SCUBA Diving in Adult Congenital Heart Disease
Abstract
1. Introduction
2. Diving Physiology: Cardiovascular and General Aspects
3. Exercise Capacity and Diving
4. Evaluating Fitness to Dive in Patients with ACHD
5. Specific Conditions
5.1. Atrial Septal Defect (ASD)
- Use of nitrox gas (higher oxygen, lower nitrogen content), with decompression times calculated on air tables
- No deep dives (>25 m)
- No repetitive dives
- Minimization of Valsalva maneuvers
- No decompression dives
- Depth of 15-meter breathing air or equivalent:
- ○
- 19 msw with nitrox 32
- ○
- 23 msw with nitrox 40
5.2. Persistent Foramen Ovale (PFO)
5.3. Ventricular Septal Defect (VSD)
5.4. Right Ventricular Outflow Tract (RVOT) Obstruction
5.5. Left Ventricular Outflow Tract (LVOT) Obstruction
5.6. Valvular Regurgitation
5.7. Mitral Valve Stenosis
5.8. Aortic Coarctation
5.9. Aortic Dilatation
5.10. Tetralogy of Fallot (ToF)
5.11. Ebstein’s Anomaly
5.12. Fontan Circulation
5.13. Transposition of the Great Arteries (TGA)
5.14. Cyanotic CHD
5.15. Left and Right Ventricular Dysfunction
5.16. Arrhythmia and Conduction Disturbances
5.17. Prosthetic Valves
- No INR out of range in past 3 months
- No combination of two anticoagulatory drugs
- No simultaneous use of NSAIDs
- No major bleeding in the last year
- No additional factors increasing risk of
- Pulmonary barotrauma (e.g., asthma, smoking)
- No hazardous diving locations
- Conservative diving profile
- ○
- No decompression dives
- ○
- No dives > 20 m
- ○
- No > 2 dives per day (>4 h interval)
- ○
- No dives without possibility direct ascent (no cave/wreck diving)
5.18. Pulmonary Hypertension
6. Cardiac Medication
6.1. ACE Inhibitors and Angiotensin II Inhibitors
6.2. Beta-Blockers
6.3. Calcium Antagonists
6.4. Other Anti-Arrhythmic Drugs
6.5. Diuretics
6.6. Anti-Thrombotic Drugs
7. Ethical Considerations
8. Future Directions
9. Conclusions
10. Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- PADI. Padi Global Statistics 2016–2021. Available online: https://www.padi.com/sites/default/files/documents/2022-08/ABOUT%20PADI%20-%20Global%20Statistics%20%20%2716-%2721.pptx%20%281%29.pdf (accessed on 24 November 2022).
- Kempny, A.; Dimopoulos, K.; Uebing, A.; Diller, G.-P.; Rosendahl, U.; Belitsis, G.; Gatzoulis, M.A.; Wort, S.J. Outcome of Cardiac Surgery in Patients with Congenital Heart Disease in England between 1997 and 2015. PLoS ONE 2017, 12, e0178963. [Google Scholar] [CrossRef]
- Khairy, P.; Ionescu-Ittu, R.; Mackie, A.S.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Changing Mortality in Congenital Heart Disease. J. Am. Coll. Cardiol. 2010, 56, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal Trends in Survival to Adulthood among Patients Born with Congenital Heart Disease from 1970 to 1992 in Belgium. Circulation 2010, 122, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Apers, S.; Kovacs, A.H.; Luyckx, K.; Thomet, C.; Budts, W.; Enomoto, J.; Sluman, M.A.; Wang, J.-K.; Jackson, J.L.; Khairy, P.; et al. Quality of Life of Adults With Congenital Heart Disease in 15 Countries: Evaluating Country-Specific Characteristics. J. Am. Coll. Cardiol. 2016, 67, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Reiner, B.; Oberhoffer, R.; Ewert, P.; Müller, J. Quality of Life in Young People with Congenital Heart Disease Is Better than Expected. Arch. Dis. Child 2019, 104, 124. