 (0.65–0.78) |
| (b) | mSOFA | NEWS2 | CART | MSI | TIMI |
| mSOFA | 0.87 (0.84–0.90) | ||||
| NEWS2 | 0.011 | 0.83 (0.79–0.86) | |||
| CART | <0.001 | <0.001 | 0.75 (0.72–0.79) | ||
| MSI | <0.001 | <0.001 | <0.001 | 0.67 (0.63–0.71) | |
| TIMI | <0.001 | <0.001 | 0.061 | <0.001 | 0.71 (0.68–0.75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Portillo, E.; López-Izquierdo, R.; Castro Villamor, M.A.; Sanz-García, A.; Martín-Conty, J.L.; Polonio-López, B.; Sánchez-Soberón, I.; del Pozo Vegas, C.; Durantez-Fernández, C.; Conty-Serrano, R.; et al. Modified Sequential Organ Failure Assessment Score vs. Early Warning Scores in Prehospital Care to Predict Major Adverse Cardiac Events in Acute Cardiovascular Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 88. https://doi.org/10.3390/jcdd10020088
Castro Portillo E, López-Izquierdo R, Castro Villamor MA, Sanz-García A, Martín-Conty JL, Polonio-López B, Sánchez-Soberón I, del Pozo Vegas C, Durantez-Fernández C, Conty-Serrano R, et al. Modified Sequential Organ Failure Assessment Score vs. Early Warning Scores in Prehospital Care to Predict Major Adverse Cardiac Events in Acute Cardiovascular Disease. Journal of Cardiovascular Development and Disease. 2023; 10(2):88. https://doi.org/10.3390/jcdd10020088
Chicago/Turabian StyleCastro Portillo, Enrique, Raúl López-Izquierdo, Miguel A. Castro Villamor, Ancor Sanz-García, José L. Martín-Conty, Begoña Polonio-López, Irene Sánchez-Soberón, Carlos del Pozo Vegas, Carlos Durantez-Fernández, Rosa Conty-Serrano, and et al. 2023. "Modified Sequential Organ Failure Assessment Score vs. Early Warning Scores in Prehospital Care to Predict Major Adverse Cardiac Events in Acute Cardiovascular Disease" Journal of Cardiovascular Development and Disease 10, no. 2: 88. https://doi.org/10.3390/jcdd10020088
APA StyleCastro Portillo, E., López-Izquierdo, R., Castro Villamor, M. A., Sanz-García, A., Martín-Conty, J. L., Polonio-López, B., Sánchez-Soberón, I., del Pozo Vegas, C., Durantez-Fernández, C., Conty-Serrano, R., & Martín-Rodríguez, F. (2023). Modified Sequential Organ Failure Assessment Score vs. Early Warning Scores in Prehospital Care to Predict Major Adverse Cardiac Events in Acute Cardiovascular Disease. Journal of Cardiovascular Development and Disease, 10(2), 88. https://doi.org/10.3390/jcdd10020088







