Sex Differences in Mouse Cardiac Electrophysiology Revealed by Simultaneous Imaging of Excitation-Contraction Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
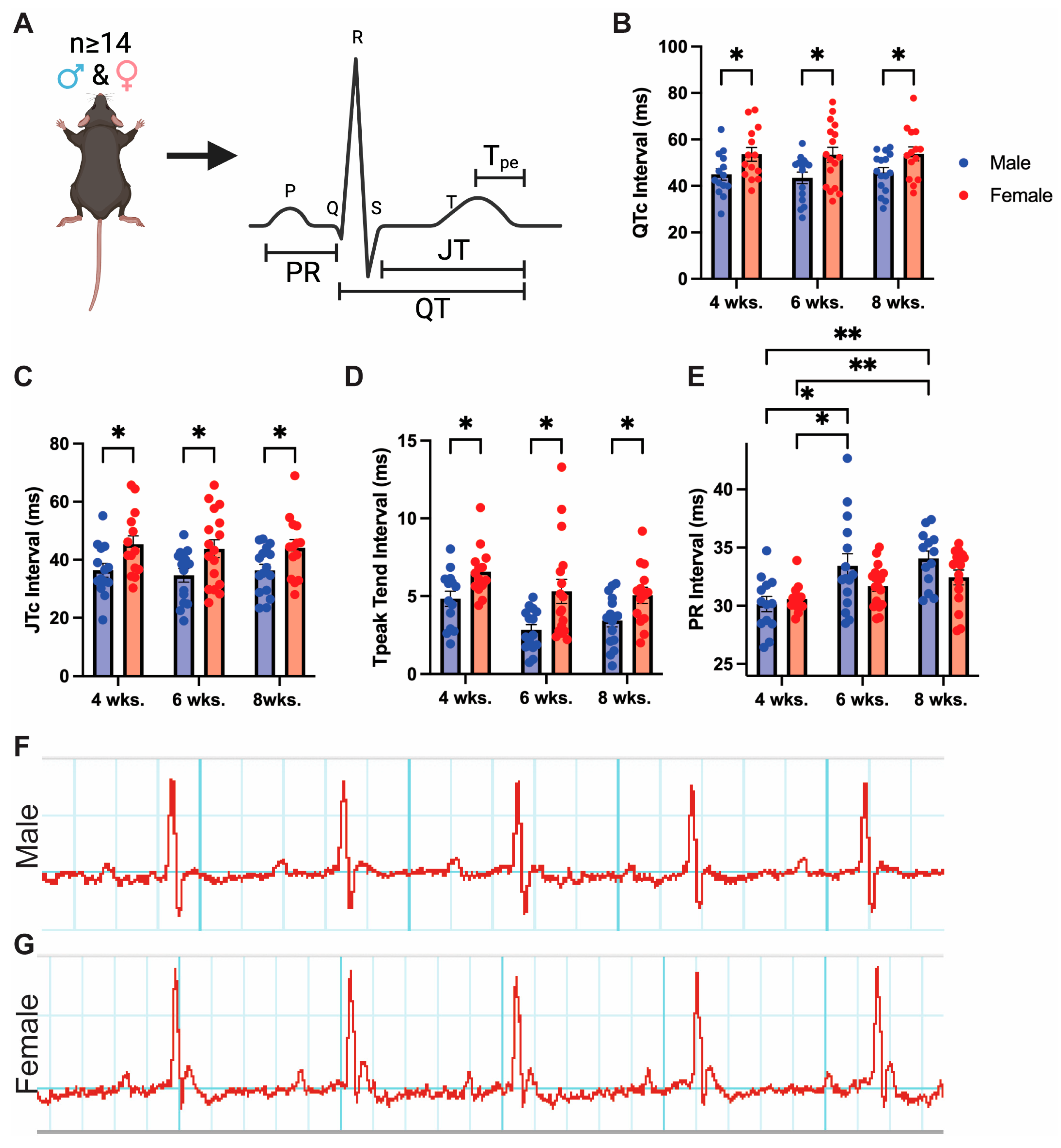
2.2. Mouse Surface Electrocardiography
2.3. Cardiomyocyte Isolation and Fluorescent Dyes
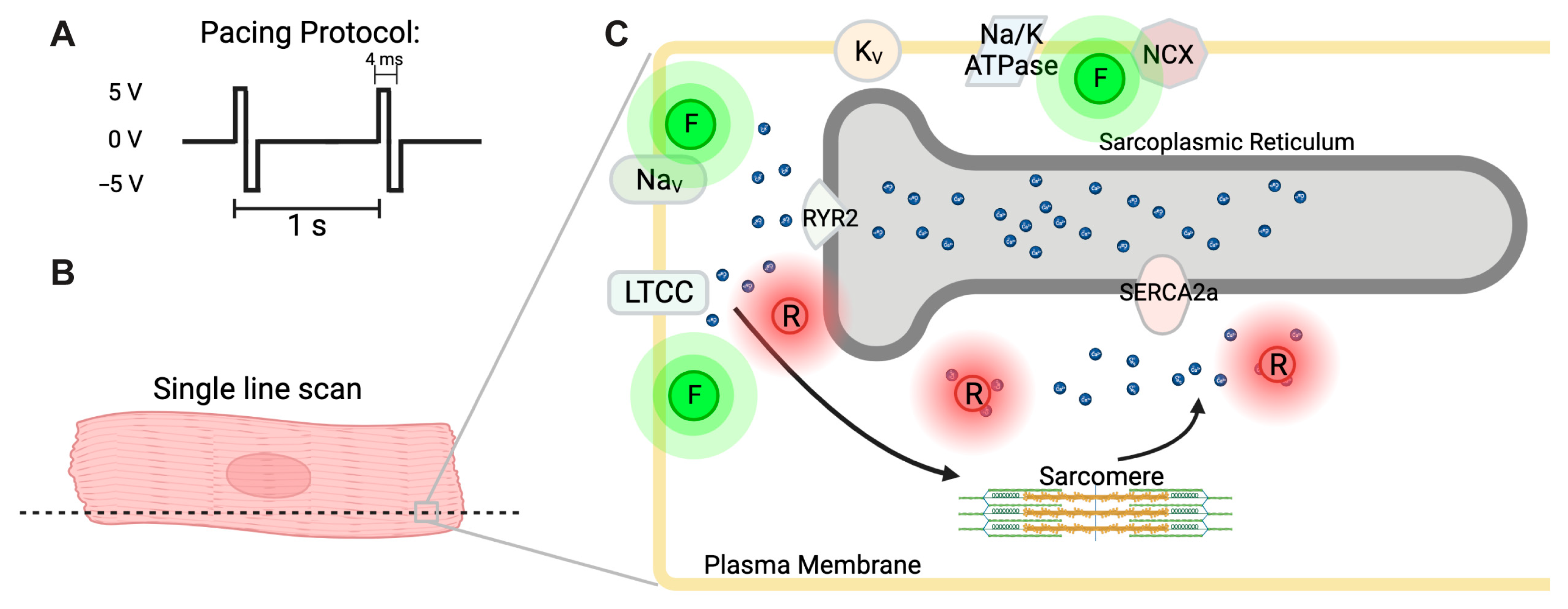
2.4. Simultaneous Imaging of Ca2+ Handling, Membrane Potential, and Contraction
2.5. Image Analysis in ImageJ and Microsoft Excel
2.6. Statistical Methods
3. Results
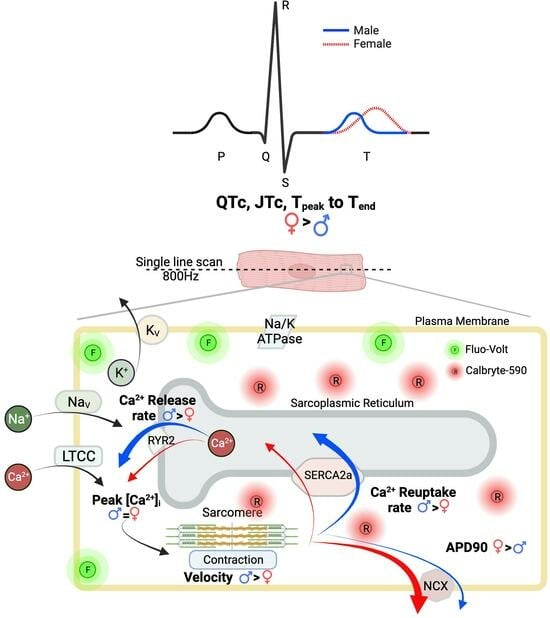
3.1. Adult Mice Demonstrate Baseline Sex Differences in Cardiac Repolarization
3.2. Simultaneous Detection of Cardiac Action Potential, Ca2+ Transient, and Contraction
3.3. Isolated Female Cardiomyocytes Exhibit Increased Action Potential Duration
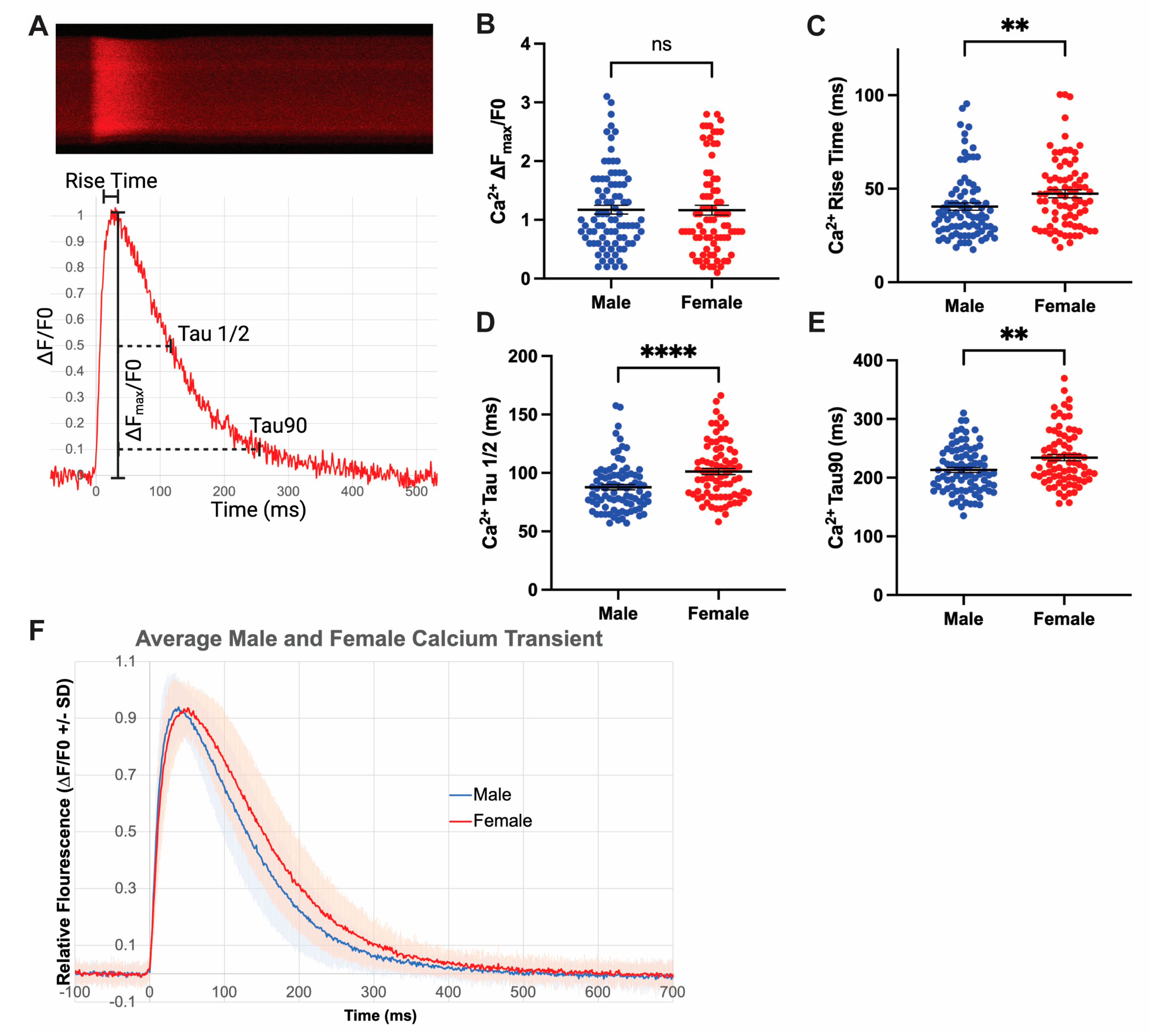
3.4. Increased Rates of Ca2+ Release and Reuptake in Male Cardiomyocytes
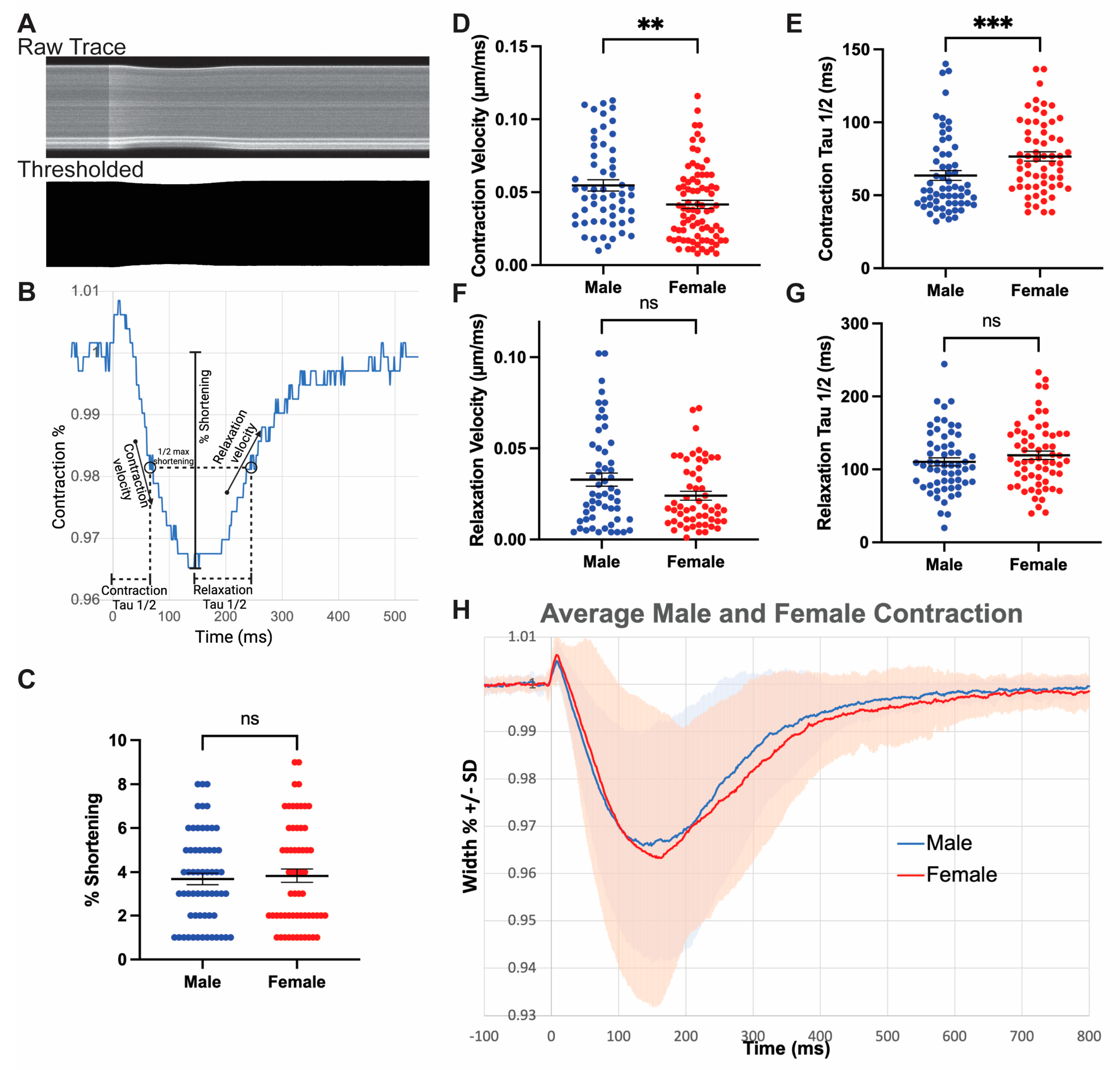
3.5. Male Myocytes Contract with Greater Velocity Compared to Females
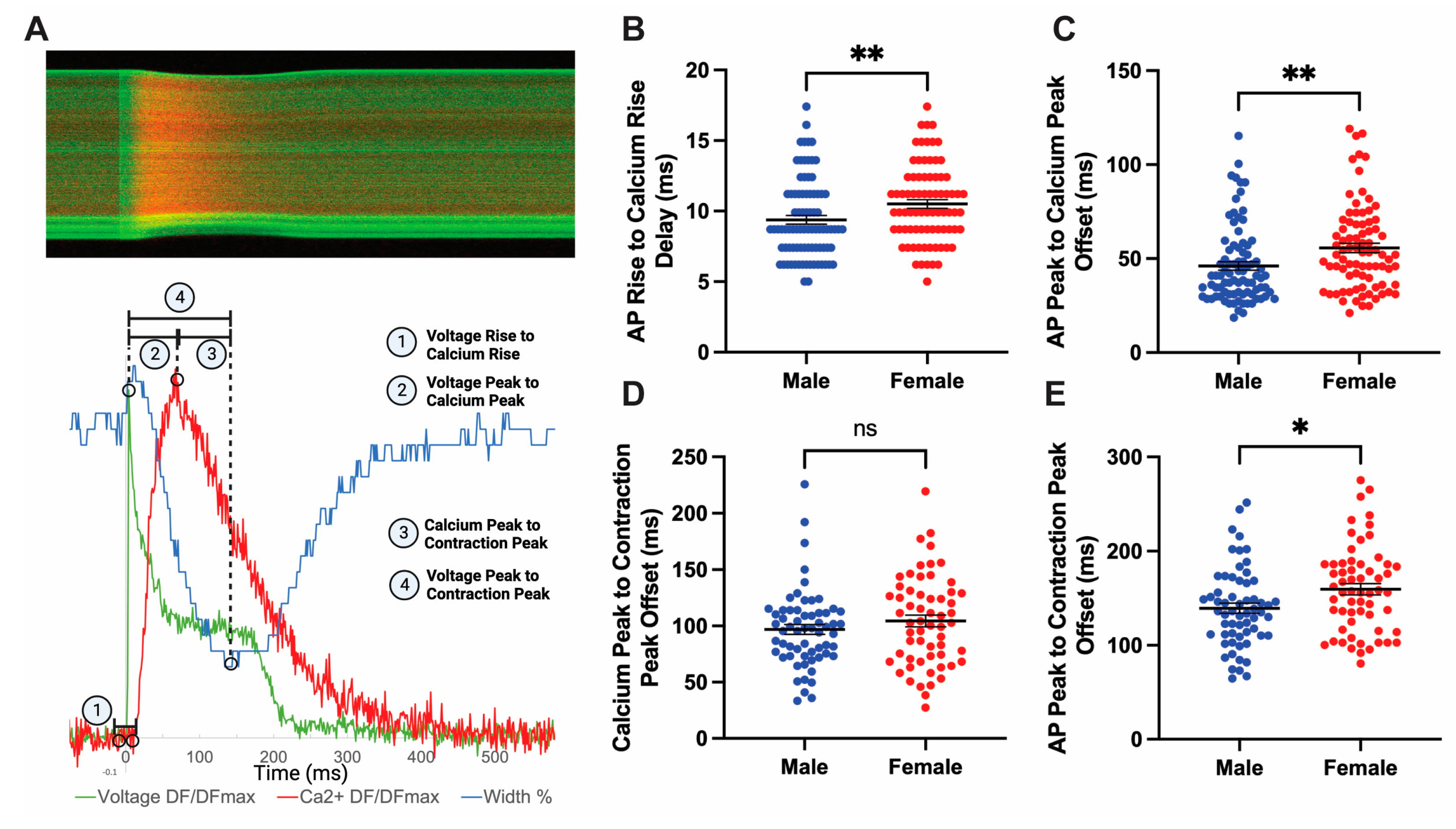
3.6. Sex Differences Exist in Coupling Times between Action Potential and Peak Contraction
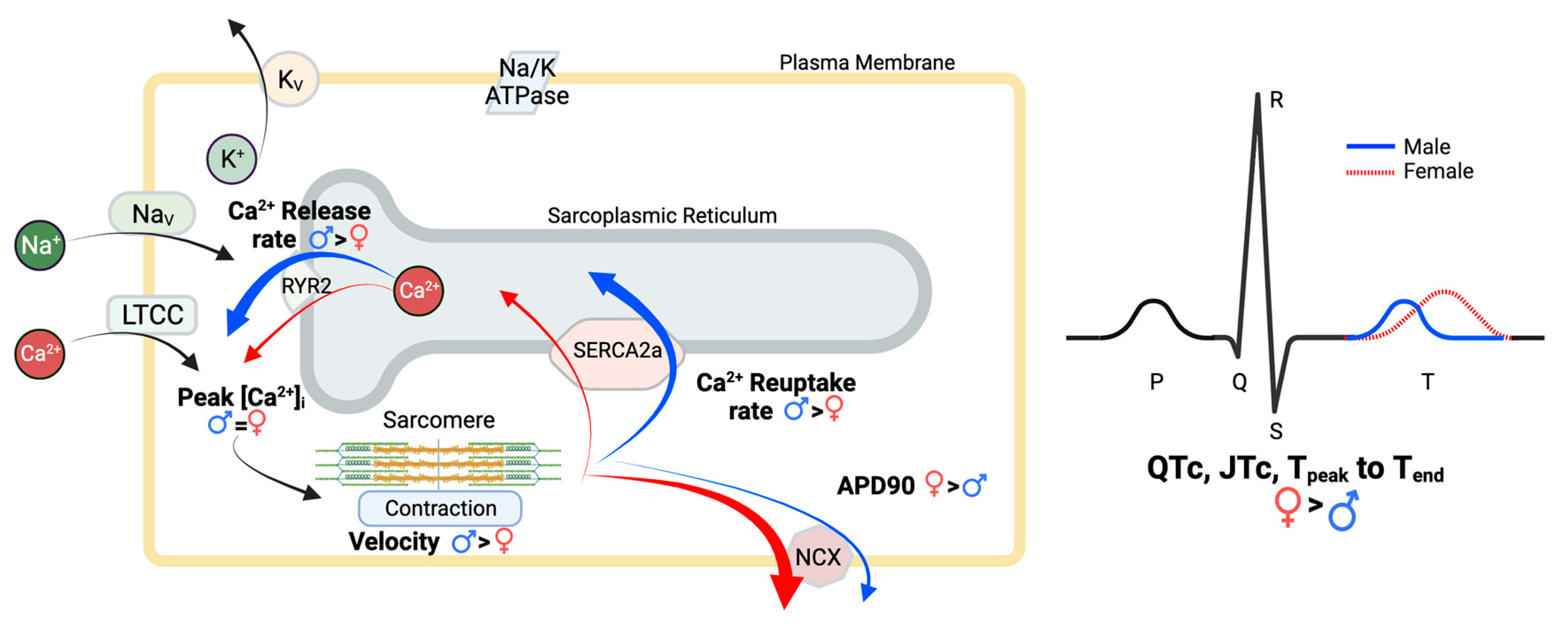
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Hodlmoser, S.; Winkelmayer, W.C.; Zee, J.; Pecoits-Filho, R.; Pisoni, R.L.; Port, F.K.; Robinson, B.M.; Ristl, R.; Krenn, S.; Kurnikowski, A.; et al. Sex differences in chronic kidney disease awareness among US adults, 1999 to 2018. PLoS ONE 2020, 15, e0243431. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Gupta, S.R.; Moustafa, A.F.; Chao, A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021, 10, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Ehdaie, A.; Cingolani, E.; Shehata, M.; Wang, X.; Curtis, A.B.; Chugh, S.S. Sex Differences in Cardiac Arrhythmias: Clinical and Research Implications. Circ. Arrhythm. Electrophysiol. 2018, 11, e005680. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Ommen, S.R.; Geske, J.B. Sex, Survival, and Cardiomyopathy: Differences Between Men and Women With Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e014448. [Google Scholar] [CrossRef]
