Physical Performance, Body Composition, and Oral Health in Community-Residing Older Adults: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
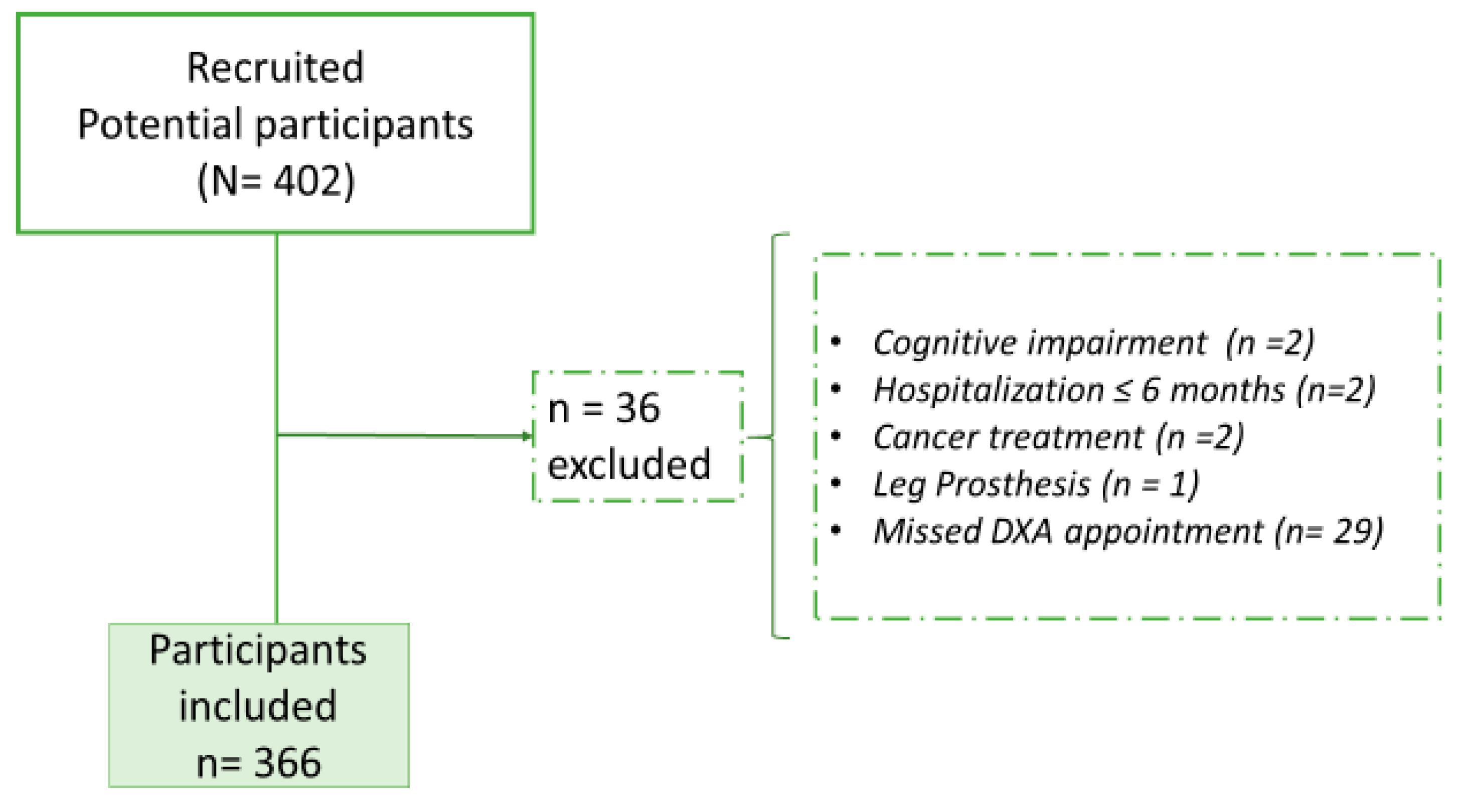
2.1. Design and Participants
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Ethics
2.5. Anthropometry
2.6. Nutritional Assessment
2.7. Physical Performance
2.8. Body Composition Assessment
2.9. Oral Health-Related Quality of Life Assessment
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2022. Summary of Results; United Nations: New York, NY, USA, 2022. [Google Scholar]
- National Institute of Statistic Geography and Informatics. Statistics on the International Day of Older Persons (October 1) National data [Estadísticas a Propósito del día Internacional de las Personas de Edad (1° de Octubre) Datos Nacionales 2019. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2019/edad2019_Nal.pdf (accessed on 17 March 2024).
- Secretaría General del Consejo Nacional de Población [SGCONAPO] Gráficas Dinámicas y Carteles Sociodemográficos. Available online: https://www.gob.mx/conapo/documentos/graficas-dinamicas-y-carteles-sociodemograficos (accessed on 9 March 2024).
