Biomarkers for Heart Failure Prediction and Prevention
Abstract
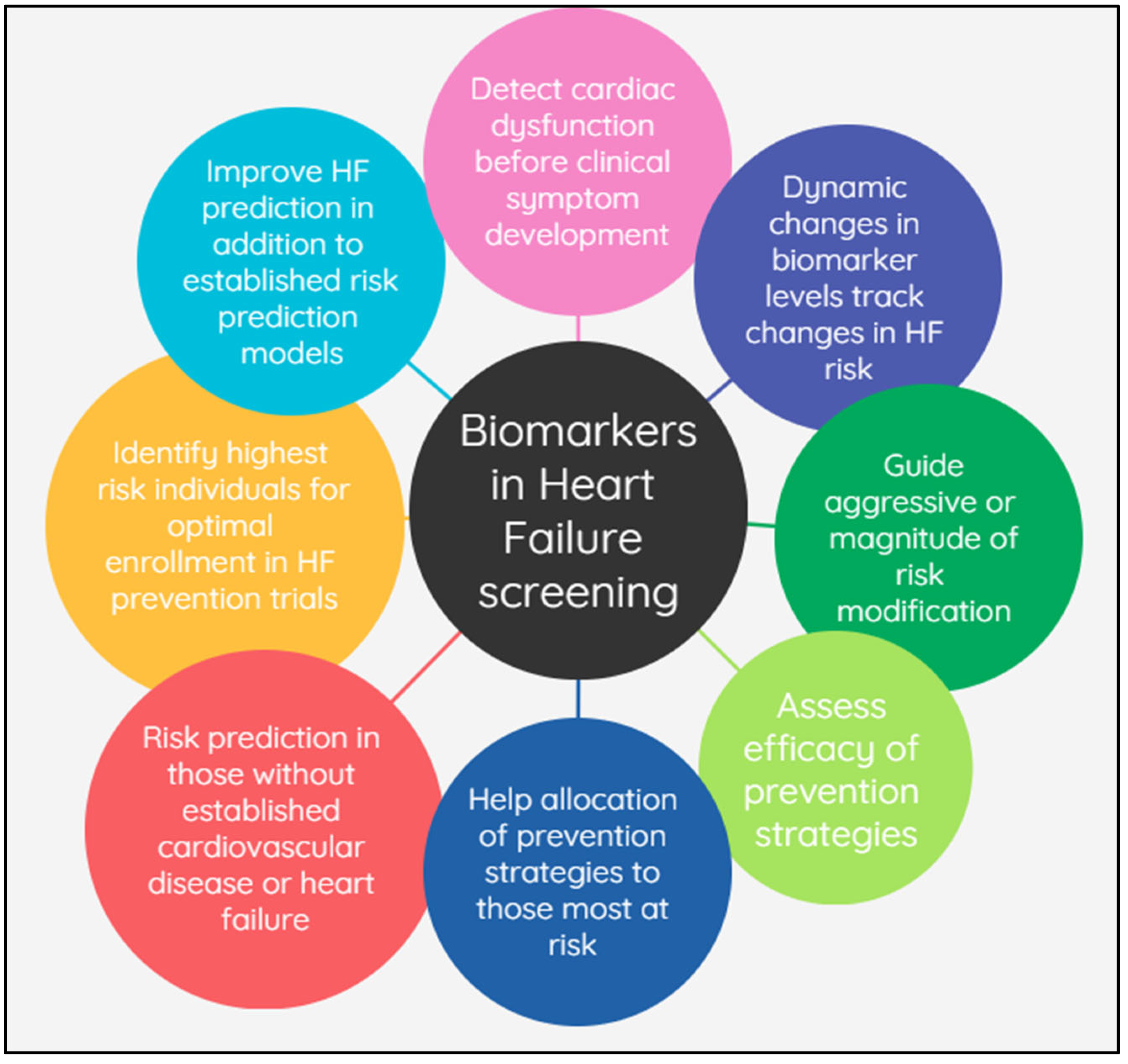
:1. Introduction
2. Biomarkers for the Prediction and Prevention of HF
2.1. Brain Natriuretic Peptide (BNP) and N-Terminal-Pro Hormone BNP (NT-proBNP)
2.1.1. HF Prediction and Prevention
2.1.2. Dynamic Changes in Biomarker Levels Reflect Changes in HF Risk
2.2. Cardiac Troponin
2.3. Albuminuria
2.4. Glomerular Filtration Rate: Cystatin C and Creatinine
2.5. Other Exploratory Biomarkers (e.g., Inflammatory and Oxidative Markers: CRP, Galectin-3, Soluble ST2 (sST2), and Growth Differentiation Factor (GDF-15))
3. Biomarkers in HFpEF vs. HFrEF
4. Race and Biomarkers
5. Multi-Biomarker and Multimodality Approach
6. Strategies to Prevent HF
7. Directions for Future Research
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bragazzi, N.L.; Zhong, W.; Shu, J.; Abu Much, A.; Lotan, D.; Grupper, A.; Younis, A.; Dai, H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021, 28, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.M.; Everitt, I.K.; Khan, S.S. New strategies and therapies for the prevention of heart failure in high-risk patients. Clin. Cardiol. 2022, 45, S13–S25. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, 895–1032. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Felker, G.M. Surfing the biomarker tsunami at JACC: Heart failure. JACC Heart Fail. 2013, 1, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma Natriuretic Peptide Levels and the Risk of Cardiovascular Events and Death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chambless, L.E.; Ballantyne, C.M.; Astor, B.; Bertoni, A.G.; Chang, P.P.; Folsom, A.R.; He, M.; Hoogeveen, R.C.; Ni, H.; et al. Prediction of incident heart failure in general practice the atherosclerosis risk in communities (ARIC) study. Circ. Heart Fail. 2012, 5, 422–429. [Google Scholar] [CrossRef]
- Chahal, H.; Bluemke, D.A.; Wu, C.O.; McClelland, R.; Liu, K.; Shea, S.J.; Burke, G.; Balfour, P.; Herrington, D.; Shi, P.B.; et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart 2015, 101, 58–64. [Google Scholar] [CrossRef]
- Jia, X.; Al Rifai, M.; Hoogeveen, R.; Echouffo-Tcheugui, J.B.; Shah, A.M.; Ndumele, C.E.; Virani, S.S.; Bozkurt, B.; Selvin, E.; Ballantyne, C.M.; et al. Association of Long-term Change in N-Terminal Pro–B-Type Natriuretic Peptide With Incident Heart Failure and Death. JAMA Cardiol. 2023, 8, 222–230. [Google Scholar] [CrossRef]
- Mishra, R.K.; Judson, G.; Christenson, R.H.; Defilippi, C.; Wu, A.H.B.; Whooley, M.A. The Association of Five-Year Changes in the Levels of N-Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP) with Subsequent Heart Failure and Death in Patients with Stable Coronary Artery Disease: The Heart and Soul Study. Cardiology 2017, 137, 201–206. [Google Scholar] [CrossRef] [PubMed]
