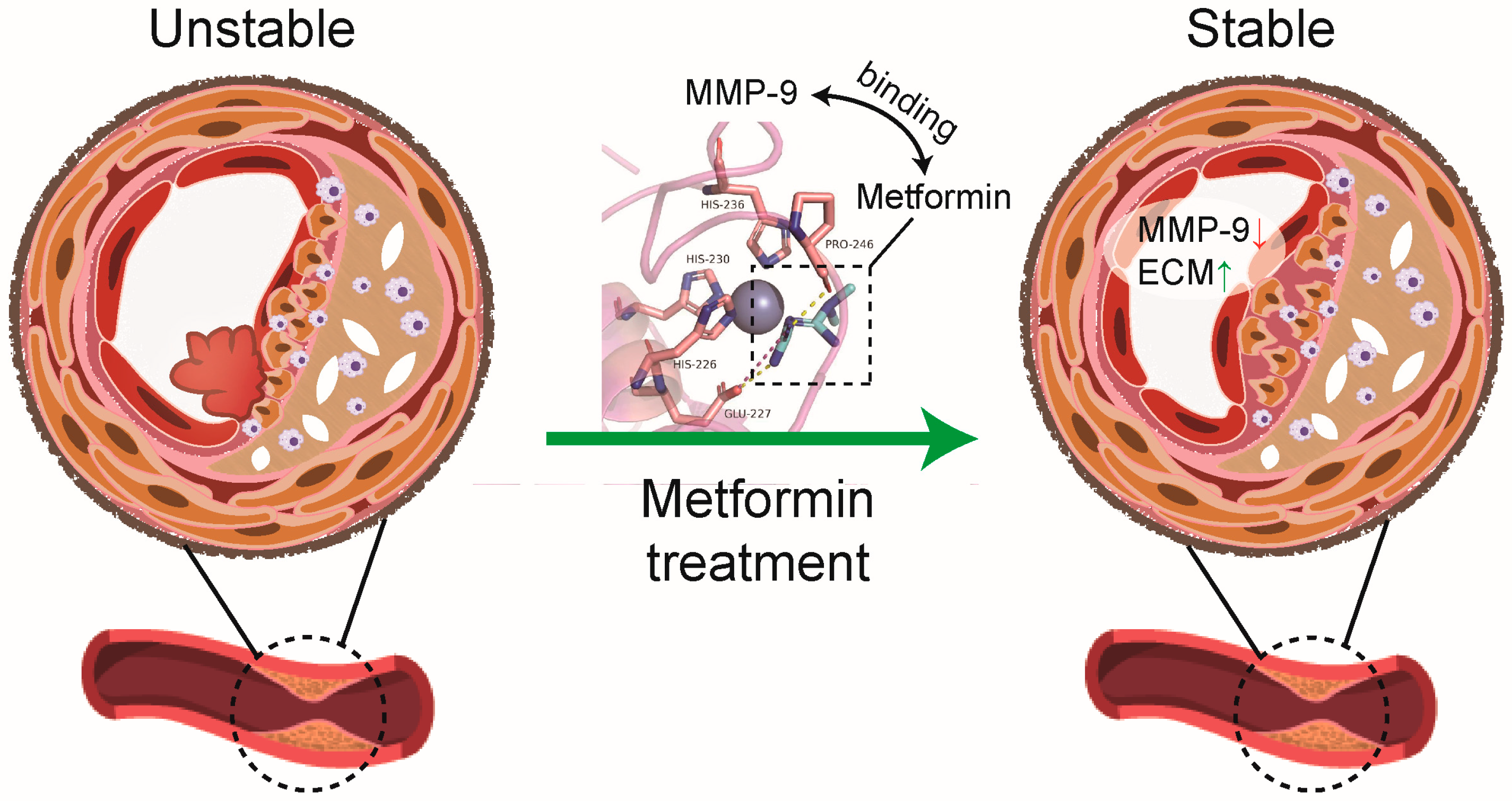
Metformin Directly Binds to MMP-9 to Improve Plaque Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
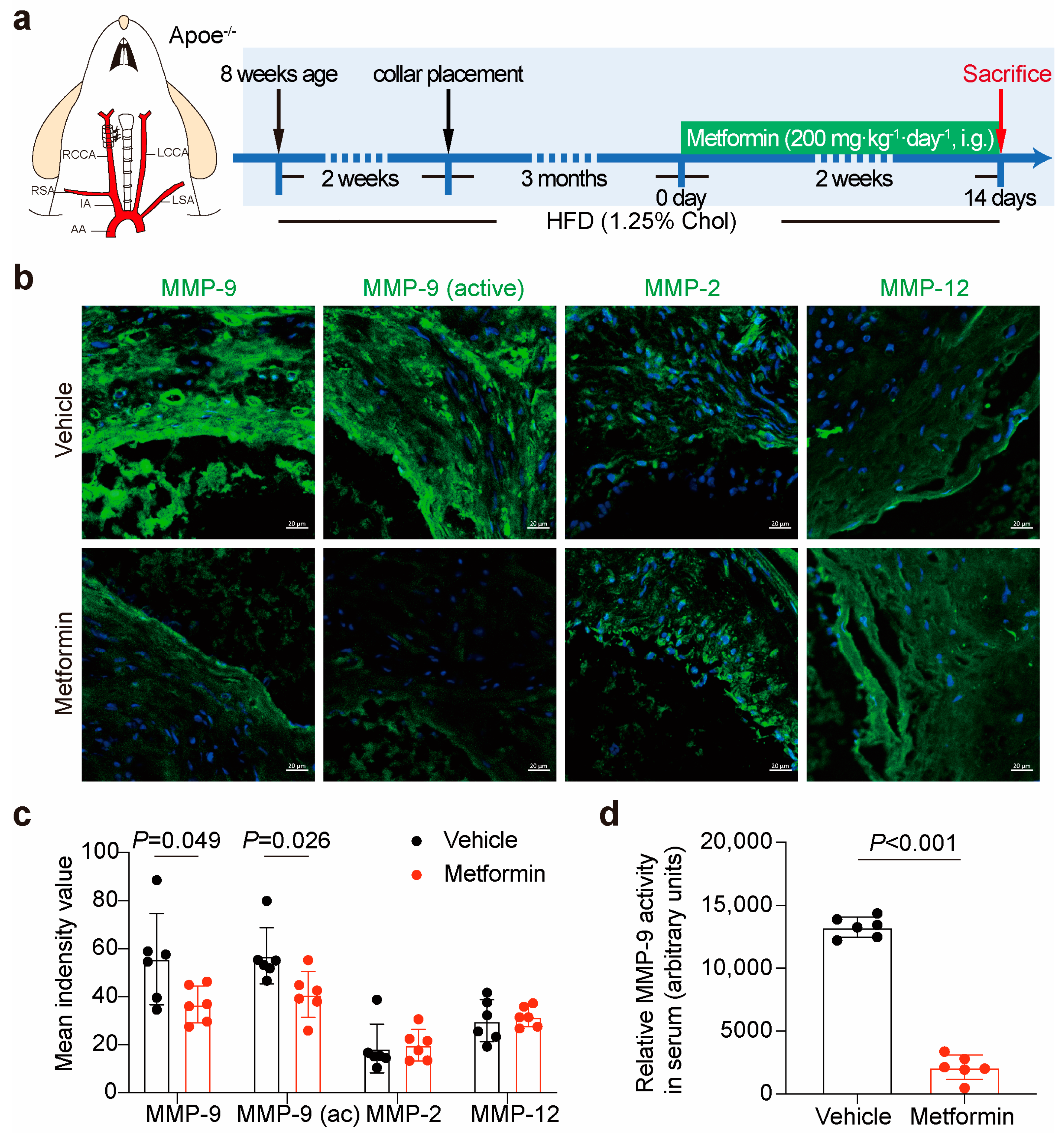
2.2. Carotid Collar Placement and Drug Treatment
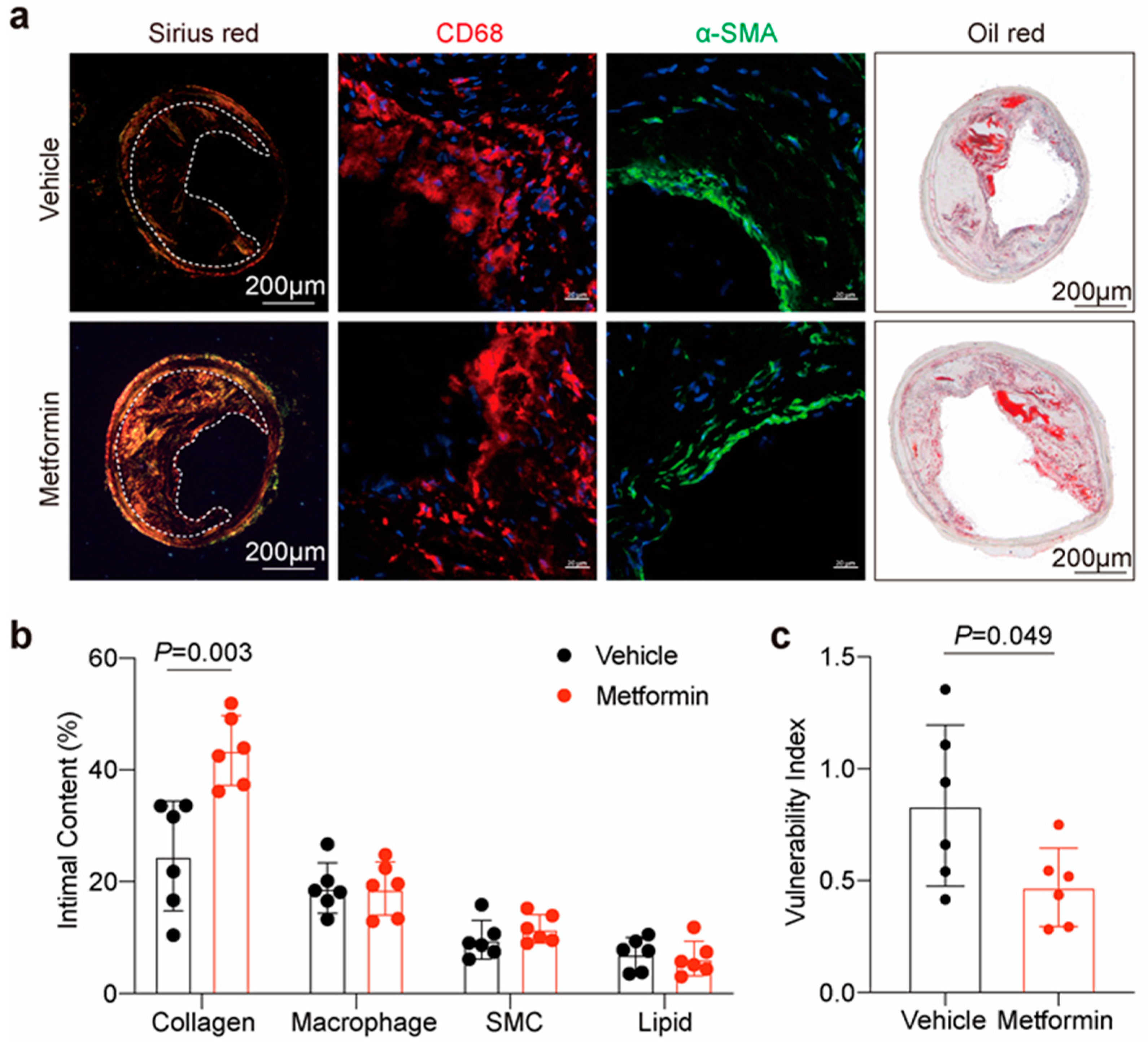
2.3. Histopathology and Immunofluorescence
2.4. Western Blotting
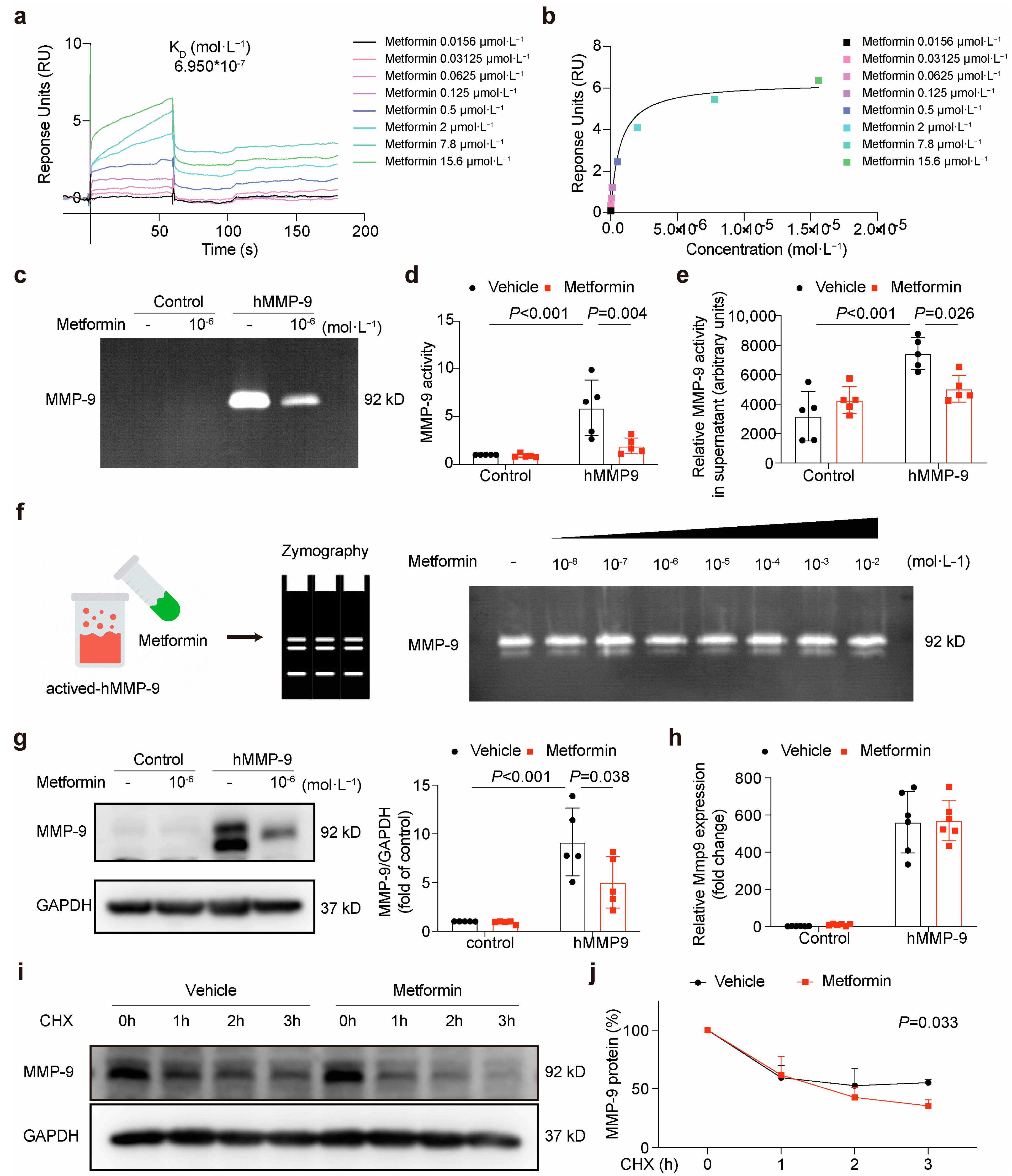
2.5. Matrix Metalloproteinases (MMPs) Activity Assay
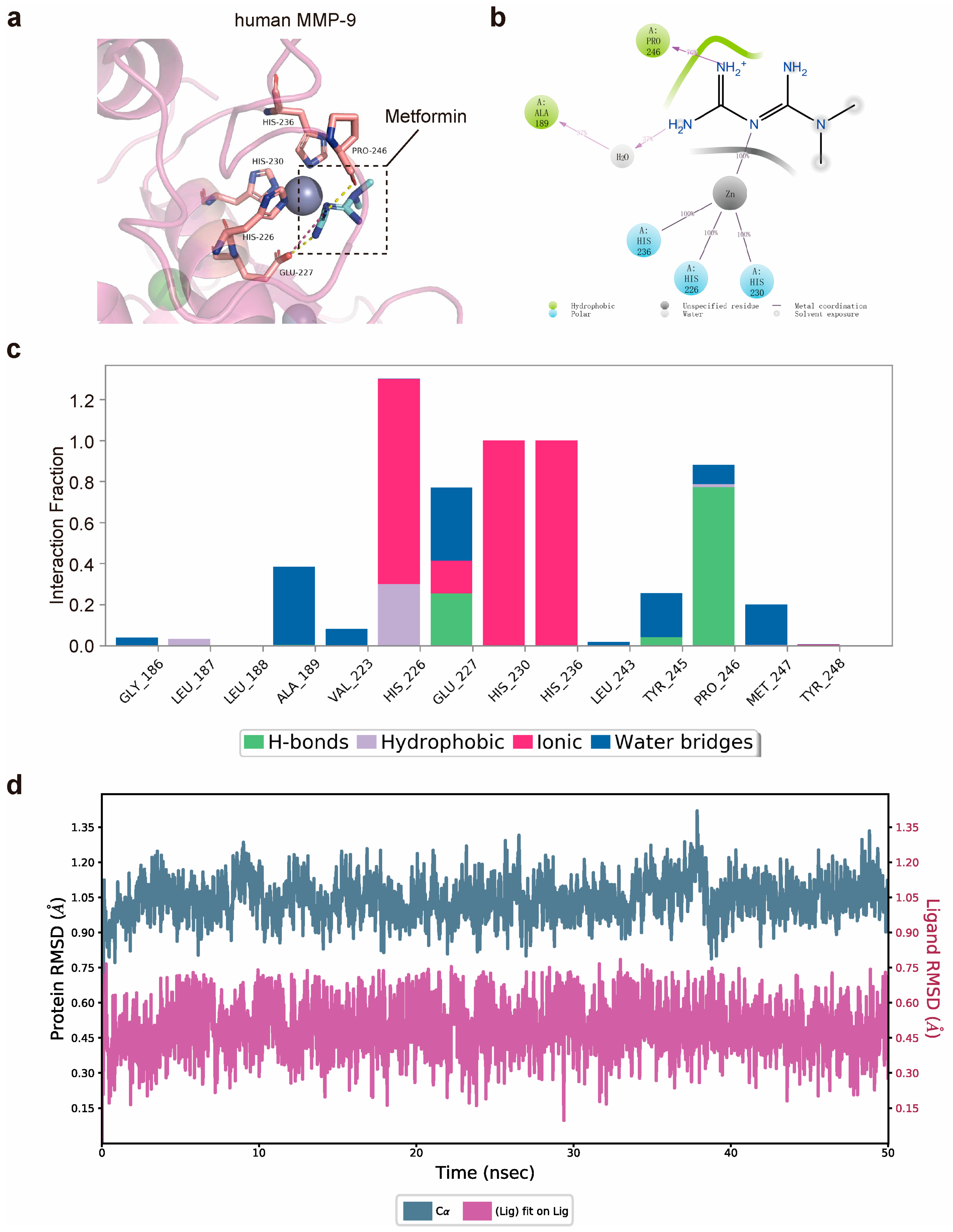
2.6. Molecular Docking and Dynamics Simulation
2.7. Cell Culture, Plasmids, and Transfection
2.8. Quantitative Real-Time PCR
2.9. Surface Plasmon Resonance (SPR) Spectroscopy
2.10. Zymography
2.11. Statistics
3. Results
3.1. Matrix Metalloproteinase-9 (MMP-9) Is Predicted to Bind Directly to Metformin
3.2. Metformin Directly Interacts with MMP-9 and Attenuates Its Activity
3.3. Metformin Inhibits Local Plaque and Circulation MMP-9 Activity in ApoE-/- Mice
3.4. Metformin Improves Atherosclerotic Plaque Stability in ApoE-/- Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Aroda, V.R.; Bluemke, D.A.; Barrett-Connor, E.; Budoff, M.; Crandall, J.P.; Dabelea, D.; Horton, E.S.; Mather, K.J.; Orchard, T.J.; et al. Effect of Long-Term Metformin and Lifestyle in the Diabetes Prevention Program and Its Outcome Study on Coronary Artery Calcium. Circulation 2017, 136, 52–64. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Dai, H.; Deng, Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res. Clin. Pract. 2020, 160, 108001. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Kalantzis, C.; Katsianos, E.; Sanoudou, D.; Vavuranakis, M.; Tousoulis, D. Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque. J. Pers. Med. 2019, 9, 8. [Google Scholar] [CrossRef]
