Ticagrelor Can Regulate the Ion Channel Characteristics of Superior Cervical Ganglion Neurons after Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Echocardiography
2.3. Preparation and Electrophysiological Recordings of SG Neurons
2.4. Intracellular Calcium Measurement in SCG Neurons
2.5. Immunofluorescence Staining
2.6. RT-PCR
2.7. ELISA
2.8. Statistical Analysis
3. Results
3.1. MI Model Validation and Changes in Cardiac Function after MI and TIC
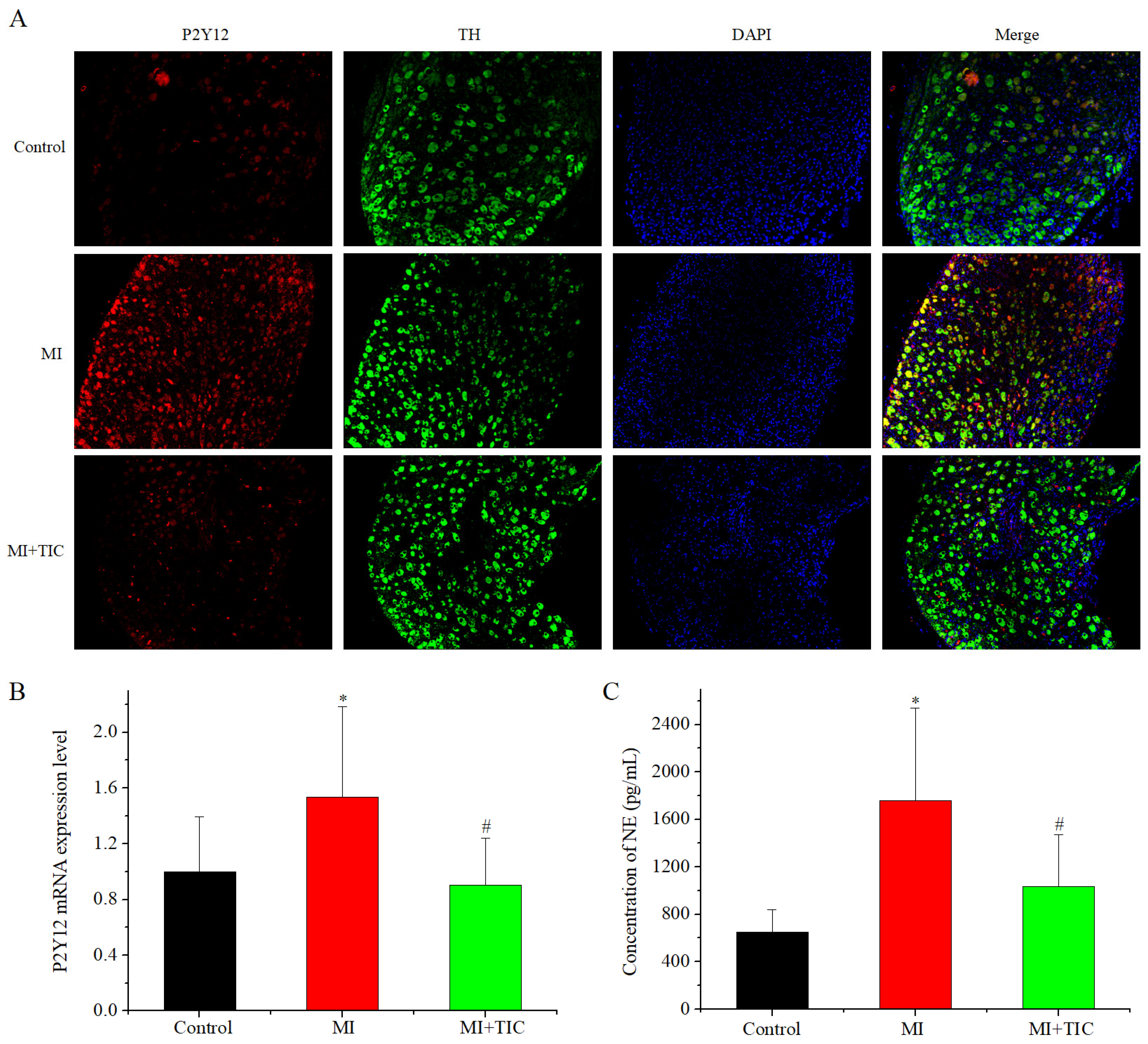
3.2. P2Y12 Expression in SCG and NE content in Serum after MI and TIC
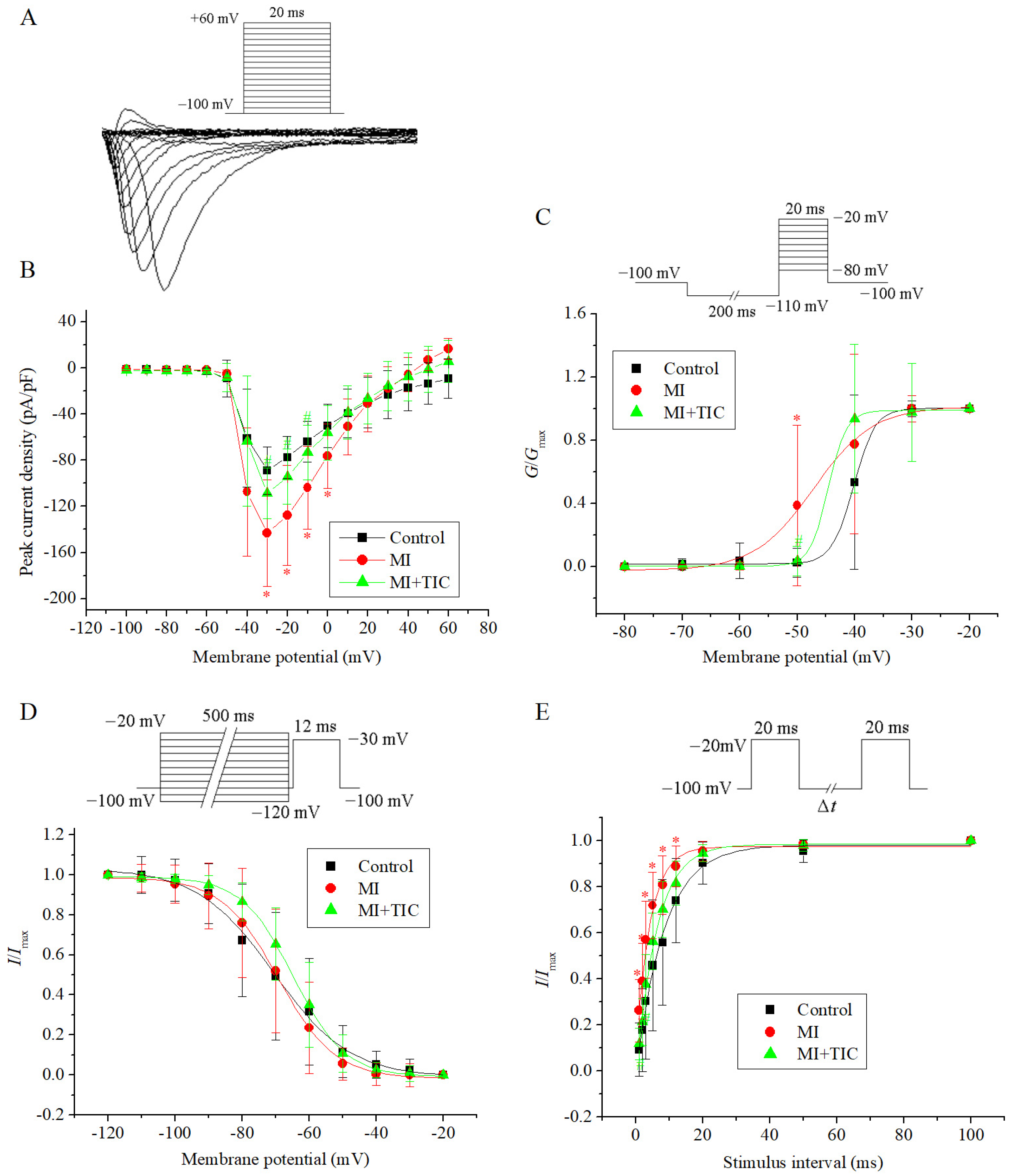
3.3. Effects of MI and TIC on Activation Kinetics of IK
3.4. Effects of MI and TIC on Activation Kinetics of INa
3.5. Effects of MI and TIC on ICa and Intracellular Calcium Concentration
4. Discussion
4.1. Remodeling in SCG Neurons following MI
4.2. Ion Channels in SCGs after MI
4.3. P2Y12 Expression and Roles of P2Y12 Receptor Antagonist Treatment in MI
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCG | superior cervical ganglion |
| MI | myocardiac infarction |
| DAPI | 4′,6-diamidino-2-phenylindole |
| TH | tyrosine hydroxylase |
| TIC | ticagrelor |
| ISO | isoproterenol |
| LAD | left atrial diameter |
| LVDD | left ventricular end-diastolic dimension |
| LVEF | left ventricular ejection fraction |
| FS | fractional shortening |
| ECG | electrocardiogram |
| CK | creatine kinase |
| CKMB | creatine kinase isoenzyme |
References
- Yu, L.; Zhou, L.; Cao, G.; Po, S.S.; Huang, B.; Zhou, X.; Wang, M.; Yuan, S.; Wang, Z.; Wang, S.; et al. Optogenetic Modulation of Cardiac Sympathetic Nerve Activity to Prevent Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2017, 70, 2778–2790. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, Y.; Zhou, M.; Wang, G.; Quan, D.; Shen, C.; Shuai, W.; Kong, B.; Huang, C.; Huang, H. Targeted ablation of cardiac sympathetic neurons improves ventricular electrical remodelling in a canine model of chronic myocardial infarction. EP Eur. 2018, 20, 2036–2044. [Google Scholar] [CrossRef]
- Tan, A.Y.; Elharrif, K.; Cardona-Guarache, R.; Mankad, P.; Ayers, O.; Joslyn, M.; Das, A.; Kaszala, K.; Lin, S.-F.; Ellenbogen, K.A.; et al. Persistent Proarrhythmic Neural Remodeling Despite Recovery From Premature Ventricular Contraction-Induced Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Q.; Lu, Y.; Li, Y.; Zhang, L.; Zhang, J.; Xing, Q.; Lv, W.; Cheng, X.; Zhang, G.; et al. Renal Denervation Reduced Ventricular Arrhythmia After Myocardial Infarction by Inhibiting Sympathetic Activity and Remodeling. J. Am. Heart Assoc. 