- Cress, M.E.; Buchner, D.M.; Questad, K.A.; Esselman, P.C.; de Lateur, B.J.; Schwartz, R.S. Continuous-scale physical functional performance in healthy older adults: A validation study. Arch. Phys. Med. Rehabil. 1996, 77, 1243–1250. [Google Scholar] [CrossRef]
- Prasad, L.; Fredrick, J.; Aruna, R. The relationship between physical performance and quality of life and the level of physical activity among the elderly. J. Educ. Health Promot. 2021, 10, 68. [Google Scholar] [CrossRef]
- Manning, K.M.; Hall, K.S.; Sloane, R.; Magistro, D.; Rabaglietti, E.; Lee, C.C.; Castle, S.; Kopp, T.; Giffuni, J.; Katzel, L.; et al. Longitudinal analysis of physical function in older adults: The effects of physical inactivity and exercise training. Aging Cell 2024, 23, e13987. [Google Scholar] [CrossRef]
- Treacy, D.; Hassett, L. The Short Physical Performance Battery. J. Physiother. 2018, 64, 61. [Google Scholar] [CrossRef]
- De Fátima Ribeiro Silva, C.; Ohara, D.G.; Matos, A.P.; Pinto, A.C.P.N.; Pegorari, M.S. Short physical performance battery as a measure of physical performance and mortality predictor in older adults: A comprehensive literature review. Int. J. Environ. Res. Public Health 2021, 18, 10612. [Google Scholar] [CrossRef]
- Jung, H.-W.; Baek, J.Y.; Jang, I.-Y.; Guralnik, J.M.; Rockwood, K.; Lee, E.; Kim, D.H. Short physical performance battery as a crosswalk between frailty phenotype and deficit accumulation frailty index. J. Gerontol. Ser. A 2021, 76, 2249–2255. [Google Scholar] [CrossRef]
- Harel, J.; Fossaert, R.; Bérard, A.; Lafargue, A.; Danet-Lamasou, M.; Poisson, P.; Dupuis, V.; Bourdel-Marchasson, I. Masticatory coefficient and physical functioning in older frail patients admitted for a Comprehensive Gerontological Assessment. Arch. Gerontol. Geriatr. 2021, 95, 104421. [Google Scholar] [CrossRef]
- Gawel, J.; Vengrow, D.; Collins, J.; Brown, S.; Buchanan, A.; Cook, C. The short physical performance battery as a predictor for long term disability or institutionalization in the community dwelling population aged 65 years old or older. Phys. Ther. Rev. 2012, 17, 37–44. [Google Scholar] [CrossRef]
- Kramer, C.S.; Groenendijk, I.; Beers, S.; Wijnen, H.H.; Van De Rest, O.; De Groot, L.C.P.G.M. The Association between malnutrition and physical performance in older adults: A systematic review and meta-analysis of observational studies. Curr. Dev. Nutr. 2022, 6, nzac007. [Google Scholar] [CrossRef]
- Amasene, M.; Besga, A.; Medrano, M.; Urquiza, M.; Rodriguez-Larrad, A.; Tobalina, I.; Barroso, J.; Irazusta, J.; Labayen, I. Nutritional status and physical performance using handgrip and SPPB tests in hospitalized older adults. Clin. Nutr. 2021, 40, 5547–5555. [Google Scholar] [CrossRef]
- Silver, A.J.; Guillen, C.P.; Kahl, M.J.; Morley, J.E. Effect of Aging on Body Fat. J. Am. Geriatr. Soc. 1993, 41, 211–213. [Google Scholar] [CrossRef]
- Samper-Ternent, R.; Al Snih, S. Obesity in older adults: Epidemiology and implications for disability and disease. Rev. Clin. Gerontol. 2012, 22, 10–34. [Google Scholar] [CrossRef]
- Malenfant, J.H.; Batsis, J.A. Obesity in the geriatric population–A global health perspective. J. Glob. Heal. Rep. 2019, 3, e2019045. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, A.; la Cruz-Góngora, V.D.; Manrique-Espinoza, B. Condiciones de salud, síndromes geriátricos y estado nutricional de los adultos mayores en México. Salud Pública México 2020, 62, 777–785. [Google Scholar] [CrossRef]
- Donini, L.M.; Savina, C.; Gennaro, E.; De Felice, M.R.; Rosano, A.; Pandolfo, M.M.; Del Balzo, V.; Cannella, C.; Ritz, P.; Chumlea, W.C. A systematic review of the literature concerning the relationship between obesity and mortality in the elderly. J. Nutr. Health Aging 2012, 16, 89–98. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Barquera, S.; Hernández-Barrera, L.; Trejo, B.; Shamah, T.; Campos-Nonato, I.; Rivera-Dommarco, J. Obesidad en México, prevalencia y tendencias en adultos. Ensanut 2018-19. Salud Pública México 2020, 62, 682–692. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S. V Aage-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Nogueira Paranhos, D.; da Nascimento, D.C.; Stone, W.; Alves, V.P.; Coelho Vilaça e Silva, K.H. Body composition and functional performance of older adults. Osteoporos. Sarcopenia 2022, 8, 86–91. [Google Scholar] [CrossRef]
