Using Transesophageal Echocardiography in Liver Transplantation with Veno-Venous Bypass Is a Tool with Many Applications: A Case Series from an Italian Transplant Center
Abstract
1. Introduction
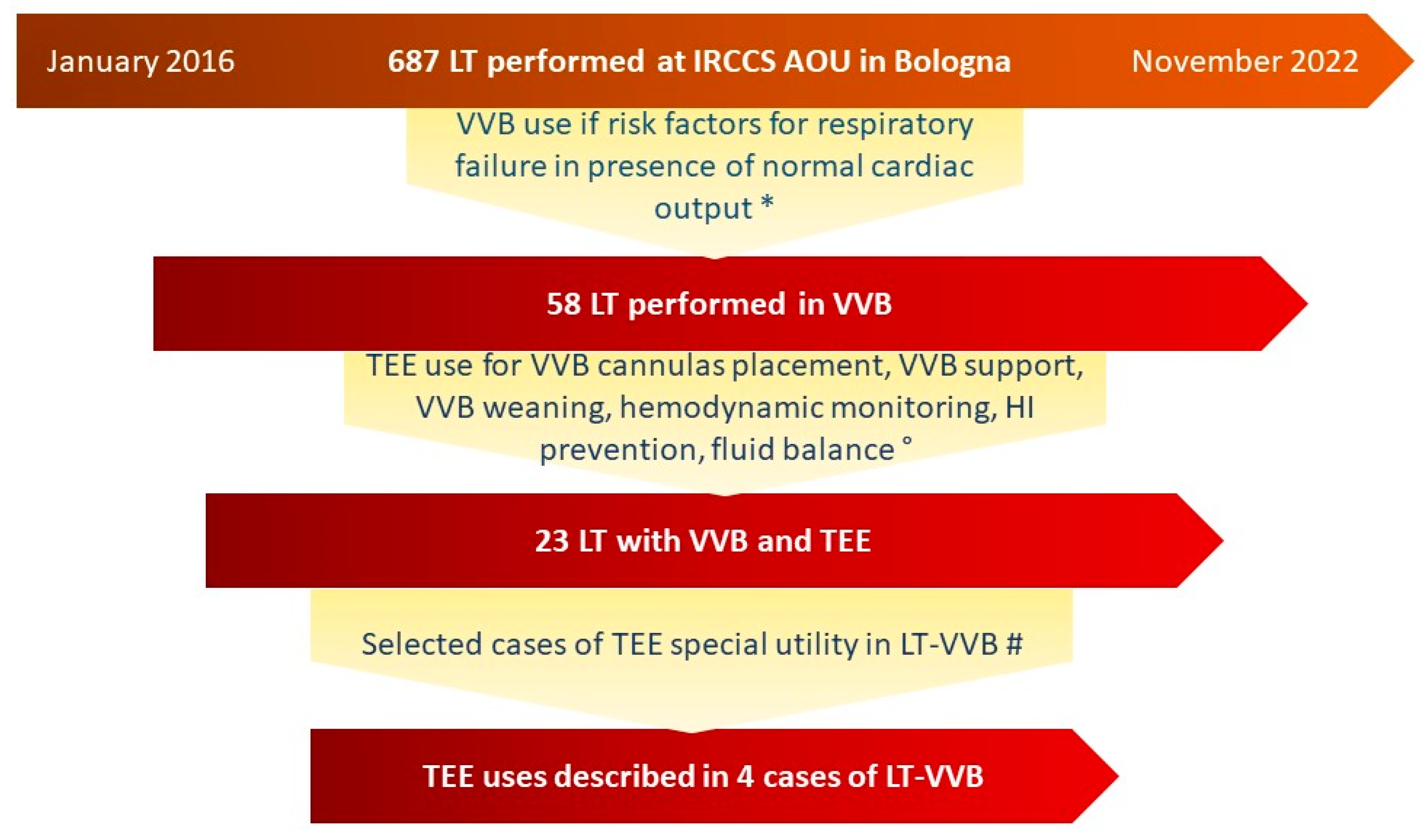
2. Materials and Methods
3. Results
3.1. Overall Results
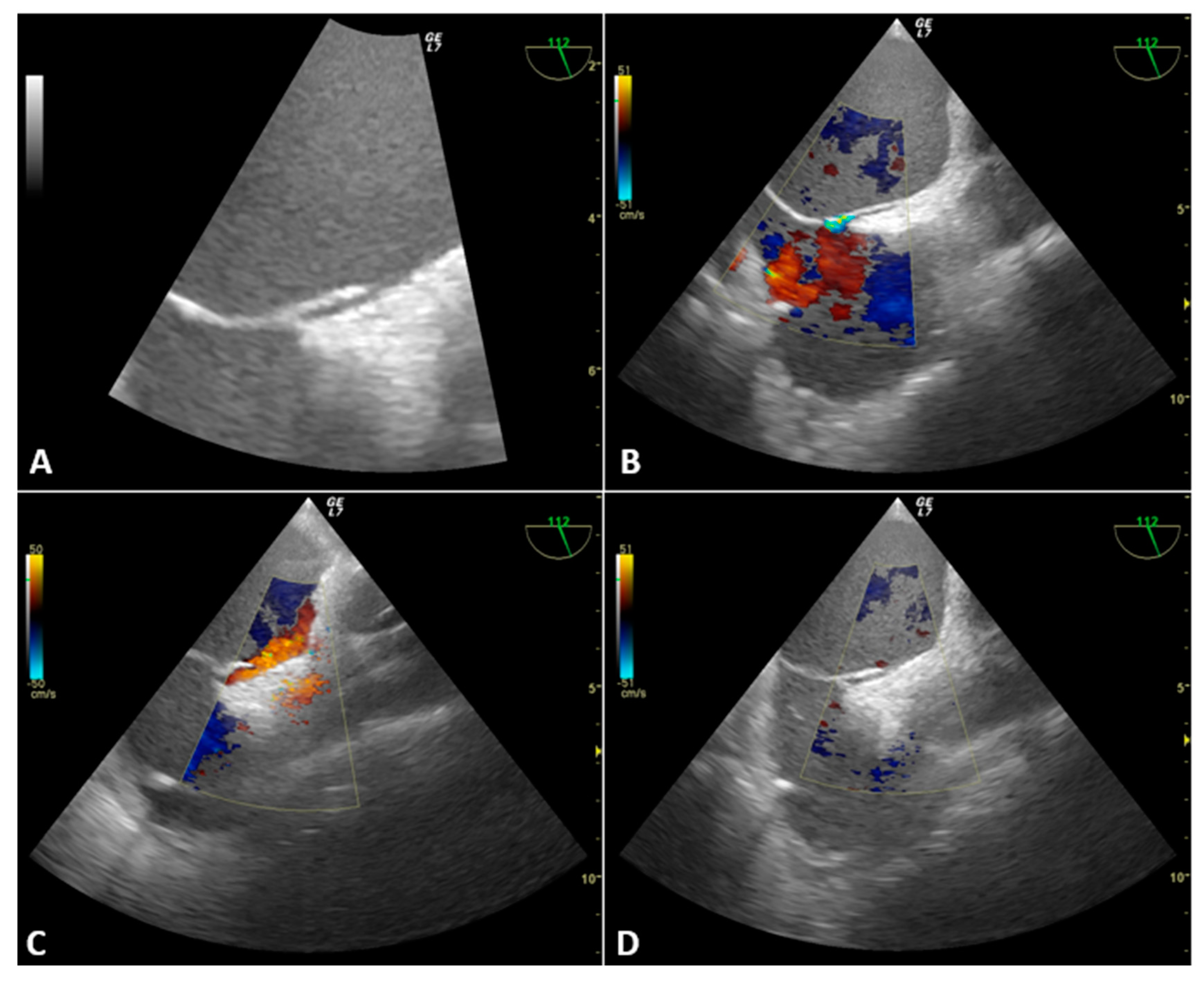
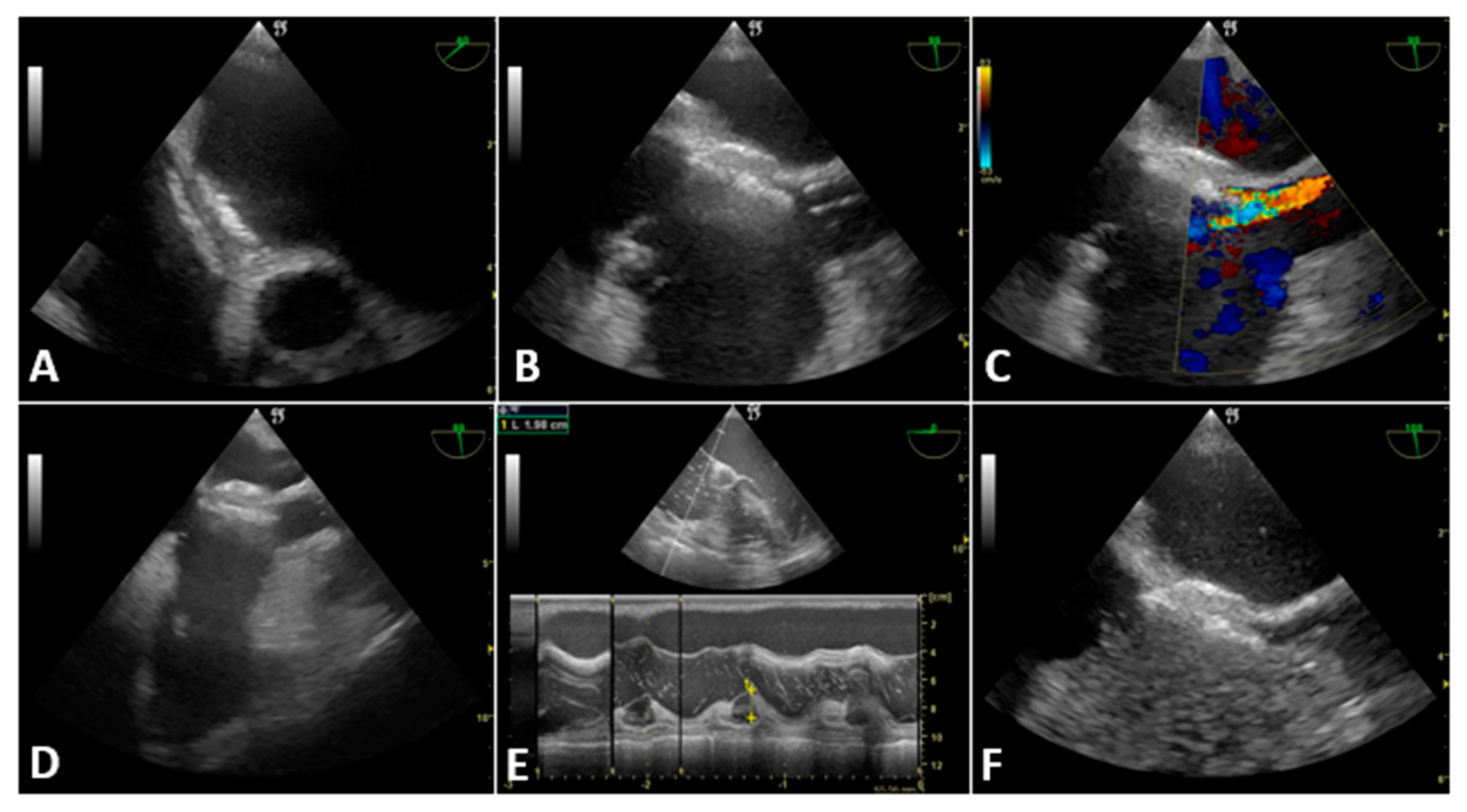
3.2. Case Presentation
3.2.1. Case 1
3.2.2. Case 2
3.2.3. Case 3
3.2.4. Case 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Activated Clotting Time |
| CVP | Central Venous Pressure |
| ESLD | End-Stage Liver Disease |
| Fr | French |
| HDI | Hemodynamic Instability |
| ICT | Intra-Cardiac Thrombosis |
| LT | Liver Transplantation |
| ME | Mid-Esophageal |
| PAC | Pulmonary Artery Catheter |