- McDermott, M.M.; Guralnik, J.M.; Corsi, A.; Albay, M.; Macchi, C.; Bandinelli, S.; Ferrucci, L. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am. Heart J. 2005, 150, 276–281. [Google Scholar] [CrossRef]
- Allison, M.A.; Criqui, M.H.; McClelland, R.L.; Scott, J.M.; McDermott, M.M.; Liu, K.; Folsom, A.R.; Bertoni, A.G.; Sharrett, A.R.; Homma, S.; et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 2006, 48, 1190–1197. [Google Scholar] [CrossRef]
- Mallat, Z.; Corbaz, A.; Scoazec, A.; Besnard, S.; Leseche, G.; Chvatchko, Y.; Tedgui, A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 2001, 104, 1598–1603. [Google Scholar] [CrossRef]
- Young, J.L.; Libby, P.; Schonbeck, U. Cytokines in the pathogenesis of atherosclerosis. Thromb. Haemost. 2002, 88, 554–567. [Google Scholar] [CrossRef]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef]
- Holm Nielsen, S.; Jonasson, L.; Kalogeropoulos, K.; Karsdal, M.A.; Reese-Petersen, A.L.; Auf dem Keller, U.; Genovese, F.; Nilsson, J.; Goncalves, I. Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. J. Intern. Med. 2020, 287, 493–513. [Google Scholar] [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef]
- Gu, C.; Wang, F.; Zhao, Z.; Wang, H.; Cong, X.; Chen, X. Lysophosphatidic Acid Is Associated with Atherosclerotic Plaque Instability by Regulating NF-kappaB Dependent Matrix Metalloproteinase-9 Expression via LPA2 in Macrophages. Front. Physiol. 2017, 8, 266. [Google Scholar] [CrossRef]