- Soh, Y.; Won, C.W. Sex differences in association between body composition and frailty or physical performance in community-dwelling older adults. Medicine 2021, 100, e24400. [Google Scholar] [CrossRef]
- Kim, J.H.; Chon, J.; Soh, Y.; Han, Y.R.; Won, C.W.; Lee, S.A. Trunk fat mass correlates with balance and physical performance in a community-dwelling elderly population. Medicine 2020, 99, e19245. [Google Scholar] [CrossRef]
- De Oliveira, L.F.S.; Wanderley, R.L.; de Medeiros, M.M.D.; de Figueredo, O.M.C.; Pinheiro, M.A.; Rodrigues Garcia, R.C.M.; de Almeida, L.F.D.; Cavalcanti, Y.W. Health-related quality of life of institutionalized older adults: Influence of physical, nutritional and self-perceived health status. Arch. Gerontol. Geriatr. 2021, 92, 104278. [Google Scholar] [CrossRef]
- Barboza-Solís, C.; Porras-Chaverri, M.; Fantin, R. Is tooth loss important when evaluating perceived general health? Findings from a nationally representative study of Costa Rican adults. Community Dent. Oral Epidemiol. 2019, 47, 358–365. [Google Scholar] [CrossRef]
- Lee, S.; Sabbah, W. Association between number of teeth, use of dentures and musculoskeletal frailty among older adults. Geriatr. Gerontol. Int. 2018, 18, 592–598. [Google Scholar] [CrossRef]
- Aida, J.; Kondo, K.; Hirai, H.; Nakade, M.; Yamamoto, T.; Hanibuchi, T.; Osaka, K.; Sheiham, A.; Tsakos, G.; Watt, R.G. Association between dental status and incident disability in an older Japanese population. J. Am. Geriatr. Soc. 2012, 60, 338–343. [Google Scholar] [CrossRef]
- Hämäläinen, P.; Rantanen, T.; Keskinen, M.; Meurman, J.H. Oral health status and change in handgrip strength over a 5-year period in 80-year-old people. Gerodontology 2004, 21, 155–160. [Google Scholar] [CrossRef]
- Welmer, A.-K.; Rizzuto, D.; Parker, M.G.; Xu, W. Impact of tooth loss on walking speed decline over time in older adults: A population-based cohort study. Aging Clin. Exp. Res. 2017, 29, 793–800. [Google Scholar] [CrossRef]
- Inui, A.; Takahashi, I.; Sawada, K.; Naoki, A.; Oyama, T.; Tamura, Y.; Osanai, T.; Satake, A.; Nakaji, S.; Kobayashi, W. Teeth and physical fitness in a community-dwelling 40 to 79-year-old Japanese population. Clin. Interv. Aging 2016, 11, 873–878. [Google Scholar] [CrossRef]
- Kimble, R.; McLellan, G.; Lennon, L.T.; Papacosta, A.O.; Weyant, R.J.; Kapila, Y.; Mathers, J.C.; Wannamethee, S.G.; Whincup, P.H.; Ramsay, S.E. Association between oral health markers and decline in muscle strength and physical performance in later life: Longitudinal analyses of two prospective cohorts from the UK and the USA. Lancet Healthy Longev. 2022, 3, e777–e788. [Google Scholar] [CrossRef]
- Okuyama, N.; Yamaga, T.; Yoshihara, A.; Nohno, K.; Yoshitake, Y.; Kimura, Y.; Shimada, M.; Nakagawa, N.; Nishimuta, M.; Ohashi, M.; et al. Influence of dental occlusion on physical fitness decline in a healthy Japanese elderly population. Arch. Gerontol. Geriatr. 2011, 52, 172–176. [Google Scholar] [CrossRef]
- Lima, C.V.; dos Santos Noronha, M.; de Menezes, E.J.M.; de Oliveira Araújo, V.S.; Mendes, P.H.C.; Ferreira, R.C.; de Barros Lima Martins, A.M.E.; Souza, J.G.S. Unraveling the signs and symptoms of oral conditions that affect daily life activities and oral health-related quality of life. Clin. Oral Investig. 2022, 27, 2725–2733. [Google Scholar] [CrossRef]
- Sischo, L.; Broder, H.L. Oral Health-related Quality of Life. J. Dent. Res. 2011, 90, 1264–1270. [Google Scholar] [CrossRef]
- Mexican Government. Data Mexico, Mexico City Government. Available online: https://www.economia.gob.mx/datamexico/en/profile/geo/coyoacan (accessed on 10 June 2024).