2018, 7, e009938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, W.; Chen, H.; Zhou, H.; Liu, Z.; Liu, Z.; Zhou, Y.; Zhou, X.; Yu, L.; Jiang, H. Sympathetic Nervous System Mediates Cardiac Remodeling After Myocardial Infarction in a Circadian Disruption Model. Front. Cardiovasc. Med. 2021, 8, 668387. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Yin, J.; Hu, H.; Xue, M.; Li, X.; Cheng, W.; Wang, Y.; Li, X.; Wang, Y.; et al. A novel sympathetic neuronal GABAergic signalling system regulates NE release to prevent ventricular arrhythmias after acute myocardial infarction. Acta Physiol. 2019, 227, e13315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Fu, H.; Wang, X.; Ye, L.; Lakhani, I.; Tse, G.; Zhang, Z.; Liu, T.; Li, G. Effects of ticagrelor pretreatment on electrophysiological properties of stellate ganglion neurons following myocardial infarction. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1932–1942. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Liu, T.; Tse, G.; Fu, H.; Li, G. Modulation of Ion Channels in the Superior Cervical Ganglion Neurons by Myocardial Ischemia and Fluvastatin Treatment. Front. Physiol. 2018, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Zhang, Y.; Li, J.; Li, G.P. Characteristics of sympathetic ganglion neuron channels in two myocardial infarction models: A comparative study. Chin. J. Biomed. Eng. 2017, 23, 193–198. (In Chinese) [Google Scholar]
- Cheng, L.-J.; Li, G.-P.; Li, J.; Chen, Y.; Wang, X.-H. Effects of Fluvastatin on Characteristics of Stellate Ganglion Neurons in a Rabbit Model of Myocardial Ischemia. Chin. Med. J. 2016, 129, 549–556. [Google Scholar] [CrossRef]
- Zou, L.; Han, X.; Liu, S.; Gong, Y.; Wu, B.; Yi, Z.; Liu, H.; Zhao, S.; Jia, T.; Li, L.; et al. Baicalin Depresses the Sympathoexcitatory Reflex Induced by Myocardial Ischemia via the Dorsal Root Ganglia. Front. Physiol. 2018, 9, 928. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, X.; Shi, H.; Tian, J.; Sun, L.; Hu, X.; Cui, W.; Du, D. P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis. 2019, 10, 165. [Google Scholar] [CrossRef]
- Swiatkowski, P.; Murugan, M.; Eyo, U.; Wang, Y.; Rangaraju, S.; Oh, S.; Wu, L.-J. Activation of microglial P2Y12 receptor is required for outward potassium currents in response to neuronal injury. Neuroscience 2016, 318, 22–33. [Google Scholar] [CrossRef]