- Loftus, I.M.; Naylor, A.R.; Goodall, S.; Crowther, M.; Jones, L.; Bell, P.R.; Thompson, M.M. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke 2000, 31, 40–47. [Google Scholar] [CrossRef]
- Jiang, X.B.; Wang, J.S.; Liu, D.H.; Yuan, W.S.; Shi, Z.S. Overexpression of matrix metalloproteinase-9 is correlated with carotid intraplaque hemorrhage in a swine model. J. Neurointerv. Surg. 2013, 5, 473–477. [Google Scholar] [CrossRef]
- de Nooijer, R.; Verkleij, C.J.; von der Thusen, J.H.; Jukema, J.W.; van der Wall, E.E.; van Berkel, T.J.; Baker, A.H.; Biessen, E.A. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 340–346. [Google Scholar] [CrossRef]
- Fukuda, D.; Shimada, K.; Tanaka, A.; Kusuyama, T.; Yamashita, H.; Ehara, S.; Nakamura, Y.; Kawarabayashi, T.; Iida, H.; Yoshiyama, M.; et al. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am. J. Cardiol. 2006, 97, 175–180. [Google Scholar] [CrossRef]
- von der Thusen, J.H.; van Berkel, T.J.; Biessen, E.A. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation 2001, 103, 1164–1170. [Google Scholar] [CrossRef]
- Daugherty, A.; Tall, A.R.; Daemen, M.; Falk, E.; Fisher, E.A.; Garcia-Cardena, G.; Lusis, A.J.; Owens, A.P., 3rd; Rosenfeld, M.E.; Virmani, R.; et al. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Circ. Res. 2017, 121, e53–e79. [Google Scholar] [CrossRef]