- INEGI. Services National Institute of Statistics and Geography (INEGI). Available online: https://inegi.org.mx/siscon/ (accessed on 9 June 2024).
- Kamdem, B.; Seematter-Bagnoud, L.; Botrugno, F.; Santos-Eggimann, B. Relationship between oral health and Fried’s frailty criteria in community-dwelling older persons. BMC Geriatr. 2017, 17, 174. [Google Scholar] [CrossRef]
- Chow, S.-C.; Wang, H.; Shao, J. Sample Size Calculations in Clinical Research, 2nd ed.; Chapman & Hall/CRC Biostatistics Series: London, UK, 2008. [Google Scholar]
- Alley, D.E.; Koster, A.; MacKey, D.; Cawthon, P.; Ferrucci, L.; Simonsick, E.M.; Yu, B.; Hardy, S.; Goodpaster, B.; Sarkisian, C.; et al. Hospitalization and change in body composition and strength in a population-based cohort of older persons. J. Am. Geriatr. Soc. 2010, 58, 2085–2091. [Google Scholar] [CrossRef]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef]
- Garg, R. Methodology for research I. Indian J. Anaesth. 2016, 60, 640. [Google Scholar] [CrossRef]
- Sanford, A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef]
- Eveleth, P.B.; Andres, R.; Chumlea, W.C.; Eiben, O.; Ge, K.; Harris, T.; Heymsfield, S.B.; Launer, L.J.; Rosenberg, I.H.; Solomons, N.W.; et al. Uses and interpretation of anthropometry in the elderly for the assessment of physical status. Report to the Nutrition Unit of the World Health Organization: The Expert Subcommittee on the Use and Interpretation of Anthropometry in the Elderly. J. Nutr. Health Aging 1998, 2, 5–17. [Google Scholar]
- Kıskaç, M.; Soysal, P.; Smith, L.; Capar, E.; Zorlu, M. What is the Optimal Body Mass Index Range for Older Adults? Ann. Geriatr. Med. Res. 2022, 26, 49–57. [Google Scholar] [CrossRef]
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the slderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 2009, 54, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Przkora, R.; Kinsky, M.P.; Fisher, S.R.; Babl, C.; Heyde, C.E.; Vasilopoulos, T.; Kaye, A.D.; Volpi, E. Functional improvements utilizing the Short Physical Performance Battery (SPPB) in the elderly after epidural steroid injections. Curr. Pain Headache Rep. 2019, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Maffi, G.; Vitale, J.A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Diagnostic imaging of osteoporosis and sarcopenia: A narrative review. Quant. Imaging Med. Surg. 2018, 8, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Atchison, K.A.; Dolan, T.A. Development of the Geriatric Oral Health Assessment Index. J. Dent. Educ. 1990, 54, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Seoane, M.; Reichenheim, M.E.; Tsakos, G.; Celeste, R.K. Adult oral health-related quality of life instruments: A systematic review. Community Dent. Oral Epidemiol. 2022, 50, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, S.; Heredia-Ponce, E.; Juárez-Cedillo, T.; Gallegos-Carrillo, K.; Espinel-Bermúdez, C.; De La Fuente-Hernández, J.; García-Peña, C. Psychometric properties of the General Oral Health Assessment Index (GOHAI) and dental status of an elderly Mexican population. J. Public Health Dent. 2010, 70, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N. Detecting Multicollinearity in Regression Analysis. Am. J. Appl. Math. Stat. 2020, 8, 39–42. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Han, P.; Liu, Y.; Ma, W.; Zhang, H.; Wu, N.; Sang, S.; Xia, Y.; Pan, J.; et al. Relationship between tooth loss and sarcopenia in suburban community-dwelling older adults in Shanghai and Tianjin of China. Sci. Rep. 2022, 12, 7618. [Google Scholar] [CrossRef]
- Albani, V.; Nishio, K.; Ito, T.; Kotronia, E.; Moynihan, P.; Robinson, L.; Hanratty, B.; Kingston, A.; Abe, Y.; Takayama, M.; et al. Associations of poor oral health with frailty and physical functioning in the oldest old: Results from two studies in England and Japan. BMC Geriatr. 2021, 21, 187. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal inflammation and systemic diseases: An overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Lee, J.; Kang, J.-H. Associations between oral health status and risk of fractures in elder adults. Sci. Rep. 2023, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Bramantoro, T.; Hariyani, N.; Setyowati, D.; Purwanto, B.; Zulfiana, A.A.; Irmalia, W.R. The impact of oral health on physical fitness: A systematic review. Heliyon 2020, 6, e03774. [Google Scholar] [CrossRef]
