Modified R-GLIM Score Is a Good Prognostic Tool to Predict a Long-Term Prognosis in Poor Conditioned Elderly Patients with Aspiration Pneumonia, a Pilot Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Patients Enrolled
- The onset of pneumonia was community-acquired pneumonia (CAP) or HCAP.
- Those who had an intervention by the NST.
- Those whom we could adequately follow the clinical course and prognosis of after discharge.
2.3. Disease Severity and Nutritional Status
2.4. The Global Leadership Initiative on Malnutrition (GLIM) Criteria
2.5. Microbial Assessment
2.6. Definition of Appropriate or Inappropriate Treatment and Initial Treatment Failure
2.7. Statistical Analyses
3. Results
3.1. Comparison of Patients’ Profiles and Conditions between Survival and Death Group
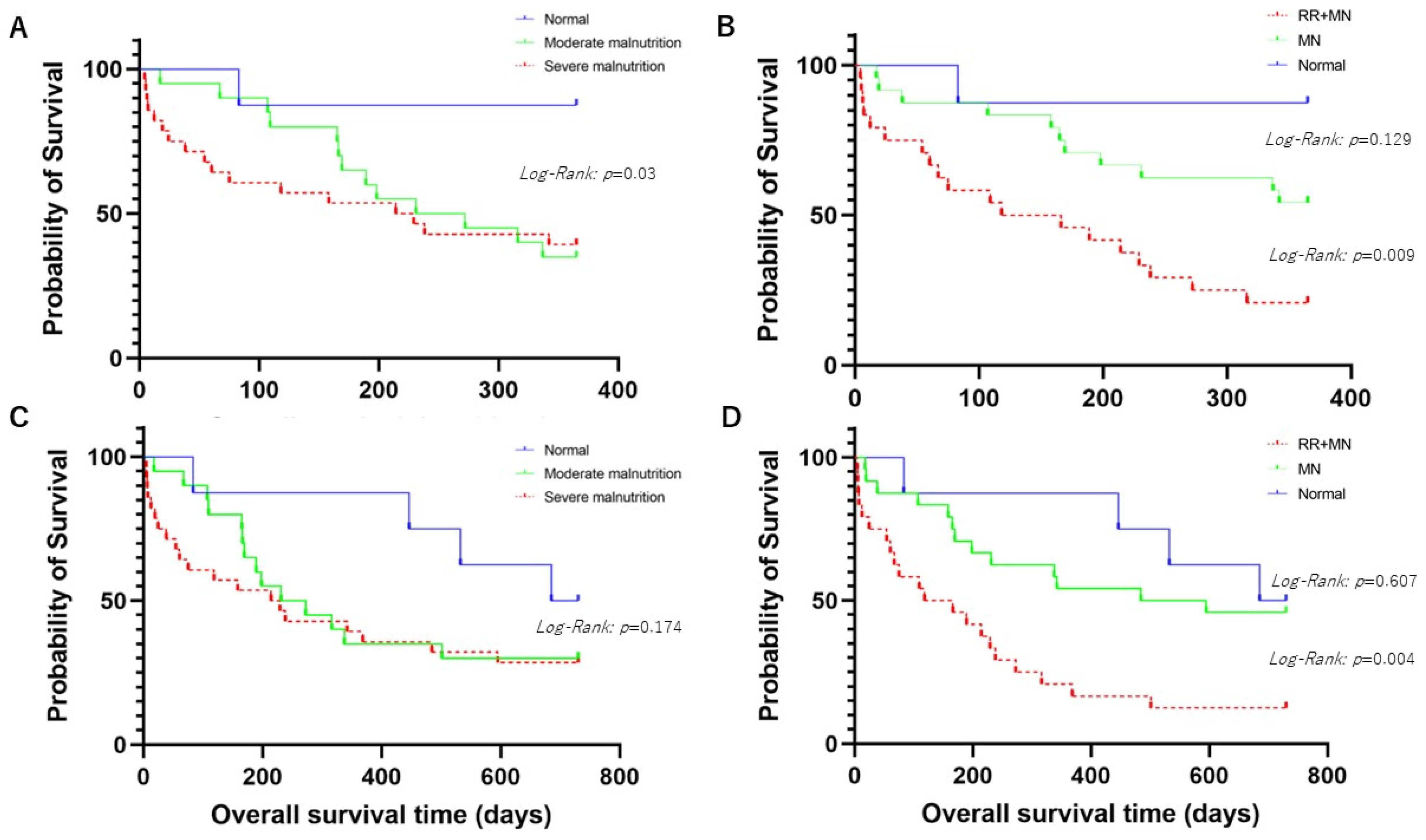
3.1.1. Comparison of Overall Survival Times for 1-Year Prognosis between Patients with Normal Nutritional Status or Malnutrition According to the GLIM Criteria
3.1.2. Comparison of Overall Survival Times for 2-Year Prognosis between Patients with Normal Nutritional Status or Malnutrition According to the GLIM Criteria
3.2. Receiver Operating Characteristic (ROC) Curves of Combined Score of RR and GLIM Criteria, the GLIM Criteria, A-DROP, CURB-65, PSI, I-ROAD, qSOFA, and SOFA Score for 1- and 2-Year Death
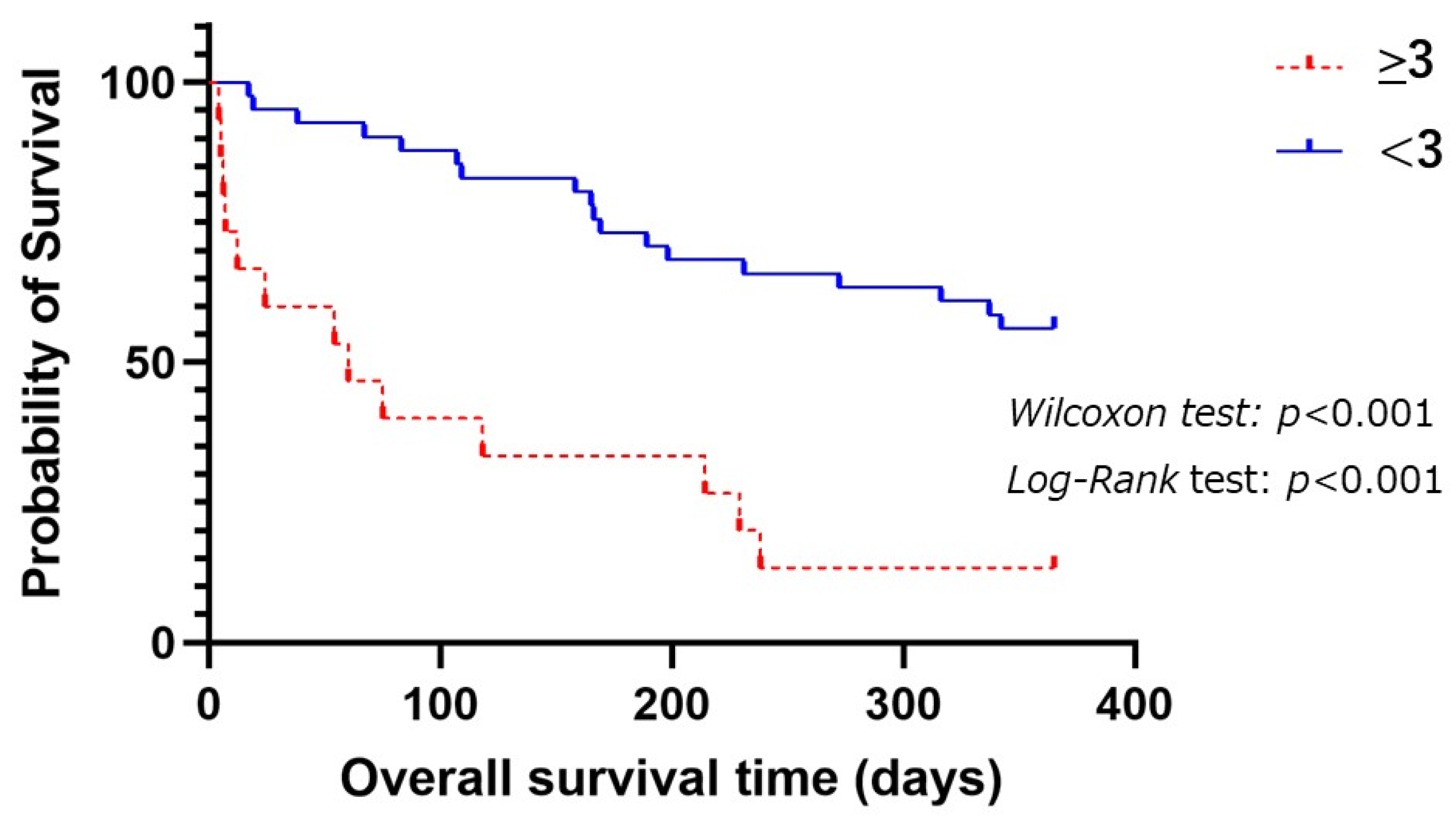
3.2.1. Prognostic Factors for 1-Year Survival among Aspiration Pneumonia
3.2.2. Prognostic Factors for 2-Year Survival among Aspiration Pneumonia Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012, 67, 71–79. [Google Scholar] [CrossRef]
- Asai, N.; Watanabe, H.; Shiota, A.; Kato, H.; Sakanashi, D.; Hagihara, M.; Koizumi, Y.; Yamagishi, Y.; Suematsu, H.; Mikamo, H. Efficacy and accuracy of qSOFA and SOFA scores as prognostic tools for community-acquired and healthcare-associated pneumonia. Int. J. Infect. Dis. 2019, 84, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Watanabe, H.; Shiota, A.; Kato, H.; Sakanashi, D.; Hagihara, M.; Koizumi, Y.; Yamagishi, Y.; Suematsu, H.; Mikamo, H. Could qSOFA and SOFA score be correctly estimating the severity of healthcare-associated pneumonia? J. Infect. Chemother. 2018, 24, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Tufa, T.B.; Horner, J.; Hurissa, Z.; Nordmann, T.; Bosselmann, M.; Abdissa, S.; Sorsa, A.; Orth, H.M.; Jensen, B.O.; et al. Clinical and microbiological characterization of sepsis and evaluation of sepsis scores. PLoS ONE 2021, 16, e0247646. [Google Scholar] [CrossRef] [PubMed]
