Comparative Analysis of Temperature Rise between Convective Heat Transfer Method and Computational Fluid Dynamics Method in an Anatomy-Based Left Atrium Model during Pulsed Field Ablation: A Computational Study
Abstract
1. Introduction
2. Materials and Methods
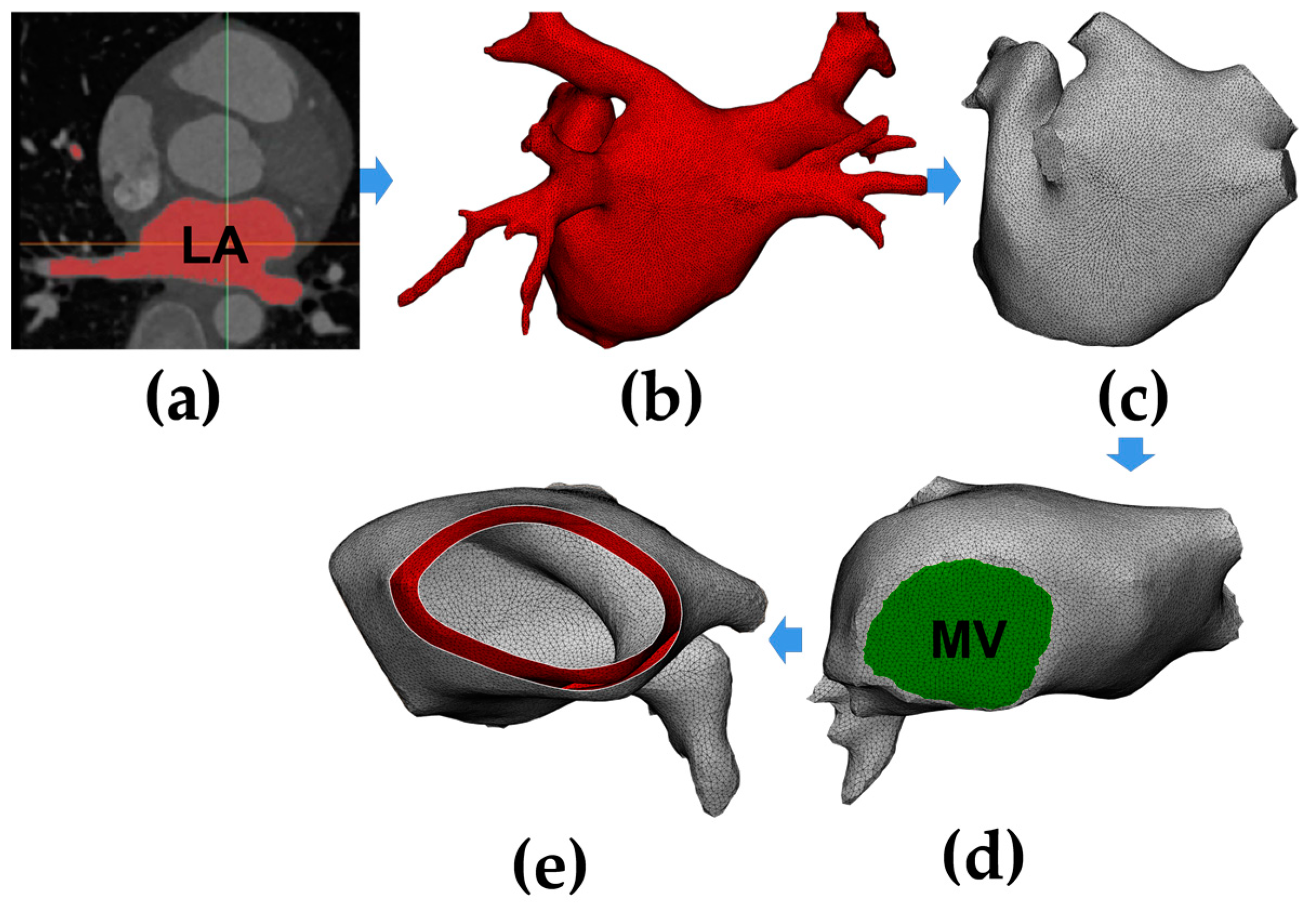
2.1. Model Construction
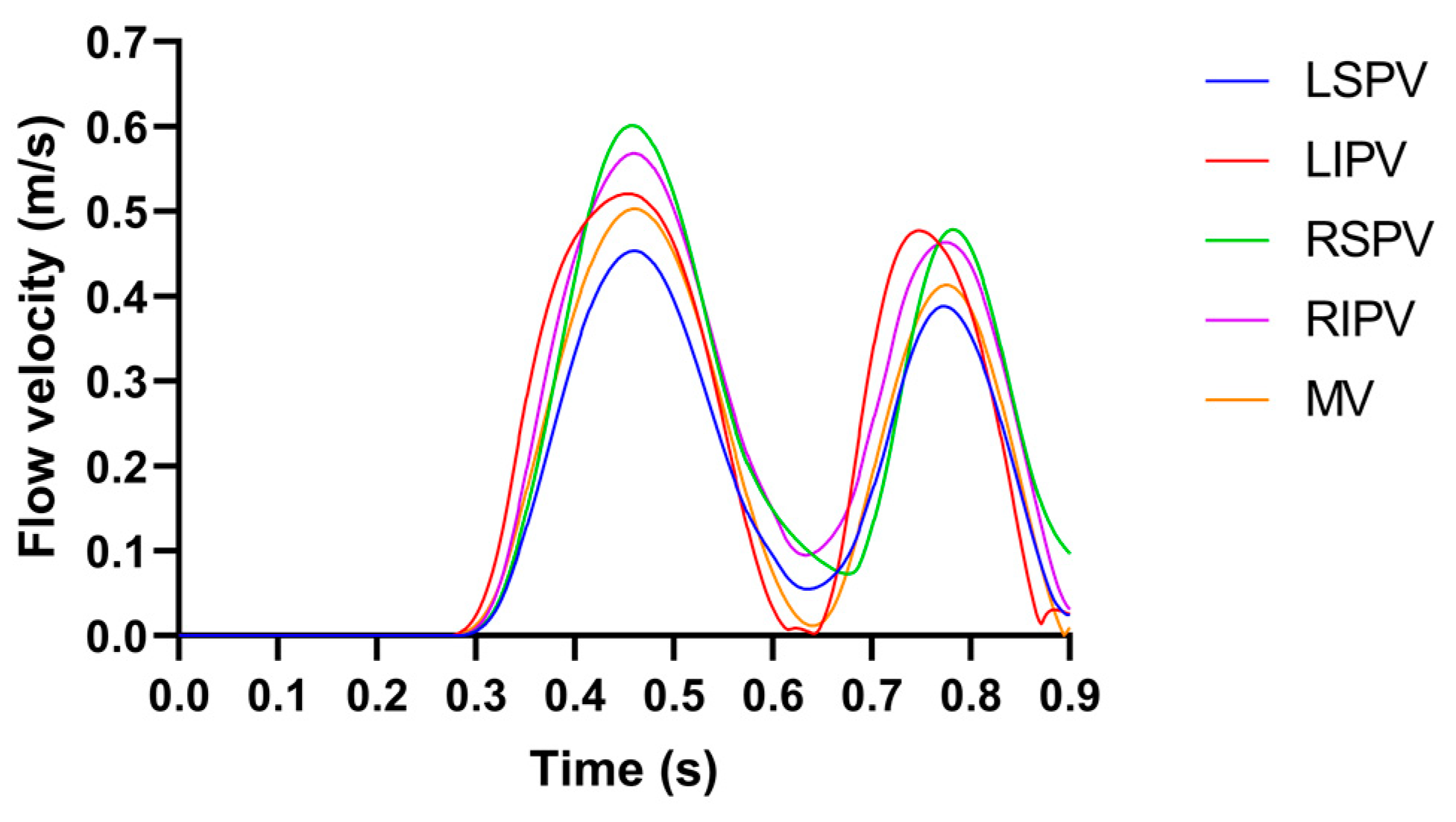
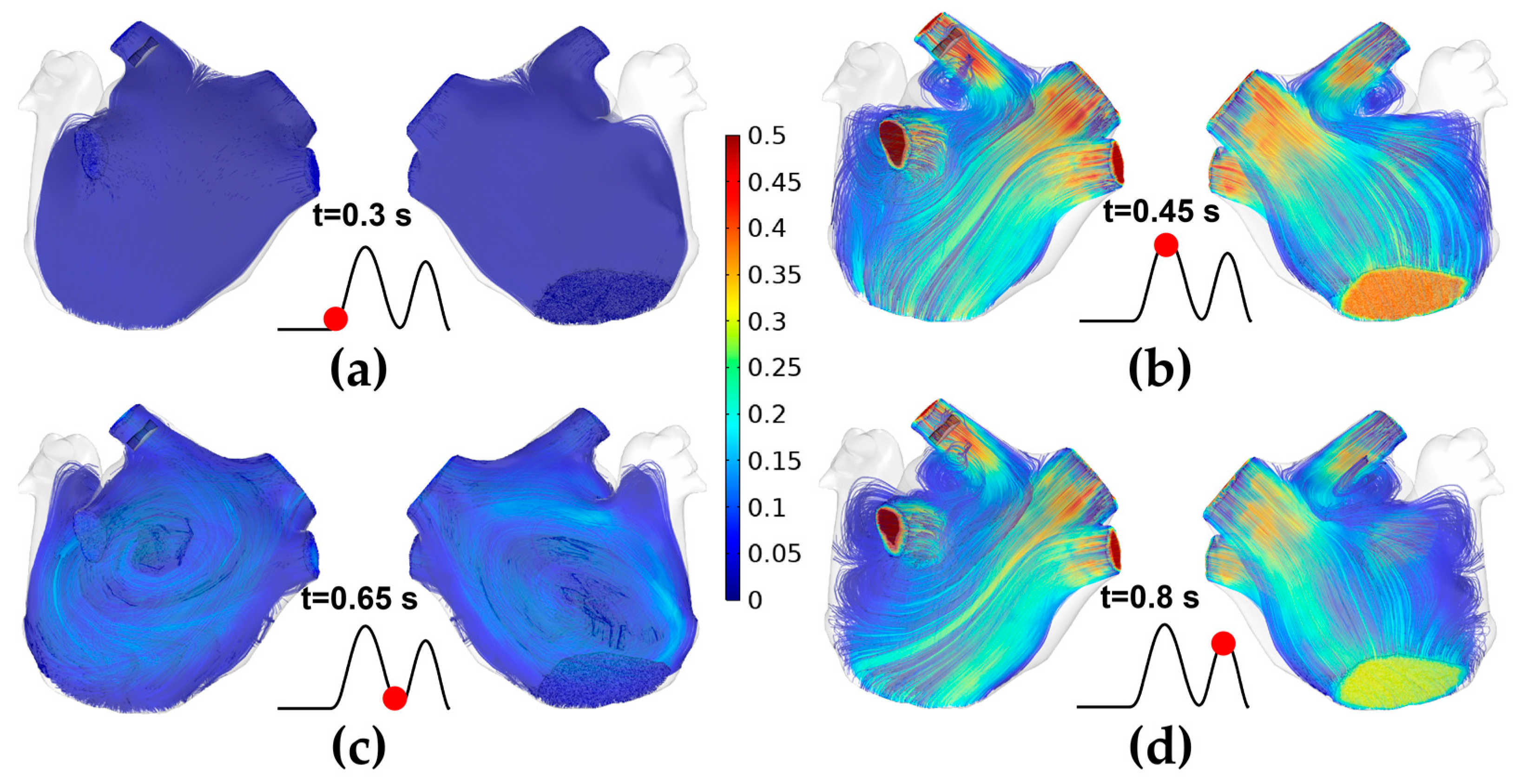
2.2. Pulsatile Blood Flow Velocity Profiles
2.3. Governing Equations
2.3.1. Electrical Equations
2.3.2. Thermal Equations
2.3.3. CFD Equations
2.4. Domain