- Gafar, H.; Rodriguez, M.D.; Chandaka, G.K.; Salzer, I.; Boehm, S.; Schicker, K. Membrane coordination of receptors and channels mediating the inhibition of neuronal ion currents by ADP. Purinergic Signal. 2016, 12, 497–507. [Google Scholar] [CrossRef]
- Olgar, Y.; Durak, A.; Degirmenci, S.; Tuncay, E.; Billur, D.; Ozdemir, S.; Turan, B. Ticagrelor alleviates high-carbohydrate intake induced altered electrical activity of ventricular cardiomyocytes by regulating sarcoplasmic reticulum–mitochondria miscommunication. Mol. Cell. Biochem. 2021, 476, 3827–3844. [Google Scholar] [CrossRef] [PubMed]
- Gelosa, P.; Lecca, D.; Fumagalli, M.; Wypych, D.; Pignieri, A.; Cimino, M.; Verderio, C.; Enerbäck, M.; Nikookhesal, E.; Tremoli, E.; et al. Microglia is a Key Player in the Reduction of Stroke Damage Promoted by the New Antithrombotic Agent Ticagrelor. J. Cereb. Blood Flow Metab. 2014, 34, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Amoni, M.; Dries, E.; Ingelaere, S.; Vermoortele, D.; Roderick, H.L.; Claus, P.; Willems, R.; Sipido, K.R. Ventricular Arrhythmias in Ischemic Cardiomyopathy—New Avenues for Mechanism-Guided Treatment. Cells 2021, 10, 2629. [Google Scholar] [CrossRef]
- Wu, P.; Vaseghi, M. The autonomic nervous system and ventricular arrhythmias in myocardial infarction and heart failure. Pacing Clin. Electrophysiol. 2020, 43, 172–180. [Google Scholar] [CrossRef]
- Yu, R.; Wang, F.; Yin, J.; Shi, Y.; Wang, Y.; Wen, S.; Hu, H.; Yan, S. Expression of oxytocin receptor in the rat superior cervical ganglion after myocardial infarction. Int. J. Clin. Exp. Pathol. 2018, 11, 739–747. [Google Scholar] [PubMed]
- Liu, J.; Li, G.; Peng, H.; Tu, G.; Kong, F.; Liu, S.; Gao, Y.; Xu, H.; Qiu, S.; Fan, B.; et al. Sensory–sympathetic coupling in superior cervical ganglia after myocardial ischemic injury facilitates sympathoexcitatory action via P2X7 receptor. Purinergic Signal. 2013, 9, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.; Zou, L.; Liu, S.; Wu, B.; Lv, Q.; Wang, S.; Xue, Y.; Zhang, C.; Yi, Z.; Zhang, X.; et al. Long noncoding NONRATT021972 siRNA normalized abnormal sympathetic activity mediated by the upregulation of P2X7 receptor in superior cervical ganglia after myocardial ischemia. Purinergic Signal. 