- Tusgul, S.; Carron, P.N.; Yersin, B.; Calandra, T.; Dami, F. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 108. [Google Scholar] [CrossRef]
- Miyashita, N.; Matsushima, T.; Oka, M. The JRS guidelines for the management of community-acquired pneumonia in adults: An update and new recommendations. Intern. Med. 2006, 45, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Pneumonia Guidelines Committee of the BTS Standards of Care Committee. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax 2001, 56 (Suppl. S4), IV1–IV64. [Google Scholar]
- Niederman, M.S.; Mandell, L.A.; Anzueto, A.; Bass, J.B.; Broughton, W.A.; Campbell, G.D.; Dean, N.; File, T.; Fine, M.J.; Gross, P.A.; et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 2001, 163, 1730–1754. [Google Scholar] [CrossRef]
- Matsunuma, R.; Asai, N.; Ohkuni, Y.; Nakashima, K.; Iwasaki, T.; Misawa, M.; Norihiro, K. I-ROAD could be efficient in predicting severity of community-acquired pneumonia or healthcare-associated pneumonia. Singap. Med. J. 2014, 55, 318–324. [Google Scholar] [CrossRef]
- Japanese Respiratory, S. Establishment of new severity ratings based on analysis of hospital-acquired pneumonia. Respirology 2009, 14 (Suppl. S2), S4–S9. [Google Scholar]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ozorio, G.A.; Ribeiro, L.M.K.; Santos, B.C.; Bruzaca, W.F.S.; Rocha, G.; Marchi, L.; Santos, F.M.; Alves de Almeida, M.M.F.; Kulcsar, M.A.V.; Junior, U.R.; et al. Exploring the use of the GLIM criteria to diagnose malnutrition in cancer inpatients. Nutrition 2023, 116, 112195. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Impact of disease burden or inflammation on nutritional assessment by the GLIM criteria in female patients with rheumatoid arthritis. Clin. Nutr. ESPEN 2022, 52, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ito, M.; Sugiyama, H.; Iwagaitsu, S.; Nobata, H.; Kinashi, H.; Katsuno, T.; Banno, S.; Ito, Y.; Ando, M.; et al. Malnutrition according to the GLIM criteria with kidney dysfunction is associated with increased mortality in hospitalized patients with cardiovascular disease: A retrospective cohort study. Clin. Nutr. ESPEN 2023, 55, 167–173. [Google Scholar] [CrossRef]