| PE | Pulmonary Embolism |
| PEEP | Positive End-Expiratory Pressure |
| PFO | Patent Foramen Ovale |
| PoPH | Porto-Pulmonary Hypertension |
| SAM | Systolic Anterior Motion |
| SATA | Society for the Advancement of Transplant Anesthesia |
| TEE | Trans-Esophageal Echocardiography |
| TEG | Thrombo-Elasto-Graphy |
| TELU | Trans Esophageal Lung Ultrasound |
| TTE | Trans-Thoracic Echocardiography |
| VVB | Veno-Venous Bypass |
References
- De Pietri, L.; Mocchegiani, F.; Leuzzi, C.; Montalti, R.; Vivarelli, M.; Agnoletti, V. Transoesophageal echocardiography during liver transplantation. World J. Hepatol. 2015, 7, 2432–2448. [Google Scholar] [CrossRef] [PubMed]
- Hofer, R.E.; Vogt, M.; Taner, T.; Findlay, J.Y. Influence of Intraoperative Transesophageal Echocardiography and Pulmonary Artery Catheter Monitoring on Outcomes in Liver Transplantation. Transplant. Direct 2020, 6, e525. [Google Scholar] [CrossRef] [PubMed]
- Fayad, A.; Shillcutt, S.K. Perioperative transesophageal echocardiography for non-cardiac surgery. Can. J. Anaesth. 2017, 65, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Bezinover, D.; Mukhtar, A.; Wagener, G.; Wray, C.; Blasi, A.; Kronish, K.; Zerillo, J.; Tomescu, D.; Pustavoitau, A.; Gitman, M.; et al. Hemodynamic Instability During Liver Transplantation in Patients with End-stage Liver Disease: A Consensus Document from ILTS, LICAGE, and SATA. Transplantation 2021, 105, 2184–2200. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Planinsic, R.M.; Hilmi, I.A.; Marsh, J.W. Complications associated with percutaneous placement of venous return cannula for venovenous bypass in adult orthotopic liver transplantation. Liver Transplant. 2007, 13, 961–965. [Google Scholar] [CrossRef]
- Kwon, H.-M.; Kim, K.-S.; Hwang, G.-S. Systolic anterior motion of mitral chordae tendineae: Prevalence and clinical implications in liver transplantation. Anesthesia Pain Med. 2020, 15, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Gligor, S.; Diulus, J.; McAffee, R.; Marsh, J.W.; Planinsic, R.M. Insertion and management of percutaneous veno-venous bypass cannula for liver transplantation: A reference for transplant anesthesiologists. Clin. Transplant. 2009, 24, 585–591. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/compliance/good-clinical-practice (accessed on 10 December 2022).