2016, 12, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Herring, N.; Paterson, D.J. Downregulation of M Current Is Coupled to Membrane Excitability in Sympathetic Neurons Before the Onset of Hypertension. Hypertension 2020, 76, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, E.N.; Davis, H.; Ajijola, O.A.; Buckler, K.J.; Ardell, J.L.; Shivkumar, K.; Paterson, D.J. RNA Sequencing Reveals Novel Transcripts from Sympathetic Stellate Ganglia During Cardiac Sympathetic Hyperactivity. Sci. Rep. 2018, 8, 8633. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, M.; Hu, D.; Huang, B.; Zhou, L.; Zhou, X.; Wang, Z.; Wang, S.; Jiang, H. Blocking the Nav1.8 channel in the left stellate ganglion suppresses ventricular arrhythmia induced by acute ischemia in a canine model. Sci. Rep. 2017, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tu, H.; Wang, C.; Cao, L.; Hu, W.; Hackfort, B.T.; Muelleman, R.L.; Wadman, M.C.; Li, Y.-L. Inhibition of N-type calcium channels in cardiac sympathetic neurons attenuates ventricular arrhythmogenesis in heart failure. Cardiovasc. Res. 2020, 117, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tu, H.; Cao, L.; Zheng, H.; Muelleman, R.L.; Wadman, M.C.; Li, Y. Reduced N-Type Ca2+ Channels in Atrioventricular Ganglion Neurons Are Involved in Ventricular Arrhythmogenesis. J. Am. Heart Assoc. 2018, 7, e007457. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. Mabs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Barker, B.S.; Nigam, A.; Ottolini, M.; Gaykema, R.P.; Hargus, N.J.; Patel, M.K. Pro-excitatory alterations in sodium channel activity facilitate subiculum neuron hyperexcitability in temporal lobe epilepsy. Neurobiol. Dis. 2017, 108, 183–194. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, J.-Q.; Tong, X.-P. Potassium channel dysfunction in neurons and astrocytes in Huntington’s disease. CNS Neurosci. Ther. 2018, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, G.-H.; Xie, D.-H.; Wu, W.-J.; Hu, P. Taurine Enhances Excitability of Mouse Cochlear Neural Stem Cells by Selectively Promoting Differentiation of Glutamatergic Neurons Over GABAergic Neurons. Neurochem. Res. 2015, 40, 924–931. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Wu, D.; Wang, Y.; Deng, A. Effects of Anthopleurin-Q on the Intracellular Free Ca2+ Concentration in Cultured Rat Cortical Neurons. Drug Res. 2014, 64, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.J.; Gerevich, Z. P2Y Receptors in Synaptic Transmission and Plasticity: Therapeutic Potential in Cognitive Dysfunction. Neural Plast. 2016, 2016, 1207393. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, J.; Guo, J.; Xu, Y.; Jiang, H.; Zheng, C.; Xu, Z.; Zhang, Y.; Che, H.; Liang, S.; et al. Effects of Baicalin on Diabetic Cardiac Autonomic Neuropathy Mediated by the P2Y12 Receptor in Rat Stellate Ganglia. Cell. Physiol. Biochem. 2018, 46, 986–998. [Google Scholar] [CrossRef]