- deFilippi, C.R.; Christenson, R.H.; Gottdiener, J.S.; Kop, W.J.; Seliger, S.L. Dynamic Cardiovascular Risk Assessment in Elderly People. The Role of Repeated N-Terminal Pro-B-Type Natriuretic Peptide Testing. J. Am. Coll. Cardiol. 2010, 55, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Nambi, V.; Ambrosius, W.T.; Chen, H.; Killeen, A.A.; Taylor, A.; Toto, R.D.; Soliman, E.Z.; McEvoy, J.W.; Pandey, A.; et al. Associations of High-Sensitivity Troponin and Natriuretic Peptide Levels with Outcomes after Intensive Blood Pressure Lowering: Findings from the SPRINT Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vaduganathan, M.; Patel, K.V.; Ayers, C.; Ballantyne, C.M.; Kosiborod, M.N.; Carnethon, M.; DeFilippi, C.; McGuire, D.K.; Khan, S.S.; et al. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail. 2021, 9, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.T.; Nambi, V.; De Lemos, J.A.; Chambless, L.E.; Virani, S.S.; Boerwinkle, E.; Hoogeveen, R.C.; Liu, X.; Astor, B.C.; Mosley, T.H.; et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation 2011, 123, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- DeFilippi, C.R.; De Lemos, J.A.; Christenson, R.H.; Gottdiener, J.S.; Kop, W.J.; Zhan, M.; Seliger, S.L. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010, 304, 2494–2502. [Google Scholar] [CrossRef]
- Nambi, V.; Liu, X.; Chambless, L.E.; De Lemos, J.A.; Virani, S.S.; Agarwal, S.; Boerwinkle, E.; Hoogeveen, R.C.; Aguilar, D.; Astor, B.C.; et al. Troponin T and N-Terminal Pro-B-type natriuretic peptide: A biomarker approach to predict heart failure risk-the atherosclerosis risk in communities study. Clin. Chem. 2013, 59, 1802–1810. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Fonarow, G.C.; Fudim, M.; Hall, M.E.; Hernandez, A.; Morris, A.A.; Shafi, T.; Weir, M.R.; et al. Albuminuria and Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 270–282. [Google Scholar] [CrossRef]
- Blecker, S.; Matsushita, K.; Kttgen, A.; Loehr, L.R.; Bertoni, A.G.; Boulware, L.E.; Coresh, J. High-normal albuminuria and risk of heart failure in the community. Am. J. Kidney Dis. 2011, 58, 47–55. [Google Scholar] [CrossRef]
- Nowak, C.; Ärnlöv, J. Kidney Disease Biomarkers Improve Heart Failure Risk Prediction in the General Population. Circ. Heart Fail. 2020, 13, E006904. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Arnold, A.M.; Aurigemma, G.P.; Polak, J.F.; Tracy, R.P.; Kitzman, D.W.; Gardin, J.M.; Rutledge, J.E.; Boineau, R.C. Predictors of congestive heart failure in the elderly: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2000, 35, 1628–1637. [Google Scholar] [CrossRef]
- Weir, R.A.P.; Miller, A.M.; Murphy, G.E.J.; Clements, S.; Steedman, T.; Connell, J.M.C.; McInnes, I.B.; Dargie, H.J.; McMurray, J.J.V. Serum Soluble ST2. A Potential Novel Mediator in Left Ventricular and Infarct Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 243–250. [Google Scholar] [CrossRef]