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease: The Task Force on Sports Cardiology and Exercise in Patients with Cardiovascular Disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Budts, W.; Pieles, G.E.; Roos-Hesselink, J.W.; Sanz de la Garza, M.; D’Ascenzi, F.; Giannakoulas, G.; Müller, J.; Oberhoffer, R.; Ehringer-Schetitska, D.; Herceg-Cavrak, V.; et al. Recommendations for Participation in Competitive Sport in Adolescent and Adult Athletes with Congenital Heart Disease (CHD): Position Statement of the Sports Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2020, 41, 4191–4199. [Google Scholar] [CrossRef]
- Boussuges, A.; Gole, Y.; Mourot, L.; Jammes, Y.; Melin, B.; Regnard, J.; Robinet, C. Haemodynamic Changes after Prolonged Water Immersion. J. Sport. Sci. 2009, 27, 641–649. [Google Scholar] [CrossRef]
- Michael Panneton, W. The Mammalian Diving Response: An Enigmatic Reflex to Preserve Life? Physiology 2013, 28, 284–297. [Google Scholar] [CrossRef]
- Shattock, M.J.; Tipton, M.J. ‘Autonomic Conflict’: A Different Way to Die during Cold Water Immersion? J. Physiol. 2012, 590, 3219–3230. [Google Scholar] [CrossRef]
- Peacher, D.F.; Martina, S.D.; Otteni, C.E.; Wester, T.E.; Potter, J.F.; Moon, R.E. Immersion Pulmonary Edema and Comorbidities. Med. Sci. Sport. Exerc. 2015, 47, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Held, H.E.; Pendergast, D.R. Relative Effects of Submersion and Increased Pressure on Respiratory Mechanics, Work, and Energy Cost of Breathing. J. Appl. Physiol. 2013, 114, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Marabotti, C.; Scalzini, A.; Chiesa, F. Increase of Pulmonary Arterial Pressure in Subjects with Venous Gas Emboli after Uncomplicated Recreational SCUBA Diving. Respir. Med. 2013, 107, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Vann, R.D.; Butler, F.K.; Mitchell, S.J.; Moon, R.E. Decompression Illness. Lancet 2011, 377, 153–164. [Google Scholar] [CrossRef]
- Buzzacott, P.; Pollock, N.W.; Rosenberg, M. Exercise Intensity Inferred from Air Consumption during Recreational Scuba Diving. Diving Hyperb. Med. 2014, 44, 74–78. [Google Scholar]
- Mitchell, S.J.; Bove, A.A. Medical Screening of Recreational Divers for Cardiovascular Disease: Consensus Discussion at the Divers Alert Network Fatality Workshop. Undersea Hyperb. Med. 2011, 38, 289–296. [Google Scholar] [PubMed]
- Rienks, R.; Buwalda, M.; Bucx, J.; Dubois, E.; Wingelaar, T.; van Hulst, R. Cardiovascular Risk Assessment in Divers: Toward Safer Diving. Undersea Hyperb. Med. 2022, 49, 355–365. [Google Scholar] [CrossRef]
- Godden, D.; Currie, G.; Denison, D.; Farrell, P.; Ross, J.; Stephenson, R.; Watt, S.; Wilmshurst, P. British Thoracic Society Guidelines on Respiratory Aspects of Fitness for Diving. Thorax 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose. Ademhaling Onder Bijzondere Omstandigheden—Duiken, Vliegen En Hoogte. Version 15-2-2021. Available online: https://www.nvalt.nl/kwaliteit/richtlijnen/overige-relevantedocumenten/_/Overige%20onderwerpen/Positionpaper%20Ademhaling%20onder%20bijzondere%20omstandigheden%20SLF%20feb%202021.pdf (accessed on 30 November 2022).