- Ghani, A.; Maas, A.H.; Delnoy, P.P.; Ramdat Misier, A.R.; Ottervanger, J.P.; Elvan, A. Sex-Based Differences in Cardiac Arrhythmias, ICD Utilisation and Cardiac Resynchronisation Therapy. Neth. Heart J. 2011, 19, 35–40. [Google Scholar] [CrossRef]
- Gillis, A.M. Atrial Fibrillation and Ventricular Arrhythmias: Sex Differences in Electrophysiology, Epidemiology, Clinical Presentation, and Clinical Outcomes. Circulation 2017, 135, 593–608. [Google Scholar] [CrossRef]
- Priori, S.G.; Schwartz, P.J.; Napolitano, C.; Bloise, R.; Ronchetti, E.; Grillo, M.; Vicentini, A.; Spazzolini, C.; Nastoli, J.; Bottelli, G.; et al. Risk stratification in the long-QT syndrome. N. Engl. J. Med. 2003, 348, 1866–1874. [Google Scholar] [CrossRef]
- Locati, E.H.; Zareba, W.; Moss, A.J.; Schwartz, P.J.; Vincent, G.M.; Lehmann, M.H.; Towbin, J.A.; Priori, S.G.; Napolitano, C.; Robinson, J.L.; et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: Findings from the International LQTS Registry. Circulation 1998, 97, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- James, A.F.; Choisy, S.C.; Hancox, J.C. Recent advances in understanding sex differences in cardiac repolarization. Prog. Biophys. Mol. Biol. 2007, 94, 265–319. [Google Scholar] [CrossRef] [PubMed]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ. Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef]
- Rutkowski, D.R.; Barton, G.P.; François, C.J.; Aggarwal, N.; Roldán-Alzate, A. Sex Differences in Cardiac Flow Dynamics of Healthy Volunteers. Radiol. Cardiothorac. Imaging 2020, 2, e190058. [Google Scholar] [CrossRef]
- Ramaekers, D.; Ector, H.; Aubert, A.E.; Rubens, A.; Van de Werf, F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur. Heart J. 1998, 19, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Bazett, H.C. An Analysis of the Time-Relations of Electrocardiograms. Heart 1920, 7, 353–370. [Google Scholar] [CrossRef]