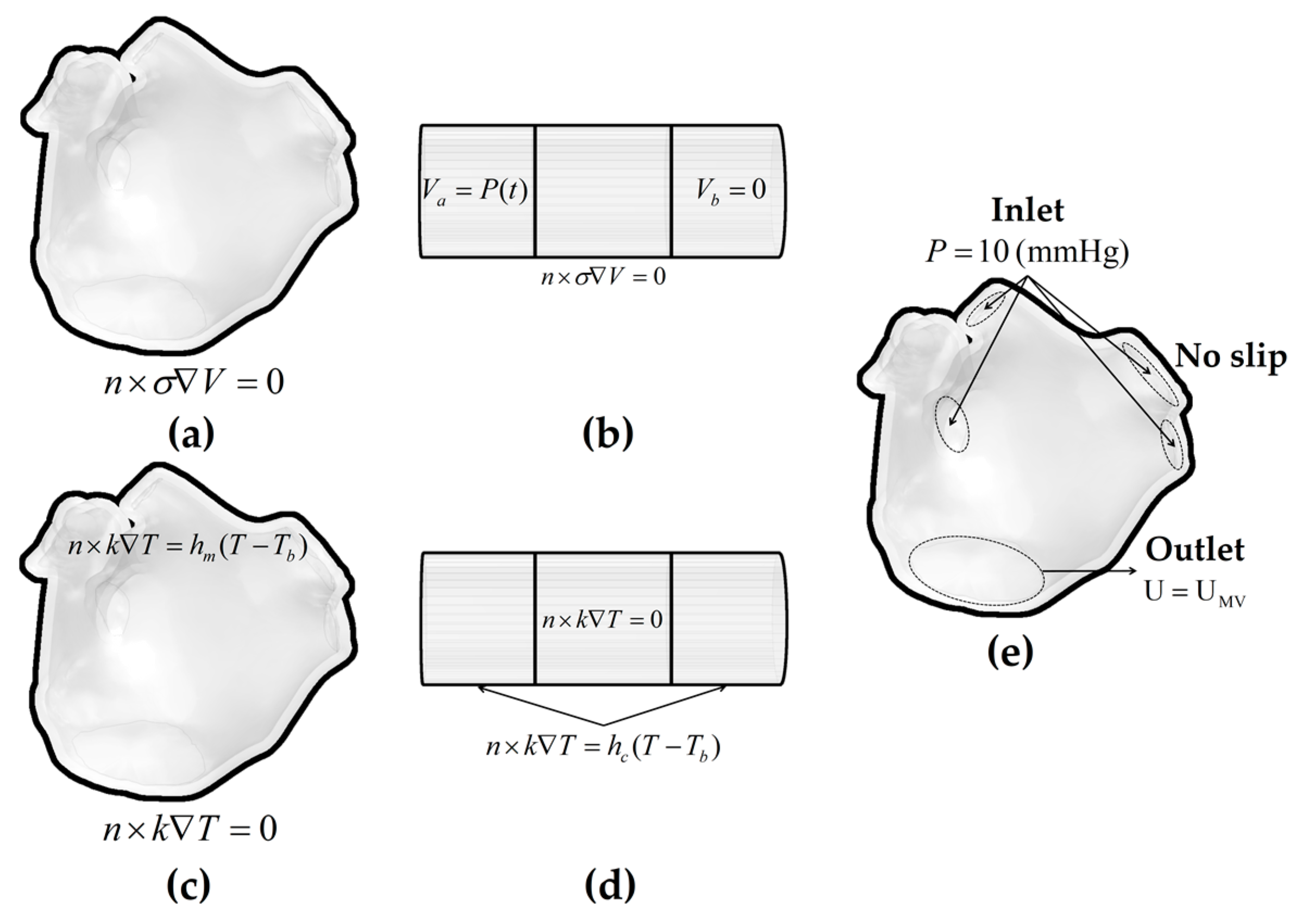
2.5. Boundary Conditions
2.5.1. Electrical Boundary Conditions
- The Parameters of
2.5.2. Thermal Boundary Conditions
- Initial temperature boundary condition: At = 0, the initial temperature of the myocardium and the blood was set to 37 °C;
- The second type of thermal boundary condition (constant heat flux boundary condition): The boundary of the plastic catheter and the epicardium represents the zero heat flux condition and is expressed as [17]:
- Methods for Simulating the Pulsatile Blood Flow
- CHT method
- 2.