- Kawakubo, N.; Miyamoto, J.J.; Katsuyama, N.; Ono, T.; Honda, E.-i.; Kurabayashi, T.; Taira, M.; Moriyama, K. Effects of cortical activations on enhancement of handgrip force during teeth clenching: An fMRI study. Neurosci. Res. 2014, 79, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Suenaga, H.; Yamabe, Y.; Torisu, T.; Fujii, H. Propagation of various tooth impacts in the human body. J. Oral Rehabil. 1998, 25, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Marchili, N.; Ortu, E.; Pietropaoli, D.; Cattaneo, R.; Monaco, A. Dental Occlusion and Ophthalmology: A Literature Review. Open Dent. J. 2016, 10, 460–468. [Google Scholar] [CrossRef]
- Corcuera-Ciudad, R.; Patiño-Villena, A.; Paima-Olivari, R.; Chambergo-Michilot, D.; Parodi, J.; Runzer-Colmenares, F. Trastornos de la marcha y el equilibrio en adultos mayores y su asociación con diabetes mellitus tipo 2. Med. Interna México 2019, 35, 676–684. [Google Scholar]
- Araki, A.; Ito, H. Diabetes mellitus and geriatric syndromes. Geriatr. Gerontol. Int. 2009, 9, 105–114. [Google Scholar] [CrossRef]
- Hewston, P.; Deshpande, N. Falls and balance impairments in older adults with type 2 diabetes: Thinking beyond diabetic peripheral neuropathy. Can. J. Diabetes 2016, 40, 6–9. [Google Scholar] [CrossRef]
- Gregg, E.W.; Menke, A. Diabetes and Disability. In Diabetes in America, 3rd ed.; Cowie, C., Ed.; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018; pp. 1–15. [Google Scholar]
- Broadwin, J.; Goodman-Gruen, D.; Slymen, D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J. Am. Geriatr. Soc. 2001, 49, 1641–1645. [Google Scholar] [CrossRef]
- Mikkola, T.M.; Kautiainen, H.; von Bonsdorff, M.B.; Salonen, M.K.; Wasenius, N.; Kajantie, E.; Eriksson, J.G. Body composition and changes in health-related quality of life in older age: A 10-year follow-up of the Helsinki Birth Cohort Study. Qual. Life Res. 2020, 29, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, J.; Stenholm, S.; Rantanen, T.; Heliöaara, M.; Sainio, P.; Koskinen, S. Effect of age on the association between body fat percentage and maximal walking speed. J. Nutr. Health Aging 2011, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Leng, X.I.; Kritchevsky, S.B. Body composition and physical function in older adults with various comorbidities. Innov. Aging 2017, 1, igx008. [Google Scholar] [CrossRef] [PubMed]
- Fico, B.G.; Maharaj, A.; Pena, G.S.; Huang, C.J. The effects of obesity on the inflammatory, cardiovascular, and neurobiological responses to exercise in older adults. Biology 2023, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.M.; Soares, R.; Carvalho, D.; Freitas, P. The impact of obesity on bone health: An overview. Endokrynol. Pol. 2022, 73, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Nicklas, B.J.; Brubaker, P.H.; Hundley, W.G.; Brinkley, T.E.; Upadhya, B.; Becton, J.T.; Nelson, M.D.; Chen, H.; Kitzman, D.W. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail. 2018, 6, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Nishizawa, H.; Otsuka, A.; Fukuda, S.; Tanaka, Y.; Nagao, H.; Sakai, Y.; Suzuki, M.; Yokota, S.; Tada, H.; et al. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc. Diabetol. 2018, 17, 112. [Google Scholar] [CrossRef]
- Tramontano, A.; Veronese, N.; Giantin, V.; Manzato, E.; Rodriguez-Hurtado, D.; Trevisan, C.; De Zaiacomo, F.; Sergi, G. Nutritional status, physical performance and disability in the elderly of the Peruvian Andes. Aging Clin. Exp. Res. 2016, 28, 1195–1201. [Google Scholar] [CrossRef]
- Jacobsen, E.L.; Brovold, T.; Bergland, A.; Bye, A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: A cross-sectional study. BMJ Open 2016, 6, e011512. [Google Scholar] [CrossRef]