- Koroneos, A.; Politis, P.; Malachias, S.; Manolis, A.S.; Vassilakopoulos, T. End-inspiratory occlusion maneuver during transesophageal echocardiography for patent foramen ovale detection in intensive care unit patients. Intensiv. Care Med. 2007, 33, 1458–1462. [Google Scholar] [CrossRef]
- Schumann, R.; Mandell, M.S.; Mercaldo, N.; Michaels, D.; Robertson, A.; Banerjee, A.; Pai, R.; Klinck, J.; Pandharipande, P.; Walia, A. Anesthesia for liver transplantation in United States academic centers: Intraoperative practice. J. Clin. Anesthesia 2013, 25, 542–550. [Google Scholar] [CrossRef]
- De Marchi, L.; Wang, C.J.; Skubas, N.J.; Kothari, R.; Zerillo, J.; Subramaniam, K.; Efune, G.E.; Braunfeld, M.Y.; Mandel, S.; Kathirvel, S. Safety and Benefit of Transesophageal Echocardiography in Liver Transplant Surgery: A Position Paper from the Society for the Advancement of Transplant Anesthesia (SATA). Liver Transplant. 2020, 26, 1019–1029. [Google Scholar] [CrossRef]
- Vetrugno, L.; Barbariol, F.; Baccarani, U.; Forfori, F.; Volpicelli, G.; Della Rocca, G. Transesophageal echocardiography in orthotopic liver transplantation: A comprehensive intraoperative monitoring tool. Crit. Ultrasound J. 2017, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Dalia, A.A.; Flores, A.; Chitilian, H.; Fitzsimons, M.G. A Comprehensive Review of Transesophageal Echocardiography During Orthotopic Liver Transplantation. J. Cardiothorac. Vasc. Anesthesia 2018, 32, 1815–1824. [Google Scholar] [CrossRef]
- Vetrugno, L.; Barnariol, F.; Bignami, E.; Centonze, G.D.; De Flaviis, A.; Piccioni, F.; Auci, E.; Bove, T. Transesophageal ultrasonography during orthotopic liver transplantation: Show me more. Echocardiography 2018, 35, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Cavayas, Y.A.; Girard, M.; Desjardins, G.; Denault, A.Y. Transesophageal lung ultrasonography: A novel technique for investigating hypoxemia. Can. J. Anaesth. 2016, 63, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.S.; Chen, R. Transesophageal lung ultrasound: To boldly go…. Can. J. Anaesth. 2016, 63, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Dunkman, W.J.; Manning, M.W.; Williams, D.A. Patterns of Use in Transesophageal Echocardiography for Liver Transplantation: A Systematic Review. Semin. Cardiothorac. Vasc. Anesthesia 2022, 26, 274–281. [Google Scholar] [CrossRef]
- Dunkman, W.J.; Williams, D.A.; Manning, M.W. Bleeding Complications from Transesophageal Echocardiography for Liver Transplantation: A Systematic Review. Semin. Cardiothorac. Vasc. Anesthesia 2022, 26, 304–309. [Google Scholar] [CrossRef]
- Fernandez, T.M.; Schofield, N.; Krenn, C.G.; Rizkalla, N.; Spiro, M.; Raptis, D.A.; De Wolf, A.M.; Merritt, W.T.; ERAS4OLT.org Working Group. What is the optimal anesthetic monitoring regarding immediate and short-term outcomes after liver transplantation?—A systematic review of the literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14643. [Google Scholar] [CrossRef]
- Planinsic, R.M.; Nicolau-Raducu, R.; Caldwell, J.C.; Aggarwal, S.; Hilmi, I. Transesophageal Echocardiography-Guided Placement of Internal Jugular Percutaneous Venovenous Bypass Cannula in Orthotopic Liver Transplantation. Obstet. Anesthesia Dig. 2003, 97, 648–649. [Google Scholar] [CrossRef]