- CFD Method
2.6. Material Properties
2.7. Data Computation and Criterion
2.7.1. Data Computation
2.7.2. Myocardial Ablation Volume Criterion
2.7.3. Difference Criterion
3. Results
3.1. The Velocity Characteristics of Pulsatile Blood
3.2. The Maximum Temperature of Myocardium and Blood
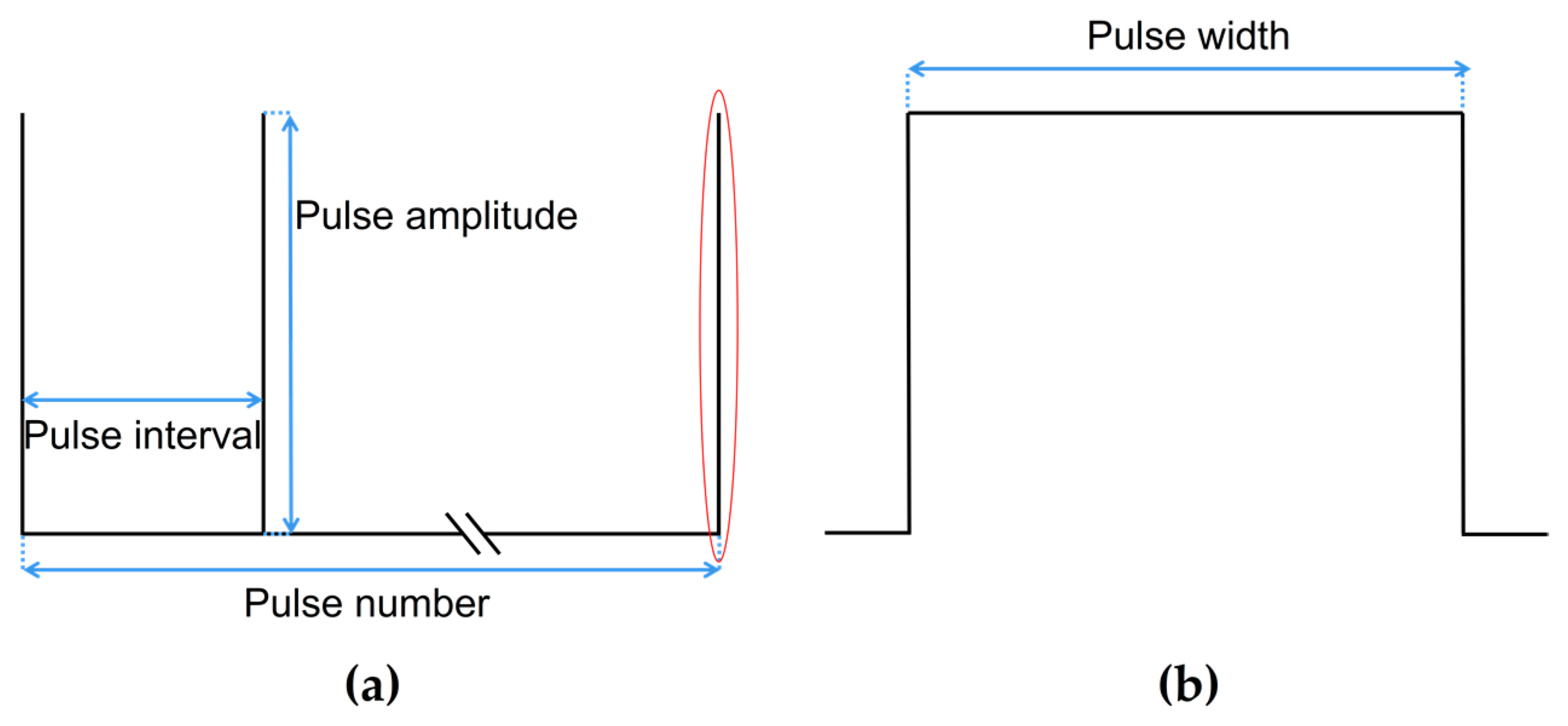
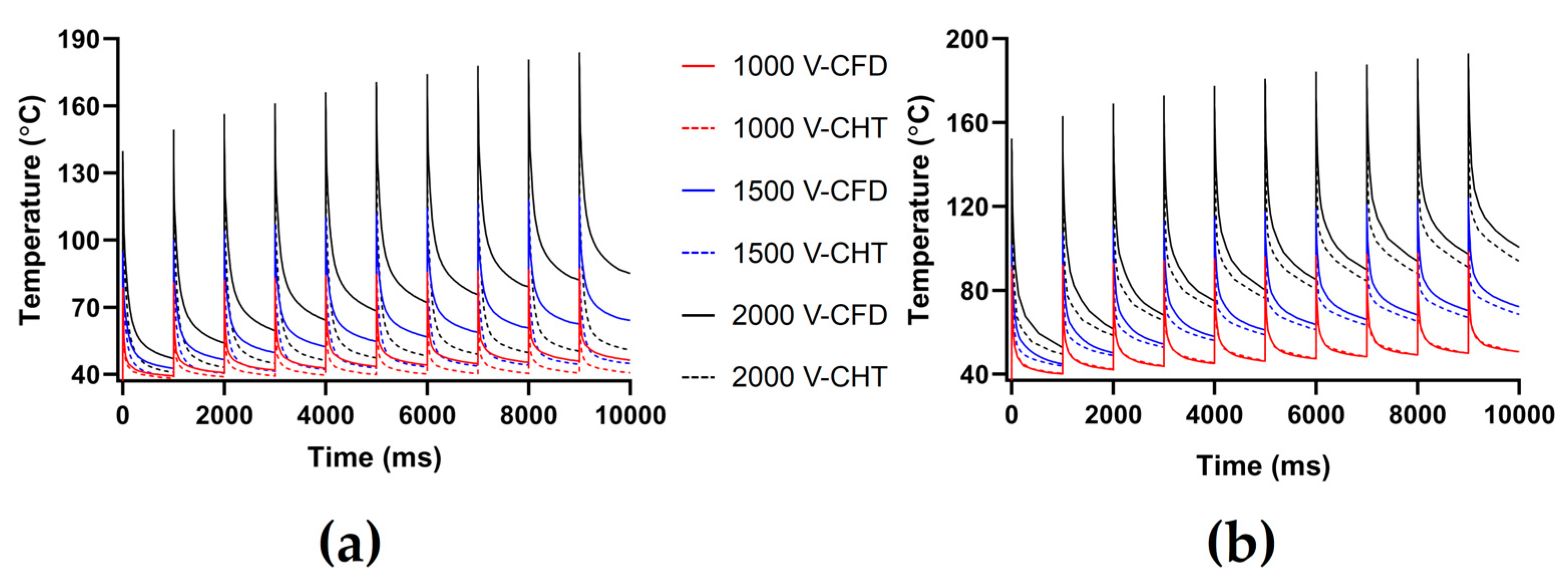
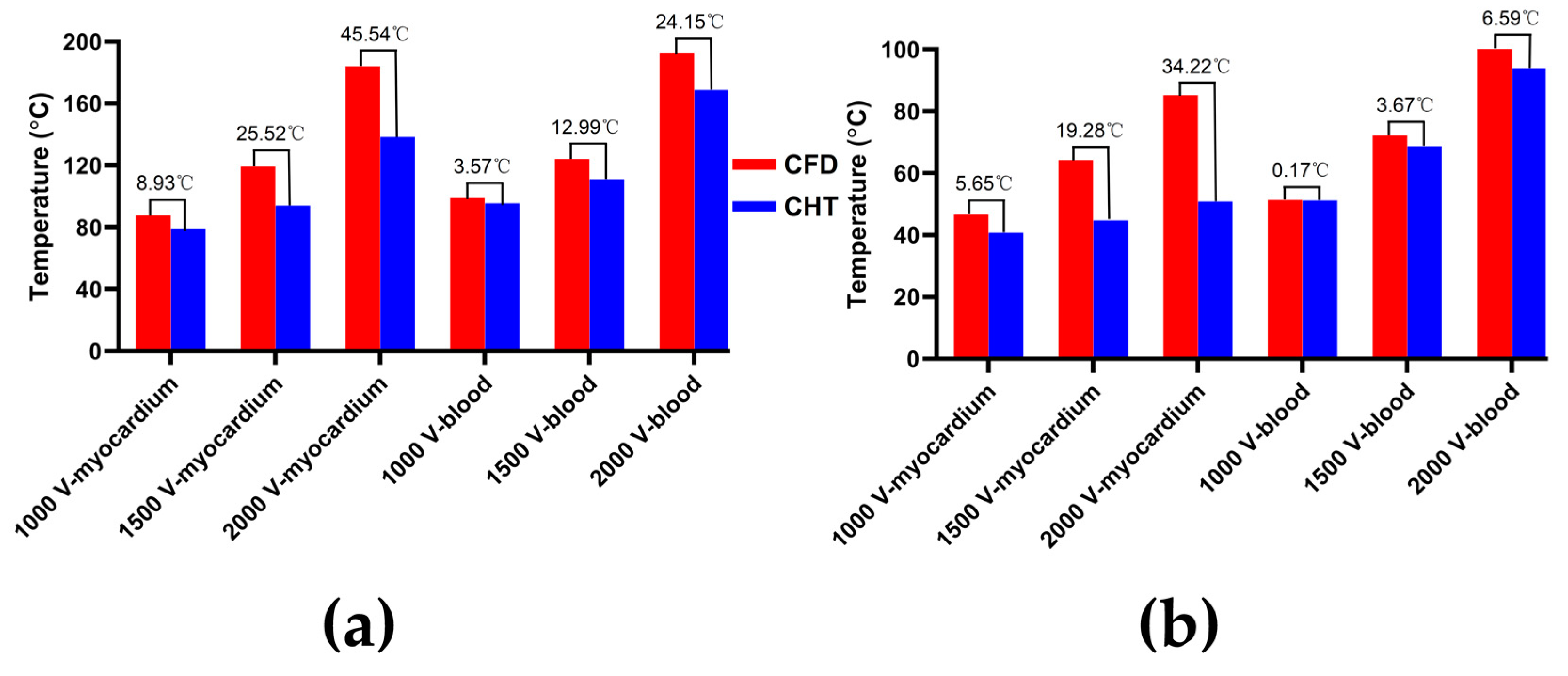
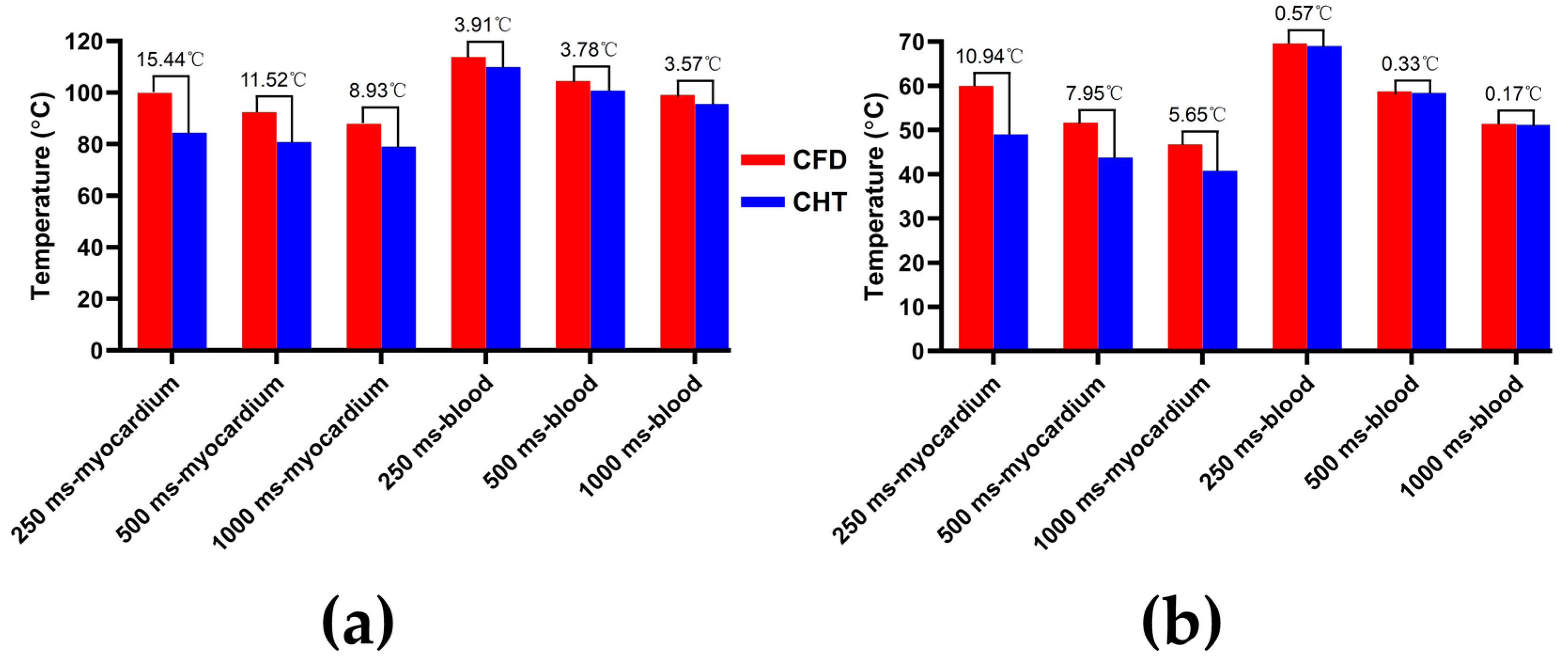
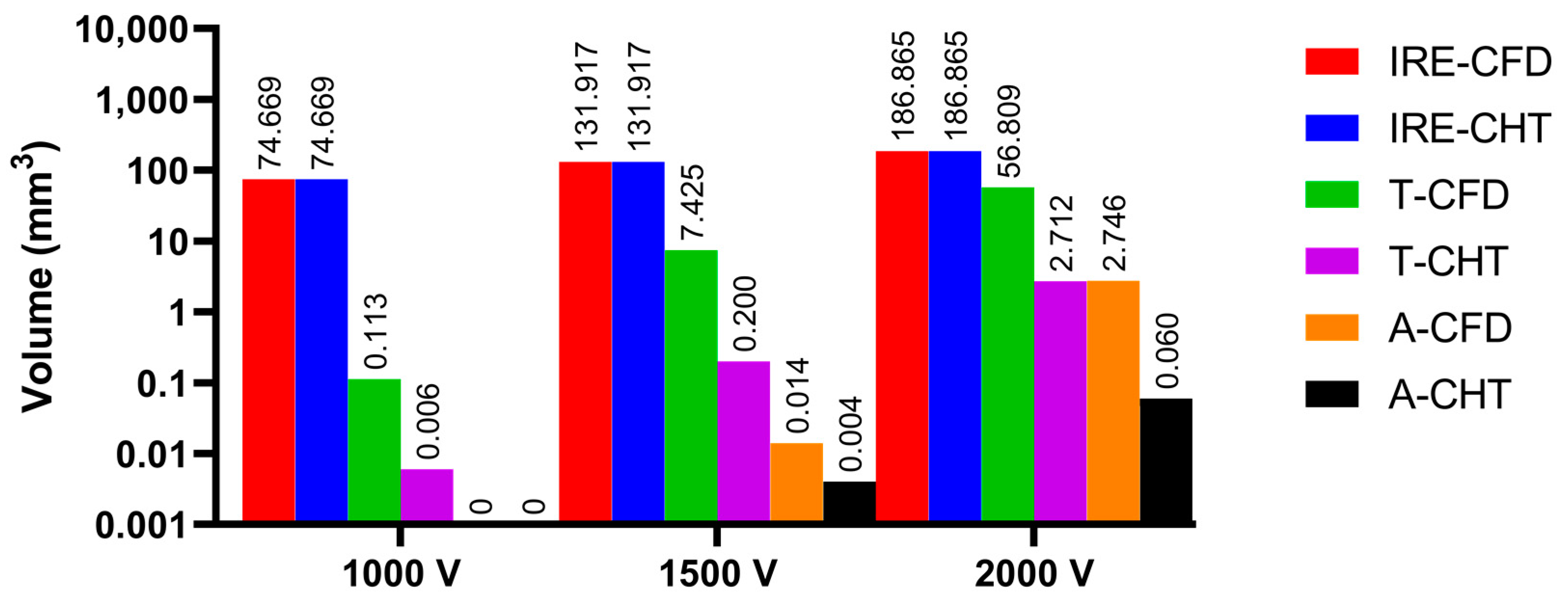
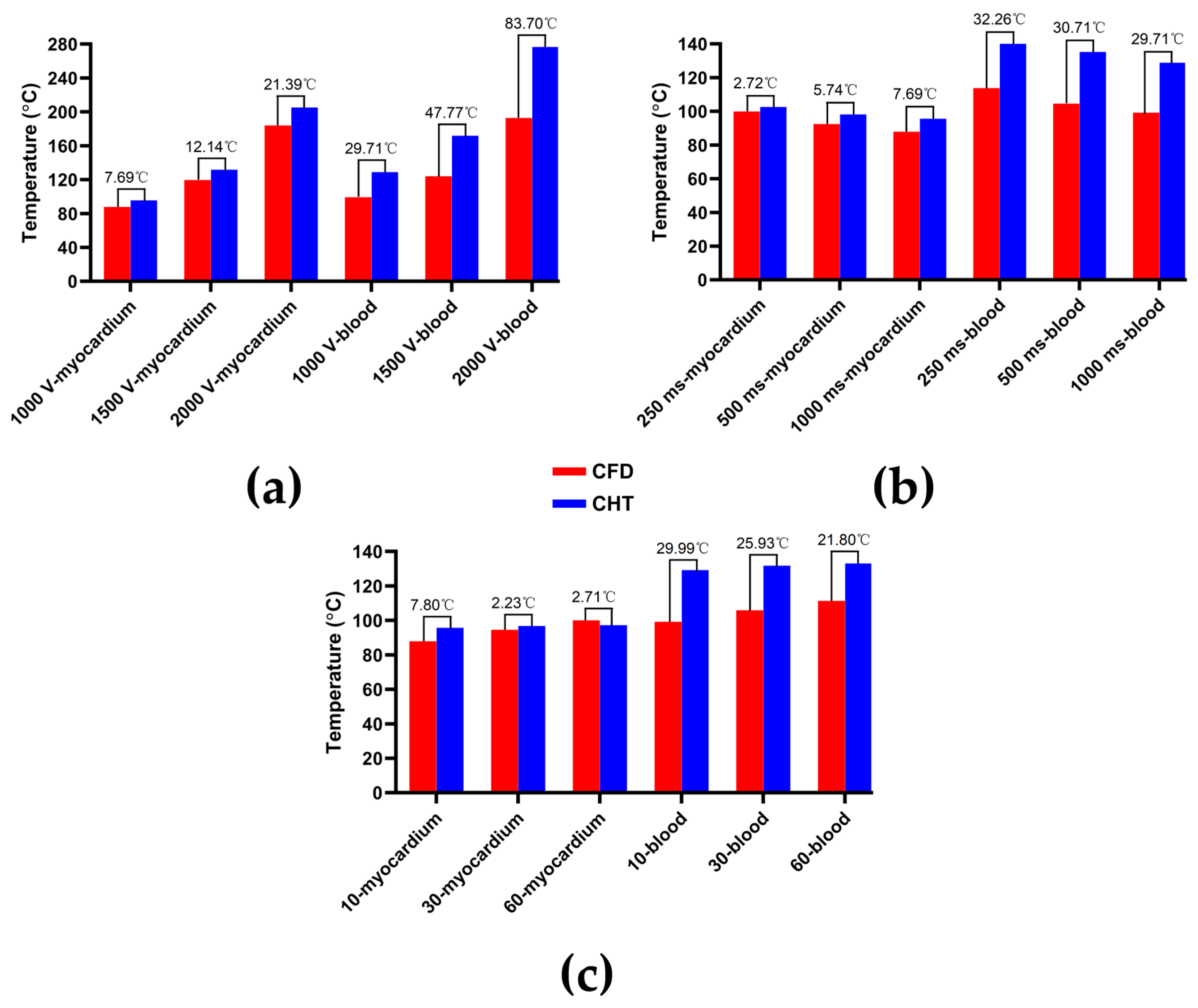
3.2.1. Influence of Different Pulse Amplitudes
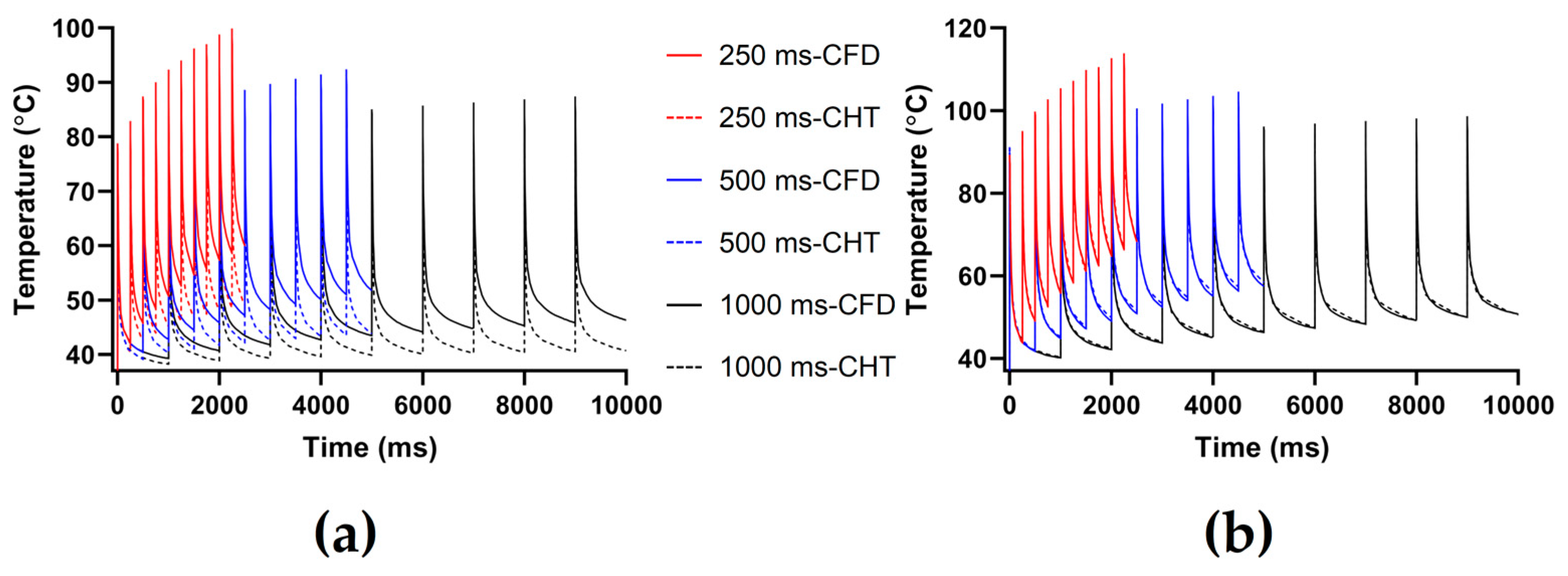
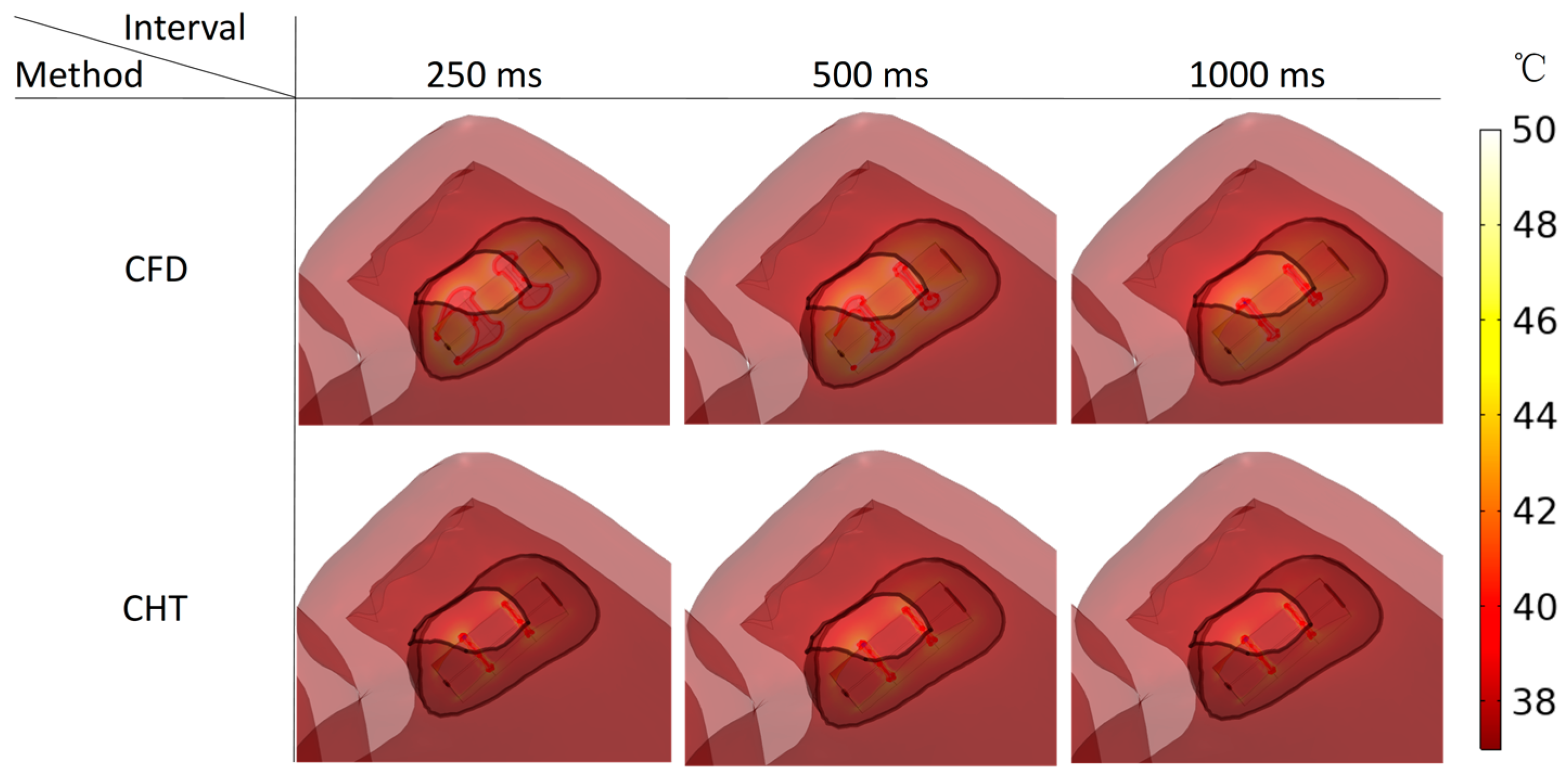
3.2.2. Influence of Different Pulse Intervals
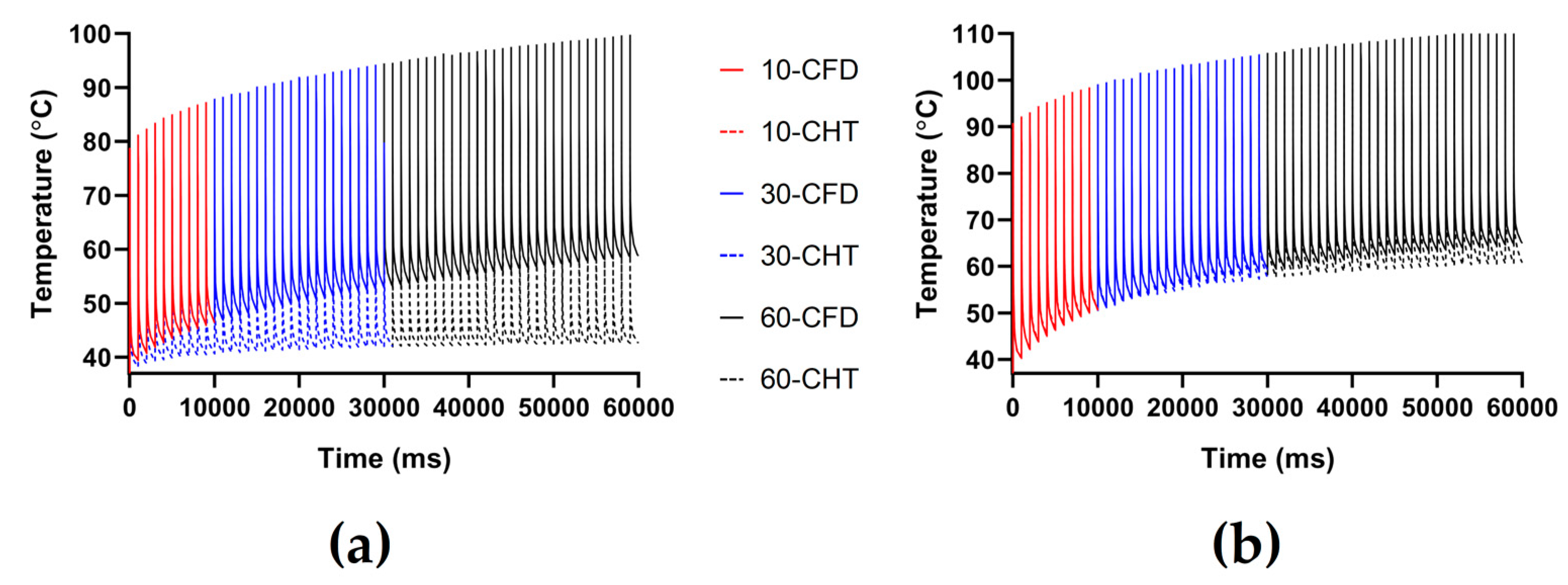
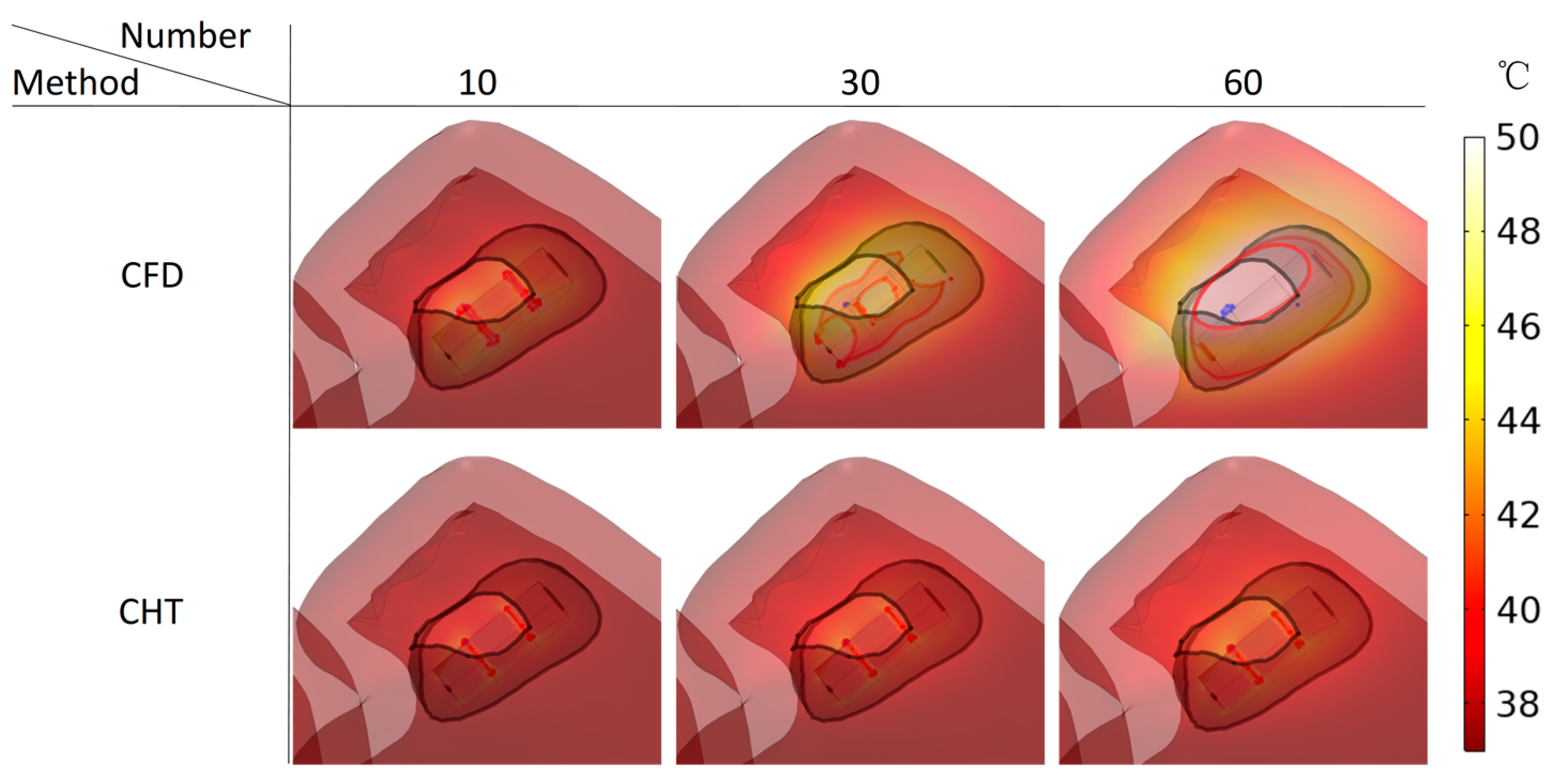
3.2.3. Influence of Different Pulse Numbers
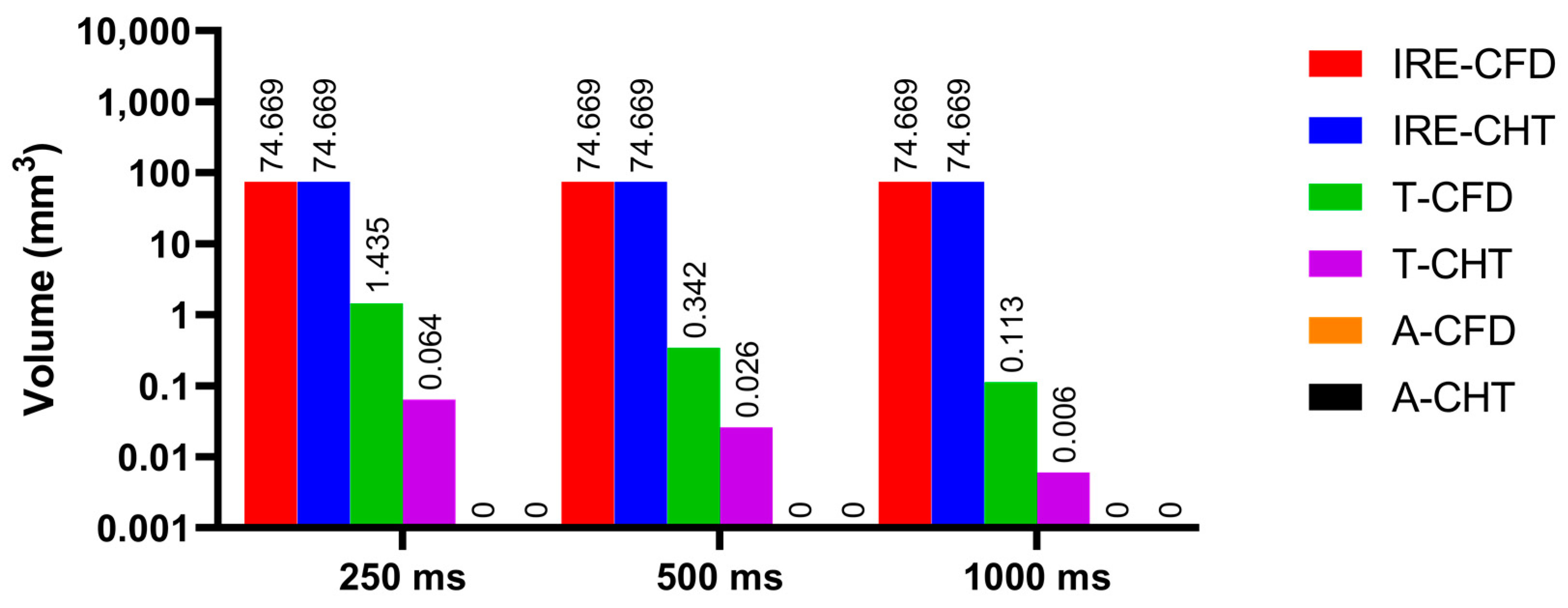
3.3. Myocardial Ablation Volume
3.3.1. Influence of Different Pulse Amplitudes
3.3.2. Influence of Different Pulse Intervals