- Rezus, C.; Moga, V.D.; Ouatu, A.; Floria, M. QT interval variations and mortality risk: Is there any relationship? Anatol. J. Cardiol. 2015, 15, 255–258. [Google Scholar] [CrossRef]
- Williams, E.S.; Thomas, K.L.; Broderick, S.; Shaw, L.K.; Velazquez, E.J.; Al-Khatib, S.M.; Daubert, J.P. Race and gender variation in the QT interval and its association with mortality in patients with coronary artery disease: Results from the Duke Databank for Cardiovascular Disease (DDCD). Am. Heart J. 2012, 164, 434–441. [Google Scholar] [CrossRef]
- Somberg, J.C.; Preston, R.A.; Ranade, V.; Cvetanovic, I.; Molnar, J. Gender differences in cardiac repolarization following intravenous sotalol administration. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 86–92. [Google Scholar] [CrossRef]
- Khatib, R.; Sabir, F.R.N.; Omari, C.; Pepper, C.; Tayebjee, M.H. Managing drug-induced QT prolongation in clinical practice. Postgrad. Med. J. 2021, 97, 452–458. [Google Scholar] [CrossRef]
- Salama, G.; Bett, G.C. Sex differences in the mechanisms underlying long QT syndrome. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H640–H648. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.V.; Rosen, M.R. Sex, hormones, and repolarization. Cardiovasc. Res. 2002, 53, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J. Evidences of the gender-related differences in cardiac repolarization and the underlying mechanisms in different animal species and human. Fundam. Clin. Pharmacol. 2006, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Trepanier-Boulay, V.; St-Michel, C.; Tremblay, A.; Fiset, C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ. Res. 2001, 89, 437–444. [Google Scholar] [CrossRef]