- Asamane, E.A.; Greig, C.A.; Thompson, J.L. The association between nutrient intake, nutritional status and physical function of community-dwelling ethnically diverse older adults. BMC Nutr. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Meskers, C.G.M.; Trappenburg, M.C.; Verlaan, S.; Reijnierse, E.M.; Whittaker, A.C.; Maier, A.B. Malnutrition is associated with dynamic physical performance. Aging Clin. Exp. Res. 2020, 32, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet dupplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, R. Dietary Management for Older Subjects with Obesity. Clin. Geriatr. Med. 2005, 21, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Gava, G.; Calvani, R.; Marzetti, E.; Coelho-Júnior, H.J.; Picca, A.; Esposito, I.; Ciciarello, F.; Salini, S.; Russo, A.; et al. Lower adherence to a mediterranean diet is associated with high adiposity in community-dwelling older adults: Results from the Longevity Check-Up (Lookup) 7+ Project. Nutrients 2023, 15, 4892. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Calvani, R.; Marzetti, E.; Picca, A.; Coelho-Júnior, H.J.; Martone, A.M.; Massaro, C.; Tosato, M.; Landi, F. Low adherence to mediterranean diet is associated with probable sarcopenia in sommunity-dwelling older adults: Results from the Longevity Check-Up (Lookup) 7+ Project. Nutrients 2023, 15, 1026. [Google Scholar] [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Voulgaridou, G.; Papadopoulou, S.D.; Spanoudaki, M.; Kondyli, F.S.; Alexandropoulou, I.; Michailidou, S.; Zarogoulidis, P.; Matthaios, D.; Giannakidis, D.; Romanidou, M.; et al. Increasing muscle mass in elders through diet and exercise: A literature review of recent RCTs. Foods 2023, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Moreno-Banda, G.L.; Carnalla, M.; Rivera, J.A.; Romero-Martinez, M.; Barquera, S.; Barrientos-Gutiérrez, T. Prevalencia de prediabetes y diabetes en México: Ensanut 2022. Salud Pública México 2023, 65, s163–s168. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Oviedo-Solís, C.; Vargas-Meza, J.; Ramírez-Villalobos, D.; Medina-García, C.; Gómez-Álvarez, E.; Hernández-Barrera, L.; Barquera, S. Prevalencia, tratamiento y control de la hipertensión arterial en adultos mexicanos: Resultados de la Ensanut 2022. Salud Pública México 2023, 65, s169–s180. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arregui, L.; Ávila Funes, J.A.; Amieva, H.; Borges-Yáñez, S.A.; Villa-Romero, A.; Aguilar-Navarro, S.; Pérez-Zepeda, M.U.; Gutierrez-Robledo, L.M.; Castrejón-Pérez, R.C. The Coyoacan Cohort Study: Design, methodology, and participants’ characteristics of a Mexican study on nutritional and psychosocial markers of frailty. J. Frailty Aging 2013, 2, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mandi, R.; Bansod, D.W.; Goyal, A.K. Exploring the association of lifestyle behaviors and healthy ageing among the older adults in India: Evidence from LASI survey. BMC Geriatr. 2023, 23, 675. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Women (74.6%) | Men (25.4%) | Total (100%) | p |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (sd±) | 73.7 (±6.3) | 74.3 (±5.82) | 73.9 (±6.2) | |
| Median (q1, q3) c | 73.0 (69.0, 78.0) | 73 (70.0, 79.0) | 73.0 (69, 78) | 0.322 a |
| Marital status | ||||
| Married | 89 (53.9) | 76 (46.1) | 165 (45.1) | <0.001 b |
| Single | 74 (93.7) | 5 (6.3) | 79 (21.6) | |
| Widow | 110 (90.2) | 12 (9.8) | 122 (33.3) | |
| Years of schooling | ||||
| ≤6 | 89 (82.4) | 19 (17.6) | 108 (29.5) | 0.065 b |
| 7–≤12 | 96 (73.3) | 35 (26.7) | 131 (35.8) | |
| 12> | 88 (69.3) | 39 (30.7) | 127 (34.7) | |
| Smoking | ||||
| Yes | 19 (61.3) | 12 (38.7) | 31 (8.5) | <0.001 b |
| No | 185 (88.5) | 24 (11.5) | 209 (57.1) | |
| Former smoker | 69 (54.8) | 57 (45.2) | 126 (34.4) | |
| More than 100 cigarettes smoked | ||||
| Yes | 66 (54.6) | 55 (45.5) | 121(33.1) | <0.001 b |
| No | 207 (84.5) | 38 (15.5) | 245 (66.9) | |