- Zou, L.; Gong, Y.; Zhao, S.; Yi, Z.; Han, X.; Wu, B.; Jia, T.; Li, L.; Yuan, H.; Shi, L.; et al. Downregulation of P2Y12 in the superior cervical ganglia alleviates abnormal sympathetic activity after myocardial ischemia. J. Cell. Physiol. 2017, 233, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Boehm, S. P2Ys go neuronal: Modulation of Ca2+ and K+ channels by recombinant receptors. Br. J. Pharmacol. 2003, 138, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.G.; Boehm, S. Regulation of neuronal ion channels via P2Y receptors. Purinergic Signal. 2004, 1, 31–41. [Google Scholar] [CrossRef]
- Zaika, O.; Tolstykh, G.P.; Jaffe, D.B.; Shapiro, M.S. Inositol Triphosphate-Mediated Ca2+ Signals Direct Purinergic P2Y Receptor Regulation of Neuronal Ion Channels. J. Neurosci. 2007, 27, 8914–8926. [Google Scholar] [CrossRef] [PubMed]
- Varenhorst, C.; Alström, U.; Braun, O.; Storey, R.; Mahaffey, K.W.; Bertilsson, M.; Cannon, C.P.; Scirica, B.M.; Himmelmann, A.; James, S.K.; et al. Causes of mortality with ticagrelor compared with clopidogrel in acute coronary syndromes. Hear 2014, 100, 1762–1769. [Google Scholar] [CrossRef]
- Lesiak, M.; Komosa, A. Dual antiplatelet therapy for reduction in mortality in patients with acute and chronic coronary syndromes. Adv. Interv. Cardiol. 2021, 17, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, L.-Y.; Li, X.-Z. Therapy with ticagrelor for ST-elevated acute coronary syndrome accompanied by diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 312–318. [Google Scholar] [PubMed]
- Lariccia, V.; Macrì, M.L.; Matteucci, A.; Maiolino, M.; Amoroso, S.; Magi, S. Effects of ticagrelor on the sodium/calcium exchanger 1 (NCX1) in cardiac derived H9c2 cells. Eur. J. Pharmacol. 2019, 850, 158–166. [Google Scholar] [CrossRef]
- Kucuk, M.; Celen, M.; Yamasan, B.E.; Olgar, Y.; Ozdemir, S. Effects of Ticagrelor on Ionic Currents and Contractility in Rat Ventricular Myocytes. Cardiovasc. Drugs Ther. 2015, 29, 419–424. [Google Scholar] [CrossRef]