- Westerweel, P.E.; Rienks, R.; Sakr, A.; Taher, A. Diving with Hypertension and Antihypertensive Drugs. Diving Hyperb. Med. J. 2020, 50, 49–53. [Google Scholar] [CrossRef]
- Gempp, E.; Demaistre, S.; Louge, P. Hypertension Is Predictive of Recurrent Immersion Pulmonary Edema in Scuba Divers. Int. J. Cardiol. 2014, 172, 528–529. [Google Scholar] [CrossRef]
- Brouant, B.; Houriez, P.; Lafay, V.; Roche, F.; Finet, G.; Grandjean, B. Pratique de La Plongée et Des Sports Subaquatiques Par Lespatients Présentant Des Troubles de La Conduction Ou Du Rythmecardiaque: Recommendations Pour La FFESSM. Bull. Medsubhyp 2009, 19, 177–184. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- di Salvo, G.; Miller, O.; Babu Narayan, S.; Li, W.; Budts, W.; Valsangiacomo Buechel, E.R.; Frigiola, A.; van den Bosch, A.E.; Bonello, B.; Mertens, L.; et al. Imaging the Adult with Congenital Heart Disease: A Multimodality Imaging Approach—Position Paper from the EACVI. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1077–1098. [Google Scholar] [CrossRef]
- Li, W.; West, C.; McGhie, J.; van den Bosch, A.E.; Babu-Narayan, S.V.; Meijboom, F.; Mongeon, F.P.; Khairy, P.; Kimball, T.R.; Beauchesne, L.M.; et al. Consensus recommendations for echocardiography in adults with congenital heart defects from the International Society of Adult Congenital Heart Disease (ISACHD). Int. J. Cardiol. 2018, 272, 77–83. [Google Scholar] [CrossRef] [PubMed]
- van Hare, G.F.; Ackerman, M.J.; Evangelista, J.-A.K.; Kovacs, R.J.; Myerburg, R.J.; Shafer, K.M.; Warnes, C.A.; Washington, R.L.; American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology; Council on Cardiovascular Disease in Young; et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 4: Congenital Heart Disease: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2015, 132, e281–e291. [Google Scholar] [CrossRef] [PubMed]
- Menting, M.E.; Cuypers, J.A.A.E.; Opić, P.; Utens, E.M.W.J.; Witsenburg, M.; van den Bosch, A.E.; van Domburg, R.T.; Meijboom, F.J.; Boersma, E.; Bogers, A.J.J.C.; et al. The Unnatural History of the Ventricular Septal Defect: Outcome up to 40 Years after Surgical Closure. J. Am. Coll. Cardiol. 2015, 65, 1941–1951. [Google Scholar] [CrossRef]
- Cuypers, J.A.A.E.; Menting, M.E.; Konings, E.E.M.; Opić, P.; Utens, E.M.W.J.; Helbing, W.A.; Witsenburg, M.; van den Bosch, A.E.; Ouhlous, M.; van Domburg, R.T.; et al. Unnatural History of Tetralogy of Fallot: Prospective Follow-up of 40 Years after Surgical Correction. Circulation 2014, 130, 1944–1953. [Google Scholar] [CrossRef]
- Cuypers, J.A.A.E.; Opić, P.; Menting, M.E.; Utens, E.M.W.J.; Witsenburg, M.; Helbing, W.A.; van den Bosch, A.E.; Ouhlous, M.; van Domburg, R.T.; Meijboom, F.J.; et al. The Unnatural History of an Atrial Septal Defect: Longitudinal 35 Year Follow up after Surgical Closure at Young Age. Heart 2013, 99, 1346–1352. [Google Scholar] [CrossRef]
- Rafiq, I.; Freeman, L.; Orzalkiewicz, M.; Hiari, N.; Lewis, C. 143 Natural History of Repaired and Unrepaired VSD—An Experience of a District General Hospital with Dedicated Adult Congenital Heart Disease Clinics. Heart 2015, 101 (Suppl. S4), A82–A83. [Google Scholar] [CrossRef]