- Olson, M.W.; Bernardo, M.M.; Pietila, M.; Gervasi, D.C.; Toth, M.; Kotra, L.P.; Massova, I.; Mobashery, S.; Fridman, R. Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase-9. Differential rates for activation by stromelysin 1. J. Biol. Chem. 2000, 275, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Tomas, L.; Edsfeldt, A.; Mollet, I.G.; Perisic Matic, L.; Prehn, C.; Adamski, J.; Paulsson-Berne, G.; Hedin, U.; Nilsson, J.; Bengtsson, E.; et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur. Heart J. 2018, 39, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef]
- Park, J.P.; Lee, B.K.; Shim, J.M.; Kim, S.H.; Lee, C.W.; Kang, D.H.; Hong, M.K. Relationship between multiple plasma biomarkers and vulnerable plaque determined by virtual histology intravascular ultrasound. Circ. J. 2010, 74, 332–336. [Google Scholar] [CrossRef]
- Chen, F.; Eriksson, P.; Hansson, G.K.; Herzfeld, I.; Klein, M.; Hansson, L.O.; Valen, G. Expression of matrix metalloproteinase 9 and its regulators in the unstable coronary atherosclerotic plaque. Int. J. Mol. Med. 2005, 15, 57–65. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Kosowski, M.; Basiak, M.; Hachula, M.; Okopien, B. Impact of Alirocumab on Release Markers of Atherosclerotic Plaque Vulnerability in Patients with Mixed Hyperlipidemia and Vulnerable Atherosclerotic Plaque. Medicina 2022, 58, 969. [Google Scholar] [CrossRef]
- Basiak, M.; Kosowski, M.; Hachula, M.; Okopien, B. Impact of PCSK9 Inhibition on Proinflammatory Cytokines and Matrix Metalloproteinases Release in Patients with Mixed Hyperlipidemia and Vulnerable Atherosclerotic Plaque. Pharmaceuticals 2022, 15, 802. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Z.; Dluski, D.F.; Wierzchowska-Opoka, M.; Ruszala, M.; Leszczynska-Gorzelak, B. Metformin as a Potential Treatment Option for Endometriosis. Cancers 2022, 14, 577. [Google Scholar] [CrossRef]