- Brunet, S.; Aimond, F.; Li, H.; Guo, W.; Eldstrom, J.; Fedida, D.; Yamada, K.A.; Nerbonne, J.M. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J. Physiol. 2004, 559, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Anderson ME. Reduced repolarization reserve in ventricular myocytes from female mice. Cardiovasc. Res. 2002, 53, 763–769. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Rodgers, L.E.; Tian, Z.; Allen-Gipson, D.; Panguluri, S.K. Sex differences in murine cardiac pathophysiology with hyperoxia exposure. J. Cell Physiol. 2019, 234, 1491–1501. [Google Scholar] [CrossRef]
- Warhol, A.; George, S.A.; Obaid, S.N.; Efimova, T.; Efimov, I.R. Differential cardiotoxic electrocardiographic response to doxorubicin treatment in conscious versus anesthetized mice. Physiol. Rep. 2021, 9, e14987. [Google Scholar] [CrossRef]
- Brouillette, J.; Trepanier-Boulay, V.; Fiset, C. Effect of androgen deficiency on mouse ventricular repolarization. J. Physiol. 2003, 546, 403–413. [Google Scholar] [CrossRef]
- Drici, M.D.; Baker, L.; Plan, P.; Barhanin, J.; Romey, G.; Salama, G. Mice display sex differences in halothane-induced polymorphic ventricular tachycardia. Circulation 2002, 106, 497–503. [Google Scholar] [CrossRef]
- Saito, T.; Ciobotaru, A.; Bopassa, J.C.; Toro, L.; Stefani, E.; Eghbali, M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ. Res. 2009, 105, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflug. Arch. 2013, 465, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Ray, G.; Bienvenu, L.A.; Rose, R.A.; Howlett, S.E. Sex differences in SR Ca2+ release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J. Mol. Cell Cardiol. 2014, 75, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Ceylan-Isik, A.F.; LaCour, K.H.; Ren, J. Sex difference in cardiomyocyte function in normal and metallothionein transgenic mice: The effect of diabetes mellitus. J. Appl. Physiol. (1985) 2006, 100, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Wasserstrom, J.A.; Kapur, S.; Jones, S.; Faruque, T.; Sharma, R.; Kelly, J.E.; Pappas, A.; Ho, W.; Kadish, A.H.; Aistrup, G.L. Characteristics of intracellular Ca2+ cycling in intact rat heart: A comparison of sex differences. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1895–H1904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curl, C.L.; Wendt, I.R.; Kotsanas, G. Effects of gender on intracellular. Pflug. Arch. 2001, 441, 709–716. [Google Scholar] [CrossRef]