- Diller, G.-P.; Dimopoulos, K.; Okonko, D.; Li, W.; Babu-Narayan, S.; Broberg, C.S.; Johansson, B.; Bouzas, B.; Mullen, M.J.; Poole-Wilson, P.A.; et al. Exercise Intolerance in Adult Congenital Heart Disease. Circulation 2005, 112, 828–835. [Google Scholar] [CrossRef]
- Kempny, A.; Dimopoulos, K.; Uebing, A.; Moceri, P.; Swan, L.; Gatzoulis, M.A.; Diller, G.-P. Reference Values for Exercise Limitations among Adults with Congenital Heart Disease. Relation to Activities of Daily Life—Single Centre Experience and Review of Published Data. Eur. Heart J. 2012, 33, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Wilmshurst, P.T.; Morrison, W.L.; Walsh, K.P. Comparison of the Size of Persistent Foramen Ovale and Atrial Septal Defects in Divers with Shunt-Related Decompression Illness and in the General Population. Diving Hyperb. Med. 2015, 45, 89–93. [Google Scholar]
- Zwijnenburg, R.D.; Baggen, V.J.M.; Witsenburg, M.; Boersma, E.; Roos-Hesselink, J.W.; van den Bosch, A.E. Risk Factors for Pulmonary Hypertension in Adults After Atrial Septal Defect Closure. Am. J. Cardiol. 2019, 123, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Amedro, P.; Guillaumont, S.; Bredy, C.; Matecki, S.; Gavotto, A. Atrial Septal Defect and Exercise Capacity: Value of Cardio-Pulmonary Exercise Test in Assessment and Follow-Up. J. Thorac. Dis. 2018, 10, S2864–S2873. [Google Scholar] [CrossRef] [PubMed]
- Koutroulou, I.; Tsivgoulis, G.; Tsalikakis, D.; Karacostas, D.; Grigoriadis, N.; Karapanayiotides, T. Epidemiology of Patent Foramen Ovale in General Population and in Stroke Patients: A Narrative Review. Front. Neurol. 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.R.; Billinger, M.; Schwerzmann, M.; Vogel, R.; Zbinden, R.; Windecker, S.; Seiler, C. Risk of Decompression Illness among 230 Divers in Relation to the Presence and Size of Patent Foramen Ovale. Eur. Heart J. 2004, 25, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Germonpré, P.; Lafère, P.; Portier, W.; Germonpré, F.-L.; Marroni, A.; Balestra, C. Increased Risk of Decompression Sickness When Diving With a Right-to-Left Shunt: Results of a Prospective Single-Blinded Observational Study (The “Carotid Doppler” Study). Front. Physiol. 2021, 12, 763408. [Google Scholar] [CrossRef]
- Pristipino, C.; Germonpré, P.; Toni, D.; Sievert, H.; Meier, B.; D’Ascenzo, F.; Berti, S.; Onorato, E.M.; Bedogni, F.; Mas, J.-L.; et al. European Position Paper on the Management of Patients with Patent Foramen Ovale. Part II—Decompression Sickness, Migraine, Arterial Deoxygenation Syndromes and Select High-Risk Clinical Conditions. Eur. Heart J. 2021, 42, 1545–1553. [Google Scholar] [CrossRef]
- Klingmann, C.; Rathmann, N.; Hausmann, D.; Bruckner, T.; Kern, R. Lower Risk of Decompression Sickness after Recommendation of Conservative Decompression Practices in Divers with and without Vascular Right-to-Left Shunt. Diving Hyperb. Med. 2012, 42, 146–150. [Google Scholar] [PubMed]