- Tay, K.C.; Tan, L.T.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Malekpour-Dehkordi, Z.; Teimourian, S.; Nourbakhsh, M.; Naghiaee, Y.; Sharifi, R.; Mohiti-Ardakani, J. Metformin reduces fibrosis factors in insulin resistant and hypertrophied adipocyte via integrin/ERK, collagen VI, apoptosis, and necrosis reduction. Life Sci. 2019, 233, 116682. [Google Scholar] [CrossRef]
- Shao, W.; Wang, D.; He, J. The role of gene expression profiling in early-stage non-small cell lung cancer. J. Thorac. Dis. 2010, 2, 89–99. [Google Scholar]
- Goldstein, B.J.; Weissman, P.N.; Wooddell, M.J.; Waterhouse, B.R.; Cobitz, A.R. Reductions in biomarkers of cardiovascular risk in type 2 diabetes with rosiglitazone added to metformin compared with dose escalation of metformin: An EMPIRE trial sub-study. Curr. Med. Res. Opin. 2006, 22, 1715–1723. [Google Scholar] [CrossRef]
- Hanefeld, M.; Pfutzner, A.; Forst, T.; Kleine, I.; Fuchs, W. Double-blind, randomized, multicentre, and active comparator controlled investigation of the effect of pioglitazone, metformin, and the combination of both on cardiovascular risk in patients with type 2 diabetes receiving stable basal insulin therapy: The PIOCOMB study. Cardiovasc. Diabetol. 2011, 10, 65. [Google Scholar] [CrossRef]