- Ceylan-Isik, A.F.; Li, Q.; Ren, J. Insulin-like growth factor I (IGF-1) deficiency ameliorates sex difference in cardiac contractile function and intracellular Ca2+ homeostasis. Toxicol. Lett. 2011, 206, 130–138. [Google Scholar] [CrossRef]
- Farrell, S.R.; Ross, J.L.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H36–H45. [Google Scholar] [CrossRef]
- Parks, R.J.; Bogachev, O.; Mackasey, M.; Ray, G.; Rose, R.A.; Howlett, S.E. The impact of ovariectomy on cardiac excitation-contraction coupling is mediated through cAMP/PKA-dependent mechanisms. J. Mol. Cell Cardiol. 2017, 111, 51–60. [Google Scholar] [CrossRef]
- Crocini, C.; Coppini, R.; Ferrantini, C.; Yan, P.; Loew, L.M.; Poggesi, C.; Cerbai, E.; Pavone, F.S.; Sacconi, L. T-Tubular Electrical Defects Contribute to Blunted beta-Adrenergic Response in Heart Failure. Int. J. Mol. Sci. 2016, 17, 1471. [Google Scholar] [CrossRef]
- Lang, D.; Sulkin, M.; Lou, Q.; Efimov, I.R. Optical mapping of action potentials and calcium transients in the mouse heart. J. Vis. Exp. 2011, 55, e3275. [Google Scholar] [CrossRef]
- George, S.A.; Lin, Z.; Efimov, I.R. Simultaneous triple-parametric optical mapping of transmembrane potential, intracellular calcium and NADH for cardiac physiology assessment. Commun. Biol. 2022, 5, 319. [Google Scholar] [CrossRef]
- van Meer, B.J.; Krotenberg, A.; Sala, L.; Davis, R.P.; Eschenhagen, T.; Denning, C.; Tertoolen, L.G.J.; Mummery, C.L. Simultaneous measurement of excitation-contraction coupling parameters identifies mechanisms underlying contractile responses of hiPSC-derived cardiomyocytes. Nat. Commun. 2019, 10, 4325. [Google Scholar] [CrossRef]
- Nguyen, C.; Upadhyay, H.; Murphy, M.; Borja, G.; Rozsahegyi, E.J.; Barnett, A.; Brookings, T.; McManus, O.B.; Werley, C.A. Simultaneous voltage and calcium imaging and optogenetic stimulation with high sensitivity and a wide field of view. Biomed. Opt. Express. 2019, 10, 789–806. [Google Scholar] [CrossRef]