- Nambi, V. Biomarkers in Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shah, R.V.; Chen-Tournoux, A.A.; Picard, M.H.; Van Kimmenade, R.R.J.; Januzzi, J.L. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ. Heart Fail. 2009, 2, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Clopton, P.; Iqbal, N.; Tran, K.; Maisel, A.S. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am. Heart J. 2010, 160, 721–728. [Google Scholar] [CrossRef]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- Jagodzinski, A.; Havulinna, A.S.; Appelbaum, S.; Zeller, T.; Jousilahti, P.; Skytte-Johanssen, S.; Hughes, M.F.; Blankenberg, S.; Salomaa, V. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int. J. Cardiol. 2015, 192, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Wollert, K.C.; Larson, M.G.; Coglianese, E.; McCabe, E.L.; Cheng, S.; Ho, J.E.; Fradley, M.G.; Ghorbani, A.; Xanthakis, V.; et al. Prognostic utility of novel biomarkers of cardiovascular stress: The framingham heart study. Circulation 2012, 126, 1596–1604. [Google Scholar] [CrossRef]
- De Boer, R.A.; Nayor, M.; DeFilippi, C.R.; Enserro, D.; Bhambhani, V.; Kizer, J.R.; Blaha, M.J.; Brouwers, F.P.; Cushman, M.; Lima, J.A.C.; et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018, 3, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults. The Health ABC (Health, Aging, and Body Composition) Study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef]
- Watson, C.J.; Gallagher, J.; Wilkinson, M.; Russell-Hallinan, A.; Tea, I.; James, S.; O’Reilly, J.; O’Connell, E.; Zhou, S.; Ledwidge, M.; et al. Biomarker profiling for risk of future heart failure (HFpEF) development. J. Transl. Med. 2021, 19, 61. [Google Scholar] [CrossRef]
- Brouwers, F.P.; Van Gilst, W.H.; Damman, K.; Van Den Berg, M.P.; Gansevoort, R.T.; Bakker, S.J.L.; Hillege, H.L.; Van Veldhuisen, D.J.; Van Der Harst, P.; De Boer, R.A. Clinical risk stratification optimizes value of biomarkers to predict new-onset heart failure in a community-based cohort. Circ. Heart Fail. 2014, 7, 723–731. [Google Scholar] [CrossRef]
- Segar, M.W.; Jaeger, B.C.; Patel, K.V.; Nambi, V.; Ndumele, C.E.; Correa, A.; Butler, J.; Chandra, A.; Ayers, C.; Rao, S.; et al. Development and Validation of Machine Learning-Based Race-Specific Models to Predict 10-Year Risk of Heart Failure: A Multicohort Analysis. Circulation 2021, 143, 2370–2383. [Google Scholar] [CrossRef]
- Jia, X.; Al Rifai, M.; Ndumele, C.E.; Virani, S.S.; de Lemos, J.A.; Lee, E.; Shah, A.M.; Echouffo-Tcheugui, J.B.; Bozkurt, B.; Hoogeveen, R.; et al. Reclassification of Pre-Heart Failure Stages Using Cardiac Biomarkers. JACC Heart Fail. 2023, 11, 440–450. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Defilippi, C.R.; Gottdiener, J.S.; Butler, J. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: The cardiovascular health study. JACC Cardiovasc. Imaging 2012, 5, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yan, I.; Börschel, C.S.; Neumann, J.T.; Sprünker, N.A.; Makarova, N.; Kontto, J.; Kuulasmaa, K.; Salomaa, V.; Magnussen, C.; Iacoviello, L.; et al. High-Sensitivity Cardiac Troponin I Levels and Prediction of Heart Failure: Results From the BiomarCaRE Consortium. JACC Heart Fail. 2020, 8, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Drazner, M.H.; Berry, J.D.; Ayers, C.R.; Defilippi, C.; Seliger, S.L.; Nambi, V.; McGuire, D.K.; Omland, T.; De Lemos, J.A. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J. Am. Coll. Cardiol. 2013, 61, 187–195. [Google Scholar] [CrossRef]