- Hammad, A.M.; Ibrahim, Y.A.; Khdair, S.I.; Hall, F.S.; Alfaraj, M.; Jarrar, Y.; Abed, A.F. Metformin reduces oxandrolone- induced depression-like behavior in rats via modulating the expression of IL-1beta, IL-6, IL-10 and TNF-alpha. Behav. Brain Res. 2021, 414, 113475. [Google Scholar] [CrossRef]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Ko, S.H.; Kim, J.S. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J. Gastroenterol. Hepatol. 2014, 29, 502–510. [Google Scholar] [CrossRef]
- Docrat, T.F.; Nagiah, S.; Chuturgoon, A.A. Metformin protects against neuroinflammation through integrated mechanisms of miR-141 and the NF-kB-mediated inflammasome pathway in a diabetic mouse model. Eur. J. Pharmacol. 2021, 903, 174146. [Google Scholar] [CrossRef]
- Mummidi, S.; Das, N.A.; Carpenter, A.J.; Kandikattu, H.; Krenz, M.; Siebenlist, U.; Valente, A.J.; Chandrasekar, B. Metformin inhibits aldosterone-induced cardiac fibroblast activation, migration and proliferation in vitro, and reverses aldosterone+salt-induced cardiac fibrosis in vivo. J. Mol. Cell Cardiol. 2016, 98, 95–102. [Google Scholar] [CrossRef]
- Chung, M.M.; Nicol, C.J.; Cheng, Y.C.; Lin, K.H.; Chen, Y.L.; Pei, D.; Lin, C.H.; Shih, Y.N.; Yen, C.H.; Chen, S.J.; et al. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp. Cell Res. 2017, 352, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Liu, M.; Liu, Z.; Yang, X.; Liu, X.; Ni, M.; Dong, M.; Luan, X.; Yuan, Y.; Xu, X.; et al. PEDF improves atherosclerotic plaque stability by inhibiting macrophage inflammation response. Int. J. Cardiol. 2017, 235, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Wang, Y.; Zhang, M.; Zhang, P.F.; Ding, S.F.; Liu, C.X.; Liu, X.L.; Zhao, Y.X.; Zhang, Y. Atherosclerotic plaque disruption induced by stress and lipopolysaccharide in apolipoprotein E knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1598–H1606. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Feng, K.; Zhang, C.; Zhang, H.; Zhang, J.; Hua, Y.; Dong, Z.; Zhu, Y.; Yang, S.; Ma, C. Metformin attenuates atherosclerosis and plaque vulnerability by upregulating KLF2-mediated autophagy in apoE(-/-) mice. Biochem. Biophys. Res. Commun. 2021, 557, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Gadalla, A.E.; Olsen, G.S.; Hardie, D.G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 2002, 51, 2420–2425. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Q.; Zheng, G.; Wang, C.; Zhou, Y.; Wu, Y.; Xuan, J.; Tian, N.; Wang, X.; Wu, Y.; et al. Metformin ameliorates BSCB disruption by inhibiting neutrophil infiltration and MMP-9 expression but not direct TJ proteins expression regulation. J. Cell Mol. Med. 2017, 21, 3322–3336. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, H.; Zhao, X.; Xin, X.; Peng, L.; Ning, Y.; Wang, Y.; Lan, Y.; Zhang, Q. Metformin treatment alleviates polycystic ovary syndrome by decreasing the expression of MMP-2 and MMP-9 via H19/miR-29b-3p and AKT/mTOR/autophagy signaling pathways. J. Cell Physiol. 2019, 234, 19964–19976. [Google Scholar] [CrossRef]
- Hershko, A. Ubiquitin-mediated protein degradation. J. Biol. Chem. 1988, 263, 15237–15240. [Google Scholar] [CrossRef]
- Chang, Y.; Jin, H.; Li, H.; Ma, J.; Zheng, Z.; Sun, B.; Lyu, Y.; Lin, M.; Zhao, H.; Shen, L.; et al. MiRNA-516a promotes bladder cancer metastasis by inhibiting MMP9 protein degradation via the AKT/FOXO3A/SMURF1 axis. Clin. Transl. Med. 2020, 10, e263. [Google Scholar] [CrossRef]