- Koopsen, R.; Stella, P.R.; Thijs, K.M.; Rienks, R. Persistent Foramen Ovale Closure in Divers with a History of Decompression Sickness. Neth. Heart J. 2018, 26, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Wilmshurst, P. Risk Mitigation in Divers with Persistent (Patent) Foramen Ovale. Diving Hyperb. Med. J. 2019, 49, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Henzel, J.; Rudziński, P.N.; Kłopotowski, M.; Konka, M.; Dzielińska, Z.; Demkow, M. Transcatheter Closure of Patent Foramen Ovale for the Secondary Prevention of Decompression Illness in Professional Divers: A Single-Centre Experience with Long-Term Follow-Up. Kardiol. Pol. 2018, 76, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Honěk, J.; Srámek, M.; Sefc, L.; Januška, J.; Fiedler, J.; Horváth, M.; Tomek, A.; Novotný, S.; Honěk, T.; Veselka, J. Effect of Catheter-Based Patent Foramen Ovale Closure on the Occurrence of Arterial Bubbles in Scuba Divers. JACC Cardiovasc. Interv. 2014, 7, 403–408. [Google Scholar] [CrossRef]
- Lammers, A.E.; Bauer, L.J.; Diller, G.-P.; Helm, P.C.; Abdul-Khaliq, H.; Bauer, U.M.M.; Baumgartner, H. Pulmonary Hypertension after Shunt Closure in Patients with Simple Congenital Heart Defects. Int. J. Cardiol. 2020, 308, 28–32. [Google Scholar] [CrossRef]
- Baumgartner, H.; de Backer, J.; Babu-Narayan, S.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- de Meester, P.; Buys, R.; van de Bruaene, A.; Gabriels, C.; Voigt, J.-U.; Vanhees, L.; Herijgers, P.; Troost, E.; Budts, W. Functional and Haemodynamic Assessment of Mild-to-Moderate Pulmonary Valve Stenosis at Rest and during Exercise. Heart 2014, 100, 1354–1359. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef]
- Michelena, H.I.; Prakash, S.K.; della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid Aortic Valve. Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef]
- Baird, C.W.; Marx, G.R.; Borisuk, M.; Emani, S.; del Nido, P.J. Review of Congenital Mitral Valve Stenosis: Analysis, Repair Techniques and Outcomes. Cardiovasc. Eng. Technol. 2015, 6, 167–173. [Google Scholar] [CrossRef]
- Wilmshurst, P.T. Immersion Pulmonary Oedema: A Cardiological Perspective. Diving Hyperb. Med. J. 2019, 49, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.; Bové, T.; Vandekerckhove, K.; de Wolf, D. Hypertension after Coarctation Repair-a Systematic Review. Transl. Pediatr. 2022, 11, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022. [Google Scholar] [CrossRef]
- Regalado, E.S.; Morris, S.A.; Braverman, A.C.; Hostetler, E.M.; de Backer, J.; Li, R.; Pyeritz, R.E.; Yetman, A.T.; Cervi, E.; Shalhub, S.; et al. Comparative Risks of Initial Aortic Events Associated With Genetic Thoracic Aortic Disease. J. Am. Coll. Cardiol. 2022, 80, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Fang, Y.; Lai, H.; Shen, Y.; Wang, H.; Lin, M.; Tan, L. Marfan Syndrome with Pneumothorax: Case Report and Review of Literatures. J. Thorac. Dis. 2017, 9, E1100–E1103. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Miranda, W.R.; Ammash, N.M.; Anavekar, N.S.; Missula, V.R.; Kothapalli, S.; Khan, A.R.; Said, S.M.; Connolly, H.M. Aortic Disease and Interventions in Adults with Tetralogy of Fallot. Heart 2019, 105, 926–931. [Google Scholar] [CrossRef]
- Hebe, J. Ebstein’s Anomaly in Adults.Arrhythmias: Diagnosis and Therapeutic Approach. Thorac. Cardiovasc. Surg. 2000, 48, 214–219. [Google Scholar] [CrossRef]
- Attenhofer Jost, C.H.; Connolly, H.M.; Dearani, J.A.; Edwards, W.D.; Danielson, G.K. Ebstein’s Anomaly. Circulation 2007, 115, 277–285. [Google Scholar] [CrossRef]