- Ackers-Johnson, M.; Li, P.Y.; Holmes, A.P.; O’Brien, S.M.; Pavlovic, D.; Foo, R.S. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circ. Res. 2016, 119, 909–920. [Google Scholar] [CrossRef]
- Shi, W.; Sheng, X.; Dorr, K.M.; Hutton, J.E.; Emerson, J.I.; Davies, H.A.; Andrade, T.D.; Wasson, L.K.; Greco, T.M.; Hashimoto, Y.; et al. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev. Cell. 2021, 56, 3019–3034.e7. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Kaltenbrun, E.; Greco, T.M.; Temple, B.; Herring, L.E.; Cristea, I.M.; Conlon, F.L. Formation of a TBX20-CASZ1 protein complex is protective against dilated cardiomyopathy and critical for cardiac homeostasis. PLoS Genet. 2017, 13, e1007011. [Google Scholar] [CrossRef] [PubMed]
- Judd, J.; Lovas, J.; Huang, G.N. Isolation, Culture and Transduction of Adult Mouse Cardiomyocytes. J. Vis. Exp. 2016, e54012. [Google Scholar] [CrossRef]
- Herron, T.J.; Lee, P.; Jalife, J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ. Res. 2012, 110, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Goudy, J.; Henley, T.; Bressan, M. Optical Electrophysiology in the Developing Heart. J. Cardiovasc. Dev. Dis. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Bedut, S.; Seminatore-Nole, C.; Lamamy, V.; Caignard, S.; Boutin, J.A.; Nosjean, O.; Stephan, J.P.; Coge, F. High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human iPSC-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H44–H53. [Google Scholar] [CrossRef] [PubMed]
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Oheim, M.; van ‘t Hoff, M.; Feltz, A.; Zamaleeva, A.; Mallet, J.M.; Collot, M. New red-fluorescent calcium indicators for optogenetics, photoactivation and multi-color imaging. Biochim. Biophys. Acta 2014, 1843, 2284–2306. [Google Scholar] [CrossRef]