- Kotra, L.P.; Zhang, L.; Fridman, R.; Orlando, R.; Mobashery, S. N-Glycosylation pattern of the zymogenic form of human matrix metalloproteinase-9. Bioorg. Chem. 2002, 30, 356–370. [Google Scholar] [CrossRef]
- Duellman, T.; Burnett, J.; Yang, J. Functional Roles of N-Linked Glycosylation of Human Matrix Metalloproteinase 9. Traffic 2015, 16, 1108–1126. [Google Scholar] [CrossRef]
- Kumar, S.; Cieplak, P. Role of N-glycosylation in activation of proMMP-9. A molecular dynamics simulations study. PLoS ONE 2018, 13, e0191157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, S.; Xu, W.; Zhao, M.; Zhang, Y.; Xiao, H. Metformin Directly Binds to MMP-9 to Improve Plaque Stability. J. Cardiovasc. Dev. Dis. 2023, 10, 54. https://doi.org/10.3390/jcdd10020054
Chen X, Wang S, Xu W, Zhao M, Zhang Y, Xiao H. Metformin Directly Binds to MMP-9 to Improve Plaque Stability. Journal of Cardiovascular Development and Disease. 2023; 10(2):54. https://doi.org/10.3390/jcdd10020054
Chicago/Turabian StyleChen, Xianda, Shuaixing Wang, Wenli Xu, Mingming Zhao, Youyi Zhang, and Han Xiao. 2023. "Metformin Directly Binds to MMP-9 to Improve Plaque Stability" Journal of Cardiovascular Development and Disease 10, no. 2: 54. https://doi.org/10.3390/jcdd10020054
APA StyleChen, X., Wang, S., Xu, W., Zhao, M., Zhang, Y., & Xiao, H. (2023). Metformin Directly Binds to MMP-9 to Improve Plaque Stability. Journal of Cardiovascular Development and Disease, 10(2), 54. https://doi.org/10.3390/jcdd10020054