3.3.3. Influence of Different Pulse Numbers
4. Discussion
4.1. Influence of Different Heat Dissipation Methods
4.2. Temperature Rise and Myocardial Ablation Volume during PFA
4.3. Compared with Experimental Results in Other Studies
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, F.; Zemlin, C.W. Effect of Twisted Fiber Anisotropy in Cardiac Tissue on Ablation with Pulsed Electric Fields. PLoS ONE 2016, 11, e0152262. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Asivatham, S.J.; Deneke, T.; Castellvi, Q.; Neal, R.E., II. Primer on Pulsed Electrical Field Ablation: Understanding the Benefits and Limitations. Circ. Arrhythm. Electrophysiol. 2021, 14, e010086. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barea, M.; García-Sánchez, T.; Ivorra, A. A computational comparison of radiofrequency and pulsed field ablation in terms of lesion morphology in the cardiac chamber. Sci. Rep. 2022, 12, 16144. [Google Scholar] [CrossRef] [PubMed]
- Petras, A.; Leoni, M.; Guerra, J.M.; Jansson, J.; Gerardo-Giorda, L. A computational model of open-irrigated radiofrequency catheter ablation accounting for mechanical properties of the cardiac tissue. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3232. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, A.; Pérez, J.J.; Berjano, E. Should fluid dynamics be included in computer models of RF cardiac ablation by irrigated-tip electrodes? Biomed. Eng. Online 2018, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, O.; Solis-Lemus, J.A.; Lee, A.W.C.; Karim, R.; Corrado, C.; Roney, C.H.; de Vecchi, A.; Niederer, S.A. CemrgApp: An interactive medical imaging application with image processing, computer vision, and machine learning toolkits for cardiovascular research. SoftwareX 2020, 12, 100570. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Xia, Q.; Hu, Z.; Huang, N.; Bian, C.; Zheng, Y.; Vesal, S.; Ravikumar, N.; Maier, A.; Yang, X.; et al. A global benchmark