- Chen, S.S.M.; Dimopoulos, K.; Sheehan, F.H.; Gatzoulis, M.A.; Kilner, P.J. Physiologic Determinants of Exercise Capacity in Patients with Different Types of Right-Sided Regurgitant Lesions: Ebstein’s Malformation with Tricuspid Regurgitation and Repaired Tetralogy of Fallot with Pulmonary Regurgitation. Int. J. Cardiol. 2016, 205, 1–5. [Google Scholar] [CrossRef]
- van de Bruaene, A.; Claessen, G.; Salaets, T.; Gewillig, M. Late Fontan Circulatory Failure. What Drives Systemic Venous Congestion and Low Cardiac Output in Adult Fontan Patients? Front. Cardiovasc. Med. 2022, 9, 825472. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Avitabile, C.M.; McBride, M.G.; Paridon, S.M. Exercise Capacity in the Fontan Circulation. Cardiol. Young 2013, 23, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Quinton, E.; Nightingale, P.; Hudsmith, L.; Thorne, S.; Marshall, H.; Clift, P.; de Bono, J. Prevalence of Atrial Tachyarrhythmia in Adults after Fontan Operation. Heart 2015, 101, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, J.A.A.E.; Eindhoven, J.A.; Slager, M.A.; Opić, P.; Utens, E.M.W.J.; Helbing, W.A.; Witsenburg, M.; van den Bosch, A.E.; Ouhlous, M.; van Domburg, R.T.; et al. The Natural and Unnatural History of the Mustard Procedure: Long-Term Outcome up to 40 Years. Eur. Heart J. 2014, 35, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Broberg, C.S.; van Dissel, A.; Minnier, J.; Aboulhosn, J.; Kauling, R.M.; Ginde, S.; Krieger, E.; Rodriguez, F.; Gupta, T.; Shah, S.; et al. Long-Term Outcomes After Atrial Switch Operation for Transposition of the Great Arteries. J. Am. Coll. Cardiol. 2022, 80, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, P.M.; Chen, A.; Veldtman, G.; Hechter, S.; Therrien, J.; Webb, G. Exercise Capacity in Adult Patients with Congenitally Corrected Transposition of the Great Arteries. Heart 2001, 85, 191–195. [Google Scholar] [CrossRef]
- Swartz, M.F.; Sena, A.; Atallah-Yunes, N.; Meagher, C.; Cholette, J.M.; Gensini, F.; Alfieris, G.M. Decreased Incidence of Supravalvar Pulmonary Stenosis After Arterial Switch Operation. Circulation 2012, 126. [Google Scholar] [CrossRef][Green Version]
- Oechslin, E. Management of Adults with Cyanotic Congenital Heart Disease. Heart 2015, 101, 485–494. [Google Scholar] [CrossRef]
- Marabotti, C.; Scalzini, A.; Menicucci, D.; Passera, M.; Bedini, R.; L’Abbate, A. Cardiovascular Changes during SCUBA Diving: An Underwater Doppler Echocardiographic Study. Acta Physiol. 2013, 209, 62–68. [Google Scholar] [CrossRef]
- Hernández-Madrid, A.; Paul, T.; Abrams, D.; Aziz, P.F.; Blom, N.A.; Chen, J.; Chessa, M.; Combes, N.; Dagres, N.; Diller, G.; et al. Arrhythmias in congenital heart disease: A position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. EP Eur. 2018, 20, 1719–1753. [Google Scholar] [CrossRef]
- Waldmann, V.; Laredo, M.; Abadir, S.; Mondésert, B.; Khairy, P. Atrial Fibrillation in Adults with Congenital Heart Disease. Int J. Cardiol. 2019, 287, 148–154. [Google Scholar] [CrossRef]