- Rabkin, S.W. Impact of Age and Sex on QT Prolongation in Patients Receiving Psychotropics. Can. J. Psychiatry 2015, 60, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Zhang, F.; Weiss, J.; Ehlert, F.A.; Rosenthal, J.E. Diurnal pattern of QTc interval: How long is prolonged? Possible relation to circadian triggers of cardiovascular events. J. Am. Coll. Cardiol. 1996, 27, 76–83. [Google Scholar] [CrossRef]
- Hnatkova, K.; Toman, O.; Sisakova, M.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotny, T.; Schmidt, G.; Malik, M. Sex and race differences in J-Tend, J-Tpeak, and Tpeak-Tend intervals. Sci. Rep. 2019, 9, 19880. [Google Scholar] [CrossRef]
- Spodick, D.H. Reduction of QT-interval imprecision and variance by measuring the JT interval. Am. J. Cardiol. 1992, 70, 103. [Google Scholar] [CrossRef]
- Prenner, S.B.; Shah, S.J.; Goldberger, J.J.; Sauer, A.J. Repolarization Heterogeneity: Beyond the QT Interval. J. Am. Heart Assoc. 2016, 5, e003607. [Google Scholar] [CrossRef]
- Baumann, P.Q.; Sobel, B.E.; Tarikuz Zaman, A.K.; Schneider, D.J. Gender-dependent differences in echocardiographic characteristics of murine hearts. Echocardiography 2008, 25, 739–748. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Pott, C.; Eckardt, L.; Goldhaber, J.I. Triple threat: The Na+/Ca2+ exchanger in the pathophysiology of cardiac arrhythmia, ischemia and heart failure. Curr. Drug Targets 2011, 12, 737–747. [Google Scholar] [CrossRef]
- Li, L.; Chu, G.; Kranias, E.G.; Bers, D.M. Cardiac myocyte calcium transport in phospholamban knockout mouse: Relaxation and endogenous CaMKII effects. Am. J. Physiol. 1998, 274, H1335–H1347. [Google Scholar] [CrossRef]
- Voorhees, A.P.; Han, H.C. Biomechanics of Cardiac Function. Compr. Physiol. 2015, 5, 1623–1644. [Google Scholar] [CrossRef]
- Salerno, S.; Garten, K.; Smith, G.L.; Stolen, T.; Kelly, A. Two-photon excitation of FluoVolt allows improved interrogation of transmural electrophysiological function in the intact mouse heart. Prog. Biophys. Mol. Biol. 2020, 154, 11–20. [Google Scholar] [CrossRef]