- Asai, N.; Suematsu, H.; Ohashi, W.; Shibata, Y.; Sakanashi, D.; Kato, H.; Shiota, A.; Watanabe, H.; Hagihara, M.; Koizumi, Y.; et al. Ceftriaxone versus tazobactam/piperacillin and carbapenems in the treatment of aspiration pneumonia: A propensity score matching analysis. J. Infect. Chemother. 2021, 27, 1465–1470. [Google Scholar] [CrossRef]
- Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K.; Mayumi, T.; Murata, A.; Ikeda, T.; Ishikura, H.; et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 2006, 34, 625–631. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Prina, E.; Menendez, R.; Ceccato, A.; Cilloniz, C.; Mendez, R.; Gabarrus, A.; Barbeta, E.; Bassi, G.L.; Ferrer, M.; et al. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality. A Validation and Clinical Decision-Making Study. Am. J. Respir. Crit. Care Med. 2017, 196, 1287–1297. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- De Groot, V.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. How to measure comorbidity. A critical review of available methods. J. Clin. Epidemiol. 2003, 56, 221–229. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Asai, N.; Shibata, Y.; Sakanashi, D.; Kato, H.; Hagihara, M.; Yamagishi, Y.; Suematsu, H.; Mikamo, H. A Large Gap in Patients’ Characteristics and Outcomes between the Real-World and Clinical Trial Settings in Community-Acquired Pneumonia and Healthcare-Associated Pneumonia. J. Clin. Med. 2022, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Kichikawa, Y.; Mataki, N.; Kanoh, S. Usefulness of SMART-COP score in prognosis of aspiration pneumonia. Med. Clin. 2023, 160, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lynn, L.A.; Curry, J.P. Patterns of unexpected in-hospital deaths: A root cause analysis. Patient Saf. Surg. 2011, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.H.A.; Aquino, J.S.; da Silva-Maia, J.K.; Vale, S.H.L.; Maciel, B.L.L.; Passos, T.S. Nutritional status, diet and viral respiratory infections: Perspectives for severe acute respiratory syndrome coronavirus 2. Br. J. Nutr. 2021, 125, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, D.C.; Shah, A.; Molinger, J.; Whittle, J.; Gupta, R.T.; Wischmeyer, P.E.; McDonald, S.R.; Inman, B.A. Assessments of frailty in bladder cancer. Urol. Oncol. 2020, 38, 698–705. [Google Scholar] [CrossRef]
- Saleedaeng, P.; Korwanich, N.; Muangpaisan, W.; Korwanich, K. Effect of Dysphagia on the Older Adults’ Nutritional Status and Meal Pattern. J. Prim. Care Commun. Health 2023, 14, 21501319231158280. [Google Scholar] [CrossRef]