of algorithms for segmenting the left atrium from late gadolinium-enhanced cardiac magnetic resonance imaging. Med. Image Anal. 2021, 67, 101832. [Google Scholar] [CrossRef] [PubMed]
- de Marchi, S.F.; Bodenmüller, M.; Lai, D.L.; Seiler, C. Pulmonary venous flow velocity patterns in 404 individuals without cardiovascular disease. Heart 2001, 85, 23–29. [Google Scholar] [CrossRef]
- Koizumi, R.; Funamoto, K.; Hayase, T.; Kanke, Y.; Shibata, M.; Shiraishi, Y.; Yambe, T. Numerical analysis of hemodynamic changes in the left atrium due to atrial fibrillation. J. Biomech. 2015, 48, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Morgan, R.; Theron, A.; Dillon-Murphy, D.; Chubb, H.; Whitaker, J.; Henningsson, M.; Aljabar, P.; Schaeffter, T.; Kolbitsch, C.; et al. Novel MRI Technique Enables Non-Invasive Measurement of Atrial Wall Thickness. IEEE Trans. Med. Imaging 2017, 36, 1607–1614. [Google Scholar] [CrossRef]
- Fyrenius, A.; Wigström, L.; Ebbers, T.; Karlsson, M.; Engvall, J.; Bolger, A.F. Three dimensional flow in the human left atrium. Heart 2001, 86, 448–455. [Google Scholar] [CrossRef]
- ISO 5840-1:2021; Cardiovascular Implants—Cardiac Valve Prostheses—Part 1: General Requirements. ISO: Geneva, Switzerland, 2021; p. 10.
- Njoku, P.; Wardley, J.; Garg, P. Streamline-based three-dimensional peak-velocity tracing of transvalvular flow using four-dimensional flow cardiac magnetic resonance imaging for left ventricular diastolic assessment in aortic regurgitation: A case report. J. Med. Case Rep. 2022, 16, 205. [Google Scholar] [CrossRef]
- Fluckiger, J.U.; Goldberger, J.J.; Lee, D.C.; Ng, J.; Lee, R.; Olsen, A.B.; Carr, J.; Markl, M. Quantification of left atrial flow velocity distribution in atrial fibrillation using 4D flow MRI. J. Cardiovasc. Magn. Reason. 2013, 15, P261. [Google Scholar] [CrossRef]
- MIT. Steady Ohmic Conduction. Available online: http://web.mit.edu/6.013_book/www/chapter7/7.2.html (accessed on 25 May 2022).