- MacDonald, J.K.; Pyle, W.G.; Reitz, C.J.; Howlett, S.E. Cardiac contraction, calcium transients, and myofilament calcium sensitivity fluctuate with the estrous cycle in young adult female mice. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H938–H953. [Google Scholar] [CrossRef]
- Macfarlane, P.W. The Influence of Age and Sex on the Electrocardiogram. Adv. Exp. Med. Biol. 2018, 1065, 93–106. [Google Scholar] [CrossRef]
- Chu, S.H.; Sutherland, K.; Beck, J.; Kowalski, J.; Goldspink, P.; Schwertz, D. Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci. 2005, 76, 2735–2749. [Google Scholar] [CrossRef]
- Little, S.C.; Biesiadecki, B.J.; Kilic, A.; Higgins, R.S.; Janssen, P.M.; Davis, J.P. The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C. J. Biol. Chem. 2012, 287, 27930–27940. [Google Scholar] [CrossRef]
- Morita, H.; Wu, J.; Zipes, D.P. The QT syndromes: Long and short. Lancet 2008, 372, 750–763. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emerson, J.I.; Ariel, P.; Shi, W.; Conlon, F.L. Sex Differences in Mouse Cardiac Electrophysiology Revealed by Simultaneous Imaging of Excitation-Contraction Coupling. J. Cardiovasc. Dev. Dis. 2023, 10, 479. https://doi.org/10.3390/jcdd10120479
Emerson JI, Ariel P, Shi W, Conlon FL. Sex Differences in Mouse Cardiac Electrophysiology Revealed by Simultaneous Imaging of Excitation-Contraction Coupling. Journal of Cardiovascular Development and Disease. 2023; 10(12):479. https://doi.org/10.3390/jcdd10120479
Chicago/Turabian StyleEmerson, James I., Pablo Ariel, Wei Shi, and Frank L. Conlon. 2023. "Sex Differences in Mouse Cardiac Electrophysiology Revealed by Simultaneous Imaging of Excitation-Contraction Coupling" Journal of Cardiovascular Development and Disease 10, no. 12: 479. https://doi.org/10.3390/jcdd10120479
APA StyleEmerson, J. I., Ariel, P., Shi, W., & Conlon, F. L. (2023). Sex Differences in Mouse Cardiac Electrophysiology Revealed by Simultaneous Imaging of Excitation-Contraction Coupling. Journal of Cardiovascular Development and Disease, 10(12), 479. https://doi.org/10.3390/jcdd10120479