- Komatsu, R.; Okazaki, T.; Ebihara, S.; Kobayashi, M.; Tsukita, Y.; Nihei, M.; Sugiura, H.; Niu, K.; Ebihara, T.; Ichinose, M. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J. Cachexia Sarcopenia Muscle 2018, 9, 643–653. [Google Scholar] [CrossRef]
- Arai, H.; Maeda, K.; Wakabayashi, H.; Naito, T.; Konishi, M.; Assantachai, P.; Auyeung, W.T.; Chalermsri, C.; Chen, W.; Chew, J.; et al. Diagnosis and outcomes of cachexia in Asia: Working Consensus Report from the Asian Working Group for Cachexia. J. Cachexia Sarcopenia Muscle 2023, 14, 1949–1958. [Google Scholar] [CrossRef]
- Ijmker-Hemink, V.; Heerschop, S.; Wanten, G.; Berg, M.v.D. Evaluation of the Validity and Feasibility of the GLIM Criteria Compared with PG-SGA to Diagnose Malnutrition in Relation to One-Year Mortality in Hospitalized Patients. J. Acad. Nutr. Diet. 2022, 122, 595–601. [Google Scholar] [CrossRef]
- Trollebo, M.A.; Tangvik, R.J.; Skeie, E.; Gronning, M.K.; Nygard, O.; Eagan, T.M.L.; Dierkes, J. Malnutrition as a prognostic factor for 2-year mortality in hospitalized patients in Norway: A matched cohort study. JPEN J. Parenter. Enteral Nutr. 2024, 48, 308–317. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients | Survival Group | Death Group | p-Value |
|---|---|---|---|---|
| (n = 56) | (n = 25) | (n = 31) | ||
| Age (mean years ± SD) | 86.4 ± 6.5 | 85.1 ± 6.2 | 87.4 ± 6.8 | 0.186 |
| Median age (years, range) | 86 (69–105) | 85 (69–91) | 86 (78–105) | - |
| Male gender (n, %) | 25 (45) | 9 (36) | 16 (51) | 0.251 |
| Smoking history (n, %) | ||||
| Current smoker | 3 (5) | 2 (8) | 1 (3) | 0.439 |
| Former smoker | 36 (64) | 20 (80) | 16 (52) | 0.027 |
| Never smoker | 33 (59) | 18 (72) | 15 (48) | 0.077 |
| Unknown | 2 (4) | 1 (4) | 1 (3) | 0.879 |
| Nutritional status (n, %) | ||||
| Normal | 8 (14) | 7 (28) | 1 (3) | 0.001 |
| Malnutrition | 48 (86) | 18 (72) | 30 (97) | 0.001 |
| Moderate malnutrition | 20 (36) | 7 (28) | 13 (42) | 0.288 |
| Severe malnutrition | 28 (50) | 11 (44) | 17 (55) | 0.429 |
| Body mass index (mean kg/m2 ± SD) | 19.5 ± 3.2 | 19.7 ± 3.6 | 19.3 ± 3.0 | 0.651 |
| Severity of pneumonia (mean points ± SD) | ||||
| A-DROP | 2.9 ± 0.9 | 2.8 ± 0.9 | 3.0 ± 0.9 | 0.506 |
| CURB-65 | 2.6 ± 1.0 | 2.5 ± 1.0 | 2.6 ± 1.0 | 0.622 |
| PSI | 144.9 ± 28.7 | 132.2 ± 28.3 | 155.3 ± 25.1 | 0.002 |
| I-ROAD * | 2.8 ± 0.9 | 2.6 ± 1.0 | 3.0 ± 0.8 | 0.087 |
| SIRS score | 1.6 ± 1.2 | 1.2 ± 1.0 | 2.0 ± 1.2 | 0.014 |
| Quick SOFA | 1.4 ± 0.7 | 1.2 ± 0.5 | 1.5 ± 0.8 | 0.055 |
| SOFA score | 3.2 ± 1.9 | 2.7 ± 1.7 | 3.6 ± 2.1 | 0.081 |