- Julliard, W.; Niles, S.; Maloney, J. Venovenous extracorporeal membrane oxygenation in patients with atrial septal defects. Perfusion 2014, 30, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Basílio, C.; Fontoura, A.; Fernandes, J.; Roncon-Albuquerque, R., Jr.; Paiva, J.A. Cardiac Tamponade Complicating Extracorporeal Membrane Oxygenation: A Single-Centre Experience. Hear. Lung Circ. 2021, 30, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Groose, M.K.; Aldred, B.N.; Mezrich, J.D.; Hammel, L.L. Risk Factors for Intracardiac Thrombus During Liver Transplantation. Liver Transplant. 2019, 25, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Dalia, A.A.; Khan, H.; Flores, A.S. Intraoperative Diagnosis of Intracardiac Thrombus During Orthotopic Liver Transplantation with Transesophageal Echocardiography: A Case Series and Literature Review. Semin. Cardiothorac. Vasc. Anesthesia 2016, 21, 245–251. [Google Scholar] [CrossRef]
- Shillcutt, S.K.; Ringenberg, K.J.; Chacon, M.M.; Brakke, T.R.; Montzingo, C.R.; Lyden, E.R.; Schulte, T.E.; Porter, T.R.; Lisco, S.J. Liver Transplantation: Intraoperative Transesophageal Echocardiography Findings and Relationship to Major Postoperative Adverse Cardiac Events. J. Cardiothorac. Vasc. Anesthesia 2016, 30, 107–114. [Google Scholar] [CrossRef]
- Peiris, P.; Pai, S.-L.; Iii, S.A.; Crawford, C.C.; Torp, K.D.; Ladlie, B.L.; Shine, T.S.; Taner, C.B.; Nguyen, J.H. Intracardiac thrombosis during liver transplant: A 17-year single-institution study. Liver Transplant. 2015, 21, 1280–1285. [Google Scholar] [CrossRef]
- Hagen, P.T.; Scholz, D.G.; Edwards, W.D. Incidence and Size of Patent Foramen Ovale During the First 10 Decades of Life: An Autopsy Study of 965 Normal Hearts. Mayo Clin. Proc. 1984, 59, 17–20. [Google Scholar] [CrossRef]
- Rais, G.; Vassallo, P.; Schorer, R.; Pinto, B.B.; Putzu, A. Patent foramen ovale and perioperative stroke in noncardiac surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 129, 898–908. [Google Scholar] [CrossRef]
- Hemamalini, P.; Dutta, P.; Attawar, S. Transesophageal Echocardiography Compared to Fluoroscopy for Avalon Bicaval Dual-Lumen Cannula Positioning for Venovenous ECMO. Ann. Card. Anaesth. 2020, 23, 283–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, A.; Laici, C.; Bordini, M.; Bianchin, M.; Silvas, C.I.I.; Cescon, M.; Ravaioli, M.; Vitale, G.; Siniscalchi, A. Using Transesophageal Echocardiography in Liver Transplantation with Veno-Venous Bypass Is a Tool with Many Applications: A Case Series from an Italian Transplant Center. J. Cardiovasc. Dev. Dis. 2023, 10, 32. https://doi.org/10.3390/jcdd10010032
Bianchini A, Laici C, Bordini M, Bianchin M, Silvas CII, Cescon M, Ravaioli M, Vitale G, Siniscalchi A. Using Transesophageal Echocardiography in Liver Transplantation with Veno-Venous Bypass Is a Tool with Many Applications: A Case Series from an Italian Transplant Center. Journal of Cardiovascular Development and Disease. 2023; 10(1):32. https://doi.org/10.3390/jcdd10010032
Chicago/Turabian StyleBianchini, Amedeo, Cristiana Laici, Martina Bordini, Matteo Bianchin, Catalin Iustin Ioan Silvas, Matteo Cescon, Matteo Ravaioli, Giovanni Vitale, and Antonio Siniscalchi. 2023. "Using Transesophageal Echocardiography in Liver Transplantation with Veno-Venous Bypass Is a Tool with Many Applications: A Case Series from an Italian Transplant Center" Journal of Cardiovascular Development and Disease 10, no. 1: 32. https://doi.org/10.3390/jcdd10010032
APA StyleBianchini, A., Laici, C., Bordini, M., Bianchin, M., Silvas, C. I. I., Cescon, M., Ravaioli, M., Vitale, G., & Siniscalchi, A. (2023). Using Transesophageal Echocardiography in Liver Transplantation with Veno-Venous Bypass Is a Tool with Many Applications: A Case Series from an Italian Transplant Center. Journal of Cardiovascular Development and Disease, 10(1), 32. https://doi.org/10.3390/jcdd10010032