- Lewis, A.A.; Ayers, C.R.; Selvin, E.; Neeland, I.; Ballantyne, C.M.; Nambi, V.; Pandey, A.; Powell-Wiley, T.M.; Drazner, M.H.; Carnethon, M.R.; et al. Racial Differences in Malignant Left Ventricular Hypertrophy and Incidence of Heart Failure: A Multicohort Study. Circulation 2020, 141, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Ledwidge, M.; Gallagher, J.; Conlon, C.; Tallon, E.; O’Connell, E.; Dawkins, I.; Watson, C.; O’Hanlon, R.; Bermingham, M.; Patle, A.; et al. Natriuretic peptide-based screening and collaborative care for heart failure: The STOP-HF randomized trial. JAMA 2013, 310, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, M.; Castle, C.H.; Elson, L. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA J. Am. Med. Assoc. 1970, 213, 1143–1152. [Google Scholar] [CrossRef]
- Sundström, J.; Arima, H.; Woodward, M.; Jackson, R.; Karmali, K.; Lloyd-Jones, D.; Baigent, C.; Emberson, J.; Rahimi, K.; Macmahon, S.; et al. Blood pressure-lowering treatment based on cardiovascular risk: A meta-analysis of individual patient data. Lancet 2014, 384, 591–598. [Google Scholar] [CrossRef]
- Harrington, J.; Anker, S.; Butler, J. Putting the puzzle together: SGLT2 inhibitors from prevention to treatment of heart failure. Eur. Heart J. 2022, 43, 4433–4435. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.M.; Rivera-Caravaca, J.M.; Underhill, P.; Fauchier, L.; Lip, G.Y.H. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: Insights from a global federated electronic medical record database. Diabetes Obes. Metab. 2023, 25, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses from the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef]
- Uijl, A.; Koudstaal, S.; Vaartjes, I.; Boer, J.M.A.; Verschuren, W.M.M.; van der Schouw, Y.T.; Asselbergs, F.W.; Hoes, A.W.; Sluijs, I. Risk for Heart Failure: The Opportunity for Prevention With the American Heart Association’s Life’s Simple 7. JACC Heart Fail. 2019, 7, 637–647. [Google Scholar] [CrossRef]
- Ascher, S.B.; de Lemos, J.A.; Lee, M.J.; Wu, E.; Soliman, E.Z.; Neeland, I.J.; Kitzman, D.W.; Ballantyne, C.M.; Nambi, V.; Killeen, A.A.; et al. Intensive Blood Pressure Lowering in Patients With Malignant Left Ventricular Hypertrophy. J. Am. Coll. Cardiol. 2022, 80, 1516–1525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotta, P.A.; Nambi, V.; Bozkurt, B. Biomarkers for Heart Failure Prediction and Prevention. J. Cardiovasc. Dev. Dis. 2023, 10, 488. https://doi.org/10.3390/jcdd10120488
Kotta PA, Nambi V, Bozkurt B. Biomarkers for Heart Failure Prediction and Prevention. Journal of Cardiovascular Development and Disease. 2023; 10(12):488. https://doi.org/10.3390/jcdd10120488
Chicago/Turabian StyleKotta, Prasanti Alekhya, Vijay Nambi, and Biykem Bozkurt. 2023. "Biomarkers for Heart Failure Prediction and Prevention" Journal of Cardiovascular Development and Disease 10, no. 12: 488. https://doi.org/10.3390/jcdd10120488
APA StyleKotta, P. A., Nambi, V., & Bozkurt, B. (2023). Biomarkers for Heart Failure Prediction and Prevention. Journal of Cardiovascular Development and Disease, 10(12), 488. https://doi.org/10.3390/jcdd10120488