- Walsh, E.P.; Cecchin, F. Arrhythmias in Adult Patients with Congenital Heart Disease. Circulation 2007, 115, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.S.; Buelow, M.W.; Aggarwal, S.; Arora, R.R.; Kovach, J.; Ginde, S. Arrhythmias in Adults with Congenital Heart Disease: What Are Risk Factors for Specific Arrhythmias? Pacing Clin. Electrophysiol. 2017, 40, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Westerweel, P.E.; Rienks, R.; Tucker, M.M.B.; van Hulst, R.A.; van Ooijn, P.J.A.M. Duiken Met Trombocytenaggregatieremmers of Anticoagulantia. Available online: https://duikgeneeskunde.nl/wp-content/uploads/2019/09/Antistolling.pdf (accessed on 30 November 2022).
- Lafay, V.; Trigano, J.A.; Gardette, B.; Micoli, C.; Carre, F. Effects of Hyperbaric Exposures on Cardiac Pacemakers. Br. J. Sport. Med. 2008, 42, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef] [PubMed]
- Vodoz, J.-F.; Cottin, V.; Glérant, J.-C.; Derumeaux, G.; Khouatra, C.; Blanchet, A.-S.; Mastroïanni, B.; Bayle, J.-Y.; Mornex, J.-F.; Cordier, J.-F. Right-to-Left Shunt with Hypoxemia in Pulmonary Hypertension. BMC Cardiovasc. Disord. 2009, 9, 15. [Google Scholar] [CrossRef]
- Hoencamp, E.; van Dongen, T.T.; van Ooij, P.-J.A.; Wingelaar, T.T.; Vervelde, M.L.; Koch, D.A.; van Hulst, R.A.; Hoencamp, R. Systematic Review on the Effects of Medication under Hyperbaric Conditions: Consequences for the Diver. Diving Hyperb. Med. J. 2019, 49, 127–136. [Google Scholar] [CrossRef]
- Frishman, W.H. Calcium Channel Blockers: Differences between Subclasses. Am. J. Cardiovasc. Drugs 2007, 7 (Suppl. S1), 17–23. [Google Scholar] [CrossRef]
- Jepson, N.; Rienks, R.; Smart, D.; Bennett, M.H.; Mitchell, S.J.; Turner, M. South Pacific Underwater Medicine Society Guidelines for Cardiovascular Risk Assessment of Divers. Diving Hyperb. Med. J. 2020, 50, 273–277. [Google Scholar] [CrossRef]
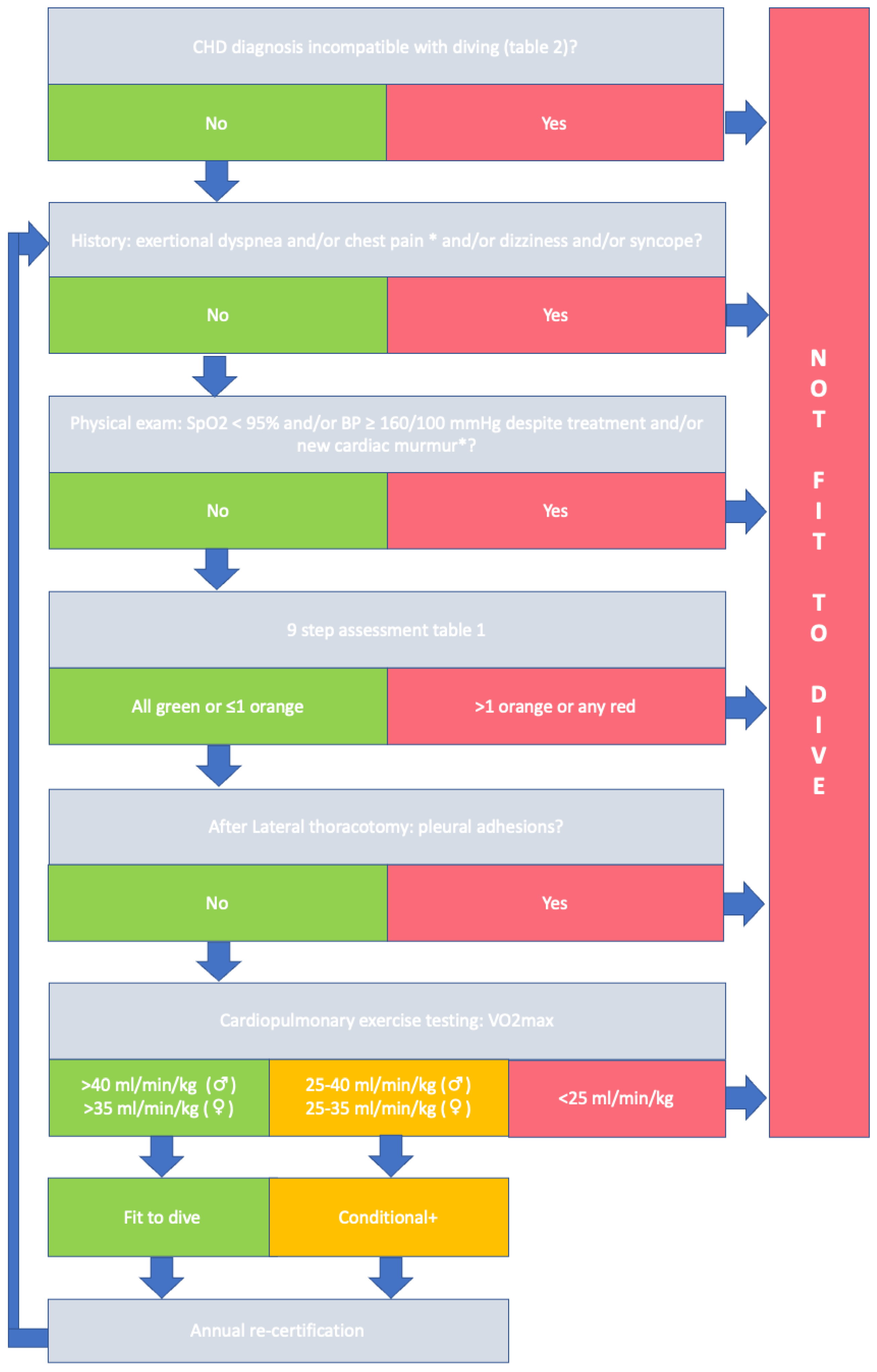
| Parameter | Definitions | ||
|---|---|---|---|
| Ventricular dysfunction | No dysfunction: LVEF > 55%, RV TAPSE > 17 mm, S’ > 10 cm/s, FAC > 35% | Mild dysfunction: 45% ≥ EF < 55% (or normal sRV function) | Moderate–severe dysfunction: EF < 45% or impaired sRV function |
| Ventricular hypertrophy | No hypertrophy: Wall thickness (cm): <1.1 (male) or <1.0 (female) | Mild hypertrophy: Wall thickness (cm): 1.1–1.3 (male) or 1.0–1.2 (female) | Moderate–severe hypertrophy: Wall thickness (cm) ≥ 1.3 (male) or ≥1.3 (female) |
| Ventricular pressure overload | No pressure overload: No RVOT or LVOT obstruction (PSV < 2.6 m/s), no coarctation | Mild pressure overload: 2.6 m/s ≤ PSV < 3 m/s for LVOT and RVOT obstructions and PPS; for CA, peak arm-leg gradient < 20 mmHg | Moderate–severe pressure overload: PSV > 3 m/s for LVOT and RVOT obstruction and PPS, CA peak arm-leg gradient ≥ 20 mmHg |
| Ventricular volume overload | No volume overload: Absent or mild to moderate valve regurgitation without LV/RV dilatation | Mild volume overload: Mild to moderate valve regurgitation with mild LV/RV dilatation (LVEDD < 61 mm/RVEDD < 42 mm with preserved systolic function) | Moderate–severe volume overload: Severe valve regurgitation or moderate–severe LV/RV dilatation (LVEDD > 61 mm/RVEDD > 42 mm) |
| Pulmonary artery pressure | Low probability PH: TVRVc ≤ 2.8 m/s and no additional echocardiographic findings suggestive of PH or invasive mPAP < 20 mmHg | Intermediate–high probability PH: TVRVc > 2.8 m/s or additional echocardiographic findings suggestive of PH or invasive mPAP > 20 mmHg | |