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. 1948. J. Appl. Physiol. 1998, 85, 5–34. [Google Scholar] [CrossRef]
- González-Suárez, A.; Berjano, E. Comparative Analysis of Different Methods of Modeling the Thermal Effect of Circulating Blood Flow During RF Cardiac Ablation. IEEE Trans. Biomed. Eng. 2016, 63, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Parés, C.; Berjano, E.; González-Suárez, A. Effect of intracardiac blood flow pulsatility during radiofrequency cardiac ablation: Computer modeling study. Int. J. Hyperth. 2021, 38, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Yan, S.; Wu, X. Influence of pulsating intracardiac blood flow on radiofrequency catheter ablation outcomes in an anatomy-based atrium model. Int. J. Hyperth. 2022, 39, 1064–1077. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef]
- Zager, Y.; Kain, D.; Landa, N.; Leor, J.; Maor, E. Optimization of Irreversible Electroporation Protocols for In-vivo Myocardial Decellularization. PLoS ONE 2016, 11, e0165475. [Google Scholar] [CrossRef]
- Heller, E.; Garcia-Sanchez, T.; Moshkovits, Y.; Rabinovici, R.; Grynberg, D.; Segev, A.; Asirvatham, S.J.; Ivorra, A.; Maor, E. Comparing High-Frequency with Monophasic Electroporation Protocols in an in vivo Beating Heart Model. JACC Clin. Electrophysiol. 2021, 7, 959–964. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, Y.H.; Mao, Y.K.; Jiang, C.Y.; Fan, J.R.; Luo, K. Numerical prediction of thrombosis risk in left atrium under atrial fibrillation. Math. Biosci. Eng. 2020, 17, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Gayatri, M.; Guha, K.; Sateesh, J. Design and Analysis of Multiple Inlet–Multiple Outlet Piezoelectric Actuated Valveless Micropump for Micro Drug Delivery Application. J. Control. Autom. Electr. Syst. 2022. [Google Scholar] [CrossRef]
- González-Suárez, A.; Irastorza, R.M.; Deane, S.; O’Brien, B.; O’Halloran, M.; Elahi, A. Full torso and limited-domain computer models for epicardial pulsed electric field ablation. Comput. Methods Programs Biomed. 2022, 221, 106886. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Available online: www.itis.ethz.ch/database (accessed on 3 August 2022).
- Yaqoob, T.; Ahsan, M.; Hussain, A.; Ahmad, I. Computational Fluid Dynamics (CFD) Modeling and Simulation of Flow Regulatory Mechanism in Artificial Kidney Using Finite Element Method. Membranes 2020, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- MUltifrontal Massively Parallel Solver Users’ Guide. Available online: https://graal.ens-lyon.fr/MUMPS/doc/userguide_5.5.1.pdf (accessed on 11 December 2022).
- Avazzadeh, S.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Keane, D.; Quinlan, L.R. Establishing Irreversible Electroporation Electric Field Potential Threshold in A Suspension in vitro Model for Cardiac and Neuronal Cells. J. Clin. Med. 2021, 10, 5443. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Melnik, R. Computational Modeling of Cardiac Ablation Incorporating Electrothermomechanical Interactions. J. Eng. Sci. Med. Diagn. 2020, 3, 041004. [Google Scholar] [CrossRef]
- Pérez, J.J.; González-Suárez, A.; Nadal, E.; Berjano, E. Thermal impact of replacing constant voltage by low-frequency sine wave voltage in RF ablation computer modeling. Comput. Methods Programs Biomed. 2020, 195, 105673. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, T.; Amorós-Figueras, G.; Jorge, E.; Campos, M.C.; Maor, E.; Guerra, J.M.; Ivorra, A. Parametric Study of Pulsed Field Ablation with Biphasic Waveforms in an in vivo Heart Model: The Role of Frequency. Circ. Arrhythm. Electrophysiol. 2022, 15, e010992. [Google Scholar] [CrossRef]
- Jain, M.K.; Wolf, P.D. A three-dimensional finite element model of radiofrequency ablation with blood flow and its experimental validation. Ann. Biomed. Eng. 2000, 28, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.J. Approximate Thermal Modeling of Radiofrequency Cardiac Ablation; Brigham Young University: Provo, UT, USA, 2005. [Google Scholar]
- Garcia, P.A.; Davalos, R.V.; Miklavcic, D. A numerical investigation of the electric and thermal cell kill distributions in electroporation-based therapies in tissue. PLoS ONE 2014, 9, e103083. [Google Scholar] [CrossRef]
- Caluori, G.; Odehnalova, E.; Jadczyk, T.; Pesl, M.; Starek, Z. AC Pulsed Field Ablation Is Feasible and Safe in Atrial and Ventricular Settings: A Proof-of-Concept Chronic Animal Study. Front. Cardiovasc. Med. 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Peyman, A.; Grant, E.H. Electrical conductivity of tissue at frequencies below 1 MHz. Phys. Med. Biol. 2009, 54, 4863–4878. [Google Scholar] [CrossRef]
- Baena-Montes, J.M.; O’Halloran, T.; Clarke, C.; Donaghey, K.; Dunne, E.; O’Halloran, M.; Quinlan, L.R. Electroporation Parameters for Human Cardiomyocyte Ablation in vitro. J. Cardiovasc. Dev. Dis. 2022, 9, 240. [Google Scholar] [CrossRef]
- Bosi, G.M.; Cook, A.; Rai, R.; Menezes, L.J.; Schievano, S.; Torii, R.; Burriesci, G. Computational Fluid Dynamic Analysis of the Left Atrial Appendage to Predict Thrombosis Risk. Front. Cardiovasc. Med. 2018, 5, 34. [Google Scholar] [CrossRef] [PubMed]




| Group | Pulse Amplitude/V | Pulse Interval/ms | Pulse Number |
|---|---|---|---|
| 1 | 1000 | 1000 | 10 |
| 2 | 1500 | 1000 | 10 |
| 3 | 2000 | 1000 | 10 |
| 4 | 1000 | 1000 | 10 |
| 5 | 1000 | 250 | 10 |
| 6 | 1000 | 500 | 10 |
| 7 | 1000 | 1000 | 10 |
| 8 | 1000 | 1000 | 30 |
| 9 | 1000 | 1000 | 60 |
| Element/Material | |||||
|---|---|---|---|---|---|
| Electrode | 21500 | 132 | 71 | 4.6 × 106 | |
| Plastic Catheter | 70 | 1045 | 0.026 | 1 × 10−5 | |
| Blood | 1000 | 4180 | 0.541 | 0.667 | |
| Myocardium | Liquid phase | 1060 | 3111 | 0.531 | 0.0537 */0.281 ** |
| Gas phase | 370.44 | 2155.92 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, L.; Gu, K.; Ji, X.; Zhang, H.; Yan, S.; Wu, X. Comparative Analysis of Temperature Rise between Convective Heat Transfer Method and Computational Fluid Dynamics Method in an Anatomy-Based Left Atrium Model during Pulsed Field Ablation: A Computational Study. J. Cardiovasc. Dev. Dis. 2023, 10, 56. https://doi.org/10.3390/jcdd10020056
Zang L, Gu K, Ji X, Zhang H, Yan S, Wu X. Comparative Analysis of Temperature Rise between Convective Heat Transfer Method and Computational Fluid Dynamics Method in an Anatomy-Based Left Atrium Model during Pulsed Field Ablation: A Computational Study. Journal of Cardiovascular Development and Disease. 2023; 10(2):56. https://doi.org/10.3390/jcdd10020056
Chicago/Turabian StyleZang, Lianru, Kaihao Gu, Xingkai Ji, Hao Zhang, Shengjie Yan, and Xiaomei Wu. 2023. "Comparative Analysis of Temperature Rise between Convective Heat Transfer Method and Computational Fluid Dynamics Method in an Anatomy-Based Left Atrium Model during Pulsed Field Ablation: A Computational Study" Journal of Cardiovascular Development and Disease 10, no. 2: 56. https://doi.org/10.3390/jcdd10020056
APA StyleZang, L., Gu, K., Ji, X., Zhang, H., Yan, S., & Wu, X. (2023). Comparative Analysis of Temperature Rise between Convective Heat Transfer Method and Computational Fluid Dynamics Method in an Anatomy-Based Left Atrium Model during Pulsed Field Ablation: A Computational Study. Journal of Cardiovascular Development and Disease, 10(2), 56. https://doi.org/10.3390/jcdd10020056