- Scirica, B.M.; Cannon, C.P.; Emanuelsson, H.; Michelson, E.L.; Harrington, R.A.; Husted, S.; James, S.; Katus, H.; Pais, P.; Raev, D.; et al. The Incidence of Bradyarrhythmias and Clinical Bradyarrhythmic Events in Patients With Acute Coronary Syndromes Treated With Ticagrelor or Clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) Trial: Results of the Continuous Electrocardiographic Assessment Substudy. J. Am. Coll. Cardiol. 2011, 57, 1908–1916. [Google Scholar] [CrossRef]
- Pujade, I.; Perino, J.; Mathieu, C.; Arnaud, M.; Raschi, E.; Gatti, M.; Bezin, J.; Salvo, F. Risk of bradyarrhythmia related to ticagrelor: A systematic review and meta-analysis. Pharmacol. Res. 2020, 160, 105089. [Google Scholar] [CrossRef] [PubMed]
- De Maria, E.; Borghi, A.; Modonesi, L.; Cappelli, S. Ticagrelor therapy and atrioventricular block: Do we need to worry? World J. Clin. Cases 2017, 5, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Rosenfeld, I.; Nordkin, I.; Halabi, M. Life-threatening complete atrioventricular block associated with ticagrelor therapy. Int. J. Cardiol. 2015, 182, 379–380. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.J.D.; Gnecchi-Ruscone, T.; Bellina, V.; Oliveira, M.; Maciel, L.; de Carvalho, A.C.C.; Salgado, H.C.; Bergamaschi, C.M.; Tobaldini, E.; Porta, A.; et al. Acute adenosine increases cardiac vagal and reduces sympathetic efferent nerve activities in rats. Exp. Physiol. 2012, 97, 719–729. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Yu, L.; Zhan, X.; Tse, G.; Liu, T.; Fu, H.; Li, G. Ticagrelor Can Regulate the Ion Channel Characteristics of Superior Cervical Ganglion Neurons after Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2023, 10, 71. https://doi.org/10.3390/jcdd10020071
Cheng L, Yu L, Zhan X, Tse G, Liu T, Fu H, Li G. Ticagrelor Can Regulate the Ion Channel Characteristics of Superior Cervical Ganglion Neurons after Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2023; 10(2):71. https://doi.org/10.3390/jcdd10020071
Chicago/Turabian StyleCheng, Lijun, Lin Yu, Xiaoping Zhan, Gary Tse, Tong Liu, Huaying Fu, and Guangping Li. 2023. "Ticagrelor Can Regulate the Ion Channel Characteristics of Superior Cervical Ganglion Neurons after Myocardial Infarction" Journal of Cardiovascular Development and Disease 10, no. 2: 71. https://doi.org/10.3390/jcdd10020071
APA StyleCheng, L., Yu, L., Zhan, X., Tse, G., Liu, T., Fu, H., & Li, G. (2023). Ticagrelor Can Regulate the Ion Channel Characteristics of Superior Cervical Ganglion Neurons after Myocardial Infarction. Journal of Cardiovascular Development and Disease, 10(2), 71. https://doi.org/10.3390/jcdd10020071