| Drinking alcoholic beverages | ||||
| Yes | 12 (40.0) | 18 (60.0) | 30 (8.2) | <0.001 b |
| No | 261(77.7) | 75 (22.3) | 336 (91.8) | |
| Hypertension | ||||
| Yes | 158 (78.6) | 43 (21.4) | 201 (54.9) | 0.051 b |
| No | 115 (69.7) | 50 (30.3) | 165 (45.1) | |
| Diabetes mellitus type 2 | ||||
| Yes | 65 (71.4) | 26 (28.6) | 91 (24.9) | 0.424 b |
| No | 208 (75.6) | 67 (24.4) | 275 (75.1) | |
| Nutritional status (MNA) | ||||
| Risk of malnutrition/Malnutrition d | 124 (83.2) | 25 (16.8) | 149 (40.71) | 0.002 b |
| Well nourished | 149 (68.7) | 68 (31.3) | 217 (59.3) | |
| Body mass index (kg/m2) Mean (sd±) | 27.5 (±4.4) | 26.5 (±3.6) | 27.3 (±4.2) | |
| Median (q1, q3) | 27.0 (24.6, 30.1) | 26.5 (24.5, 29.0) | 26.9 (24.6, 29.9) | 0.130 a |
| Underweight | 0 | 1 (100) | 1 (0.27) | 0.077 b |
| Normal | 65 (73.9) | 23 (26.1) | 88 (24.0) | |
| Overweight | 133 (71.5) | 53 (28.5) | 186 (50.82) | |
| Obese | 75 (82.4) | 16 (17.6) | 91 (24.86) | |
| Body fat % | ||||
| Mean (sd±) | 39.8 (±5.5) | 32.2 (±4.0) | 37.5 (±7.4) | |
| Median (q1, q3) | 40 (36.2, 43.3) | 32.5 (29.8, 34.8) | 37.9 (33.4, 42.5) | <0.001 a |
| Fat-free mass (lean + BMC) e (kg) mean (sd±) | 36.5 (±3.39) | 49.4 (±5.17) | 39.8 (±6.85) | |
| Median (q1, q3) | 36.2 (34.1, 38.5) | 49.9 (45.4, 58.2) | 49.0 (45.0, 52.8) | <0.001 a |
| Oral health quality of life (GOHAI) | ||||
| Mean (sd±) | 38.05 (±8.81) | 39.37(±8.61) | 39.05 (±8.75) | |
| Median (q1, q3) | 41 (34.0, 47.0) | 42 (33.0, 46.0) | 42.0 (34.0, 47.0) | 0.772 a |
| Physical component | ||||
| Mean (sd±) | 16.4 (±3.8) | 17.0 (±3.2) | 16.6 (±3.7) | |
| Median (q1, q3) | 17.0 (14.0, 20.0) | 17.0 (15.0, 20.0) | 17 (14.0, 20.0) | 0.360 a |
| Psychosocial component | ||||
| Mean (sd±) | 21.3 (±4.06) | 21.2 (±4.45) | 21.3 (±4.15) | |
| Median (q1, q3) | 22.0 (19.0, 25.0) | 23 (19.0, 26.0) | 22.5 (19.0, 25.0) | 0.955 a |
| Oral pain/discomfort component | ||||
| Mean (sd±) | 13.2 (±2.21) | 13.2 (±1.93) | 13.2 (±2.14) | |
| Median (q1, q3) | 14.0 (11.0, 15.0) | 14.0 (12.0, 15.0) | 14.0 (12.0, 15.0) | 0.650 a |
| Short physical performance battery | ||||
| Mean (sd±) | 8.3 (±2.72) | 9.2 (±2.49) | 8.5 (±2.69) | |
| Median (q1, q3) | 9.0(7.0, 10.0) | 10.0 (8.0, 11.0) | 9.0 (7.0, 11.0) | 0.005 a |
| SPPB ≤ 8 | ||||
| Yes | 128 (80.5) | 31 (19.5) | 159 (43.4) | 0.023 b |
| No | 145 (70.0) | 62 (30.0) | 207 (56.6) | |
| SPPB subtest | ||||
| Chair stand scores | ||||
| Mean (sd±) | 2.7 (±1.29) | 2.9 (±1.23) | 2.7 (±1.27) | |
| Median (q1, q3) | 3 (2, 4) | 3 (2, 5) | 3 (2, 4) | 0.187 a |
| Balance scores mean (sd±) | ||||
| Mean (sd±) | 2.6 (±1.38) | 3.0 (±1.21) | 2.7 (±1.35) | |
| Median (q1, q3) | 3 (1, 4) | 3 (2, 4) | 3 (1, 4) | 0.011 a |
| Gait speed scores mean (sd±) | ||||
| Mean (sd±) | 3.1 (±1.00) | 3.2 (±0.95) | 3.1 (±0.99) | |
| Median (q1, q3) | 3 (2, 4) | 4 (3, 4) | 3 (2, 4) | 0.042 a |
| Characteristics | SPPB > 8 | SPPB ≤ 8 | p |
|---|---|---|---|
| (207) (56.6%) | (159) (43.4%) | ||
| Age | |||
| Mean (sd±) | 72.5 (±5.6) | 75.8 (±6.5) | |
| Median (q1, q3) c | 72 (68.0, 77.0) | 76 (70.0, 80.0) | <0.001 a |
| Sex | n (%) | n (%) | |
| Women | 145 (53.1) | 128 (46.9) | 0.023 b |
| Men | 62 (66.7) | 31(33.3) | |
| Marital status | |||
| Married | 107 (64.9) | 58 (35.1) | 0.015 b |
| Single | 39 (49.4) | 40 (50.6) | |
| Widow | 61(50.0) | 61(50.0) | |
| Years of schooling | |||
| ≤6 | 43 (39.8) | 65 (60.2) | <0.001 b |
| 7–≤12 | 77 (58.8) | 54 (41.2) | |
| 12> | 87 (68.5) | 40 (31.5) | |
| Tobacco use | |||
| Current smoker | 15 (48.4) | 16 (51.6) | 0.556 b |
| Non-smoker | 122 (58.4) | 87 (41.6) | |
| Former smoker | 70 (55.6) | 56 (44.4) | |
| Ever smoked | |||
| ≥100 cigarettes | 140 (57.1) | 105 (42.9) | 0.748 b |
| <100 cigarette | 67 (55.4) | 54 (44.6) | |
| Drinking alcoholic beverages | |||
| Yes | 20 (66.7) | 10 (33.3) | 0.244 b |
| No | 187 (55.6) | 149 (44.4) | |
| Hypertension | |||
| Yes | 100 (49.7) | 101 (50.3) | 0.004 b |
| No | 107 (64.9) | 58 (35.1) | |
| Diabetes mellitus type 2 | |||