| Underlying diseases (n, %) | ||||
| Cardiac disease | 22 (39) | 11 (44) | 11 (35) | 0.525 |
| Pulmonary diseases | 11 (17) | 4 (16) | 7 (23) | 0.546 |
| Diabetes mellitus | 12 (21) | 3 (12) | 9 (29) | 0.127 |
| Cerebrovascular diseases | 30 (25) | 14 (28) | 16 (20) | 0.749 |
| Paralysis | 14 (25) | 5 (20) | 9 (29) | 0.447 |
| Dementia | 37 (66) | 16 (64) | 21 (68) | 0.774 |
| Collagen diseases/auto immune disease | 5 (9) | 3 (12) | 2 (6) | 0.478 |
| Kidney diseases | 9 (16) | 4 (16) | 5 (16) | 0.990 |
| Hemodialysis | 5 (9) | 5 (20) | 0 (0) | 0.269 |
| Malignancy | 15 (27) | 4 (10) | 11 (33) | 0.105 |
| Gastroesophageal reflux disease | 3 (5) | 1 (4) | 2 (6) | 0.692 |
| Proton pump inhibitor user | 21 (38) | 11 (44) | 10 (32) | 0.376 |
| Sleeping pill user | 13 (23) | 6 (24) | 7 (23) | 0.903 |
| Home oxygen therapy | 1 (2) | 1 (4) | 0 (0) | 0.269 |
| CCI score (mean ± SD) | 2.6 ± 1.4 | 2.3 ± 1.4 | 2.9 ± 1.4 | 0.107 |
| Higher CCI (≥3) (n, %) | 28 (50) | 10 (40) | 18 (58) | 0.185 |
| Patients’ condition (n, %) | ||||
| Respiratory rate (≥22 times/min) | 24 (43) | 5 (20) | 19 (61) | 0.003 |
| Loss of consciousness | 50 (89) | 21 (84) | 29 (94) | 0.259 |
| Shock vital | 8 (14) | 3 (12) | 5 (16) | 0.661 |
| Bacteremia ** | 17 (34) | 6 (32) | 11 (35) | 1.000 |
| PDR pathogen isolation | 14 (25) | 6 (24) | 8 (26) | 0.877 |
| Treatment (n, %) | ||||
| Mechanical ventilation | 1 (2) | 0 (0) | 1 (3) | 0.374 |
| Noninvasive positive pressure ventilation | 4 (7) | 0 (0) | 4 (13) | 0.064 |
| Vasopressor | 4 (7) | 0 (0) | 4 (13) | 0.064 |
| DNAR order | 49 (88) | 20 (80) | 29 (94) | 0.132 |
| ICU admission | 0 | 0 | 0 | - |
| Inappropriate treatment *** | 10 (23) | 4 (21) | 6 (25) | 1.000 |
| Initial treatment failure | 2 (4) | 1 (4) | 1 (3) | 0.877 |
| Antibiotics initially used (n, %) | ||||
| Monotherapy | ||||
| Penicillins alone | 40 (71) | 15 (60) | 25 (81) | 0.089 |
| Cephalosporins alone | 14 (25) | 8 (32) | 6 (19) | 0.277 |
| Carbapenems alone | 0 | 0 | 0 | - |
| Macrolide alone | 0 | 0 | 0 | - |
| Fluoroquinolones alone | 1 (2) | 1 (4) | 0 | 0.261 |
| Combination therapy | ||||
| β-lactams plus macrolide | 0 | 0 | 0 | - |
| β-lactams plus fluoroquinolones | 0 | 0 | 0 | - |
| Anti-pseudomonal agents | 12 (21) | 4 (16) | 8 (26) | 0.374 |
| Anti-MRSA agents | 0 | 0 | 0 | - |
| Outcomes (mean days ± SD) | ||||
| Duration of antibiotics | 13.3 ± 10.2 | 8.6 ± 6.6 | 17.1 ± 11.2 | 0.002 |