| Aorta (non-syndromic) | No dilatation: Aorta size ≤ 35 mm, z-score < 3 | Mild dilatation: Aorta size ≤ 45 mm, z-score ≤ 4 | Moderate–severe dilatation: Aorta size ≥ 45 mm, z-score >4 Any syndromic aorta syndrome |
| Arrhythmia | No arrhythmia: Absence of arrhythmia or infrequent PVCs (<500/24 h) that do not worsen during exercise | Mild arrhythmia: Frequent PVC not worsening during exercise Controlled AF/AFl or other SVT without incapacitating symptoms | Clinically important arrythmia: Any ventricular arrhythmia Any previously incapacitating SVT Pre-excitation pattern without EP study |
| Arterial oxygen saturation at rest/during exercise | Normal: SaO2 > 95% in rest or during exercise | Abnormal: SaO2 < 95% in rest or during exercise | |
| Shunts | No shunt: No residual ASD or VSD after closure | Shunt: Small, restrictive VSD without LV dilatation PFO | Shunt: ASD with R—L shunt VSD with LV dilatation |
| CHD Diagnosis | Feature Relevant to Scuba Diving |
|---|---|
| Unrepaired Atrial septal defect | R–L shunting, volume overload |
| Moderate and severe RVOT and LVOT obstruction | Pressure overload, subendocardial ischemia |
| Severe valvular regurgitation | Volume overload, ventricular dysfunction |
| Moderate or severe mitral valve stenosis | Impaired cardiac output, pulmonary hypertension, thrombosis |
| Ebstein anomaly | TR regurgitation, RV dysfunction, ASD, accessory pathway |
| Unrepaired ToF | R–L shunting, RVOT obstruction, RV dysfunction Pulmonary regurgitation, RV dysfunction, arrhythmia |
| TGA atrial switch (Mustard/Senning) | sRV dysfunction, arrhythmia, baffle leak |
| ccTGA | sRV dysfunction, AV conduction disorders |
| Fontan circulation and cyanotic heart disease | Impaired cardiac output, R–L shunt, arrhythmia, increase pulmonary artery pressure |
| Unrepaired aortic coarctation or significant re-coarctation | Arterial hypertension |
| Marfan syndrome or other syndromal aortopathy | Aorta dilatation, increased risk pneumothorax |
| Non syndromal aortic dilatation With moderate aorta dilatation (aorta ≥ 45 mm, z-score > 4) | Aorta dilatation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauling, R.M.; Rienks, R.; Cuypers, J.A.A.E.; Jorstad, H.T.; Roos-Hesselink, J.W. SCUBA Diving in Adult Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 20. https://doi.org/10.3390/jcdd10010020
Kauling RM, Rienks R, Cuypers JAAE, Jorstad HT, Roos-Hesselink JW. SCUBA Diving in Adult Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2023; 10(1):20. https://doi.org/10.3390/jcdd10010020
Chicago/Turabian StyleKauling, Robert M., Rienk Rienks, Judith A. A. E. Cuypers, Harald T. Jorstad, and Jolien W. Roos-Hesselink. 2023. "SCUBA Diving in Adult Congenital Heart Disease" Journal of Cardiovascular Development and Disease 10, no. 1: 20. https://doi.org/10.3390/jcdd10010020
APA StyleKauling, R. M., Rienks, R., Cuypers, J. A. A. E., Jorstad, H. T., & Roos-Hesselink, J. W. (2023). SCUBA Diving in Adult Congenital Heart Disease. Journal of Cardiovascular Development and Disease, 10(1), 20. https://doi.org/10.3390/jcdd10010020