| Yes | 40 (44.0) | 51 (56.0) | 0.005 b |
| No | 167 (60.7) | 108 (39.3) | |
| Mini Nutritional Assessment (MNA) | |||
| Malnutrition/risk of malnutrition | 70 (47.0) | 79 (53.0) | 0.002 b |
| Normal | 137 (63.1) | 80 (36.9) | |
| Body mass index (kg/m2) | |||
| Mean (sd±) | 26.8 (± 3.9) | 27.8 (±4.6) | |
| Median (q1, q3) | 26.7 (24.3, 29.4) | 27.3 (24.8, 30.5) | 0.063 a |
| Underweight | 1 (100) | 0 (0. 0) | 0.167 b |
| Normal | 56 (63.6) | 32 (36.4) | |
| Overweight | 106 (57.0) | 80 (43.0) | |
| Obese | 44 (48.4) | 47 (51.6) | |
| Body fat % | |||
| Mean (sd±) | 32.2 (±4.0) | 39.8 (±5.5) | |
| Median (q1, q3) | 36.3 (32.8, 41.4) | 39.5 (35.3, 43.3) | <0.001 a |
| Fat-free mass (lean + BMC) d (kg) | |||
| Mean (sd±) | 40.4 (±7.1) | 39.0 (±6.5) | |
| Median (q1, q3) | 38.1 (35.4, 44.9) | 37.1 (34.3, 41.9) | <0.001 a |
| Oral health quality of life (GOHAI) | |||
| Mean (sd±) | 40.5 (±7.96) | 37.1 (±9.36) | |
| Median (q1, q3) | 43.0 (36.0, 47.0) | 39.0 (29.0, 46.0) | 0.009 a |
| Physical component | |||
| Mean (sd±) | 17.2 (±3.3) | 15.70 (±4.0) | <0.001 a |
| Median (q1, q3) | 19.0 (15.0, 20.0) | 16 (13.0, 20.0) | |
| Psychosocial component | |||
| Mean (sd±) | 21.8 (±4.0) | 20.6 (±4.3) | |
| Median (q1, q3) | 23.0 (20.0, 25.0) | 21.0 (18.0, 25.0) | 0.007 a |
| Oral pain/discomfort component | |||
| Mean (sd±) | 13.5 (±1.9) | 12.8 (±2.4) | |
| Median (q1, q3) | 14.0 (12.0, 15.0) | 13.0(11.0, 15.0) | 0.006 a |
| Bivariate Models a | Multiple Model c | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR b | (95%CI) | p | OR d | (95%CI) | p |
| Age | 1.10 | (1.06–1.14) | <0.001 | 1.13 | (0.70–2.22) | <0.001 |
| Sex | ||||||
| Men | 1 | 1 | ||||
| Women | 1.7 | (1.08–2.89) | 0.024 | 1.13 | (1.08–1.18) | 0.732 |
| Tobacco consumption | ||||||
| Smoking < 100 cigarettes | 1 | 1 | ||||
| Smoking ≥ 100 cigarettes | 1.37 | (0.84–2.24) | 0.206 | 1.29 | (0.77–2.18) | 0.333 |
| Alcohol consumption | ||||||
| No | 1 | 1 | ||||
| Yes | 0.72 | (0.31–1.68) | 0.440 | 1.01 | (0.41–2.43) | 0.991 |
| Hypertension | ||||||
| No | 1 | 1 | ||||
| Yes | 1.59 | (1.02–2.48) | 0.041 | 1.09 | (0.66–1.78) | 0.740 |
| Type 2 diabetes mellitus | ||||||
| No | 1 | 1 | ||||
| Yes | 2.45 | (1.46–4.11) | 0.001 | 2.10 | (1.20–3.67) | 0.009 |
| Nutritional status normal MNA ≥ 24 e | 1 | 1 | ||||
| Risk of malnutrition/malnutrition MNA ≤ 23.5 | 1.92 | (1.22 -3.01) | 0.005 | 1.76 | (1.01–3.09) | 0.047 |
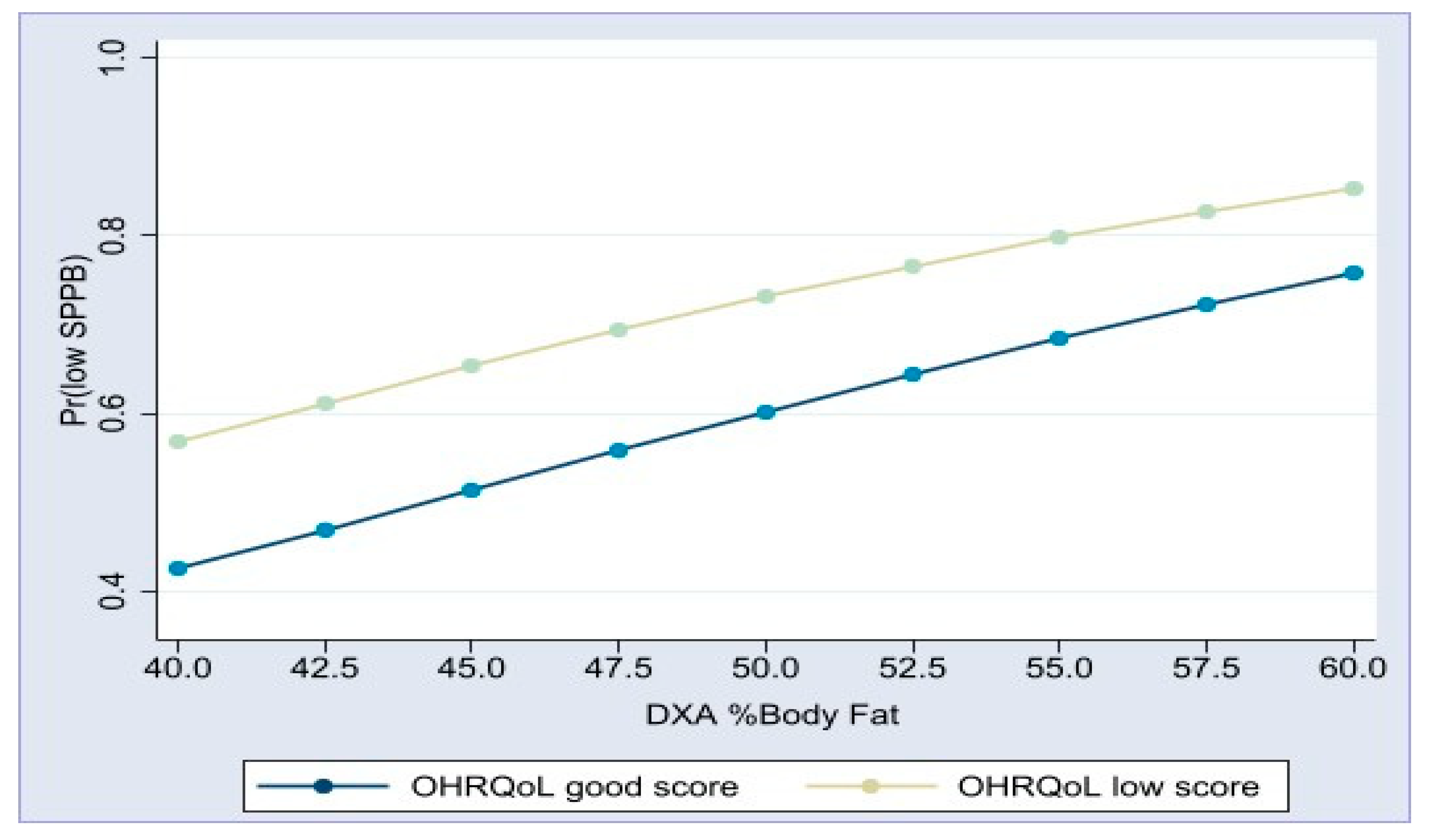
| DXA body fat% f | 1.08 | (1.03–1.13) | 0.001 | 1.09 | (1.04–1.14) | <0.001 |
| Oral health-related quality of life | ||||||
| GOHAI ≥ 37 g | 1 | 1 | ||||
| GOHAI < 37 | 2.18 | (1.35–3.51) | 0.001 | 1.96 | (1.18–3.24) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irigoyen-Camacho, M.E.; Velazquez-Alva, M.C.; Zepeda-Zepeda, M.A.; Lazarevich, I.; Castano-Seiquer, A.; Flores-Fraile, J. Physical Performance, Body Composition, and Oral Health in Community-Residing Older Adults: A Cross-Sectional Study. Geriatrics 2024, 9, 89. https://doi.org/10.3390/geriatrics9040089
Irigoyen-Camacho ME, Velazquez-Alva MC, Zepeda-Zepeda MA, Lazarevich I, Castano-Seiquer A, Flores-Fraile J. Physical Performance, Body Composition, and Oral Health in Community-Residing Older Adults: A Cross-Sectional Study. Geriatrics. 2024; 9(4):89. https://doi.org/10.3390/geriatrics9040089
Chicago/Turabian StyleIrigoyen-Camacho, Maria Esther, Maria Consuelo Velazquez-Alva, Marco Antonio Zepeda-Zepeda, Irina Lazarevich, Antonio Castano-Seiquer, and Javier Flores-Fraile. 2024. "Physical Performance, Body Composition, and Oral Health in Community-Residing Older Adults: A Cross-Sectional Study" Geriatrics 9, no. 4: 89. https://doi.org/10.3390/geriatrics9040089
APA StyleIrigoyen-Camacho, M. E., Velazquez-Alva, M. C., Zepeda-Zepeda, M. A., Lazarevich, I., Castano-Seiquer, A., & Flores-Fraile, J. (2024). Physical Performance, Body Composition, and Oral Health in Community-Residing Older Adults: A Cross-Sectional Study. Geriatrics, 9(4), 89. https://doi.org/10.3390/geriatrics9040089