| Duration of admission | 27.1 ± 20.0 | 23.3 ± 19.0 | 30.1 ± 20.6 | 0.212 |
| Survival rate (n, %) | ||||
| 7-day mortality rate | 5 (9) | - | - | - |
| 30-day mortality rate | 11 (20) | - | - | - |
| In-hospital mortality rate | 6 (11) | - | - | - |
| One-year mortality rate | 31 (55) | - | - | - |
| Two-year mortality rate | 38 (68) | - | - | - |
| Variables | Scores |
|---|---|
| Respiratory rate (times/min) | |
| RR < 22 | 0 |
| RR ≥ 22 | 1 |
| Nutritional status | |
| Normal | 0 |
| Moderate malnutrition | 1 |
| Severe malnutrition | 2 |
| Modified R-GLIM Score | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | YI |
|---|---|---|---|---|---|
| 1 | 96.8 | 28 | 62.5 | 12.5 | 24.8 |
| 2 | 74.2 | 46.2 | 62.2 | 33.3 | 20.3 |
| 3 | 41.9 | 92.3 | 86.7 | 43.9 | 34.2 |
| Variables | Univariate Analysis * | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Respiratory rate ≥ 22 | 6.3 | 1.9–21.4 | 0.002 | 2.6 | 1.2–5.6 | 0.012 |
| Malnutrition | 11.7 | 1.3–102.7 | 0.017 | 4.3 | 0.6–33.6 | 0.16 |
| Variables | Univariate Analysis * | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Respiratory rate ≥ 22 | 6.2 | 1.5–24.9 | 0.006 | 4.5 | 1.0–2.2 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakita, Y.; Asai, N.; Ohashi, W.; Mori, N.; Maekawa, M.; Mikamo, H. Modified R-GLIM Score Is a Good Prognostic Tool to Predict a Long-Term Prognosis in Poor Conditioned Elderly Patients with Aspiration Pneumonia, a Pilot Study. Geriatrics 2024, 9, 118. https://doi.org/10.3390/geriatrics9050118
Wakita Y, Asai N, Ohashi W, Mori N, Maekawa M, Mikamo H. Modified R-GLIM Score Is a Good Prognostic Tool to Predict a Long-Term Prognosis in Poor Conditioned Elderly Patients with Aspiration Pneumonia, a Pilot Study. Geriatrics. 2024; 9(5):118. https://doi.org/10.3390/geriatrics9050118
Chicago/Turabian StyleWakita, Yoshinori, Nobuhiro Asai, Wataru Ohashi, Naoharu Mori, Masato Maekawa, and Hiroshige Mikamo. 2024. "Modified R-GLIM Score Is a Good Prognostic Tool to Predict a Long-Term Prognosis in Poor Conditioned Elderly Patients with Aspiration Pneumonia, a Pilot Study" Geriatrics 9, no. 5: 118. https://doi.org/10.3390/geriatrics9050118
APA StyleWakita, Y., Asai, N., Ohashi, W., Mori, N., Maekawa, M., & Mikamo, H. (2024). Modified R-GLIM Score Is a Good Prognostic Tool to Predict a Long-Term Prognosis in Poor Conditioned Elderly Patients with Aspiration Pneumonia, a Pilot Study. Geriatrics, 9(5), 118. https://doi.org/10.3390/geriatrics9050118





