Abstract
This systematic review and meta-analysis discusses the available data on the efficacy of diet, food intake, and exercise mixed interventions (DEMI) for community-dwelling older adults in Japan and assesses the evidence level. We searched the literature regarding the research questions using electronic and hand-searching methods. To ensure the reliability and quality of the evidence, we used the Cochrane risk of bias tool and GRADE system. All studies included DEMI; other interventions included group activities, health education, and community participation. All interventions were categorized into three classifications, namely “Diet and food intake”, “Exercise”, and “Other”. Programs included lectures, practical exercises, group activities, consulting, and programs that could be implemented at home. By comparing groups and measuring outcomes at various time points, most studies reported positive results regarding the impact of the interventions. Specifically, usual gait speed, Food Frequency Questionnaire Score, and Diet Variety Score demonstrated significant improvement. Additionally, three studies demonstrated improvement in frailty. This review suggests that DEMI resulted in improvements in some outcome variables. However, the efficacy of all variables was not fully examined. The results of the meta-analysis revealed positive outcomes for some variables, although the evidence level for these outcomes was considered moderate.
1. Introduction
In an aging society, older adults face various challenges related to their physical functioning in daily life, as well as issues concerning nutrition. To address these concerns, a mixed intervention involving nutrition and exercise has been implemented to improve conditions such as sarcopenia, frailty, and the aging process in older individuals [1,2]. Numerous studies have demonstrated the efficacy of such interventions for older adults [3].
Japan garners significant attention for having one of the highest levels of long-term healthy life expectancy and overall life expectancy at birth worldwide [4]. Distinctive initiatives in Japan, such as the “Smart Life Project of Japan”, ref. [5] affirm that the older population in Japan enjoys exceptional health and functional status due to health and care services that emphasize exercise and dietary practices. We believe that researching nutritional intake and exercise interventions for community-dwelling older adults in Japan is extremely important for the future of their health.
The innovative long-term care insurance system (LTC) in Japan provides comprehensive coverage for older adults. The health status of these individuals is influenced by both health and care services and the compensation system within the social and healthcare framework, particularly in terms of nutritional intake.
According to many studies previously reported in Japan [6,7], significant concerns regarding nutrition and exercise mixed interventions exist. A prior systematic review highlighted the low level of evidence available and the necessity for future research [8]. Earlier studies have reported promising results for community-dwelling older individuals. However, the number of eligible, well-designed studies has been limited. Most prior studies had poor study designs, a small number of participants, or were lacking a comparison group. Furthermore, these studies often lacked comprehensive details about the interventions employed.
In the daily lives of the older adults, it is crucial to consider their habitual activities, especially those related to daily eating. When implementing interventions for this population, it is advisable to address not only nutritional aspects but also the broader context of eating, food, and other relevant psychosocial factors. Despite the importance of dietary and exercise factors for community-dwelling older adults, many studies [6,7,9,10,11,12,13,14] have not emphasized the significance. However, there is a noticeable gap when it comes to investigating the efficacy of interventions that combine both food and exercise for the older adults. This gap is largely due to the lack of well-designed studies; lack of comprehensive information about the participants, their characteristics, and dietary and food interventions; and missing details of the specific interventions themselves.
In Japan, the policy task force overseeing care programs [15,16] has shown a clear commitment to improving nutrition. However, studies addressing the impact of this policy on nutritional improvement have been insufficiently examined and discussed [8]. One study with a substantial sample size investigated both formal and informal programs that involved exercise and nutrition interventions for community-dwelling older adults [16]. Especially, the LTC of Japan offers care services for individuals with various degrees of functional disabilities, including food and exercise mixed interventions [15,16]. These services are accessible to older individuals upon certification of their needs. However, despite the availability of these services, there remains a lack of sufficient evidence regarding the efficacy of these interventions. While a few studies, such as the one conducted in Kameoka on LTC in Japan, attempted to verify their efficacy, the authors lacked comprehensive evaluations, control groups, and the revelation of individual factors or detailed issues [16].
We hypothesize that DEMI is effective for community-dwelling older adults in Japan. This review aims to discuss the efficacy of diet, food intake, and exercise mixed intervention (DEMI) for community-dwelling older adults in Japan and assesses the supporting level of evidence for these interventions. To this end, this systematic review and meta-analysis was conducted to investigate the efficacy of DEMI for community-dwelling older adults in Japan and determine the level of supporting evidence. We scoped and systematically reviewed previous articles related to this thesis, exploring the efficacy, specific approaches, and future research directions.
2. Materials and Methods
2.1. Protocol of Systematic Review
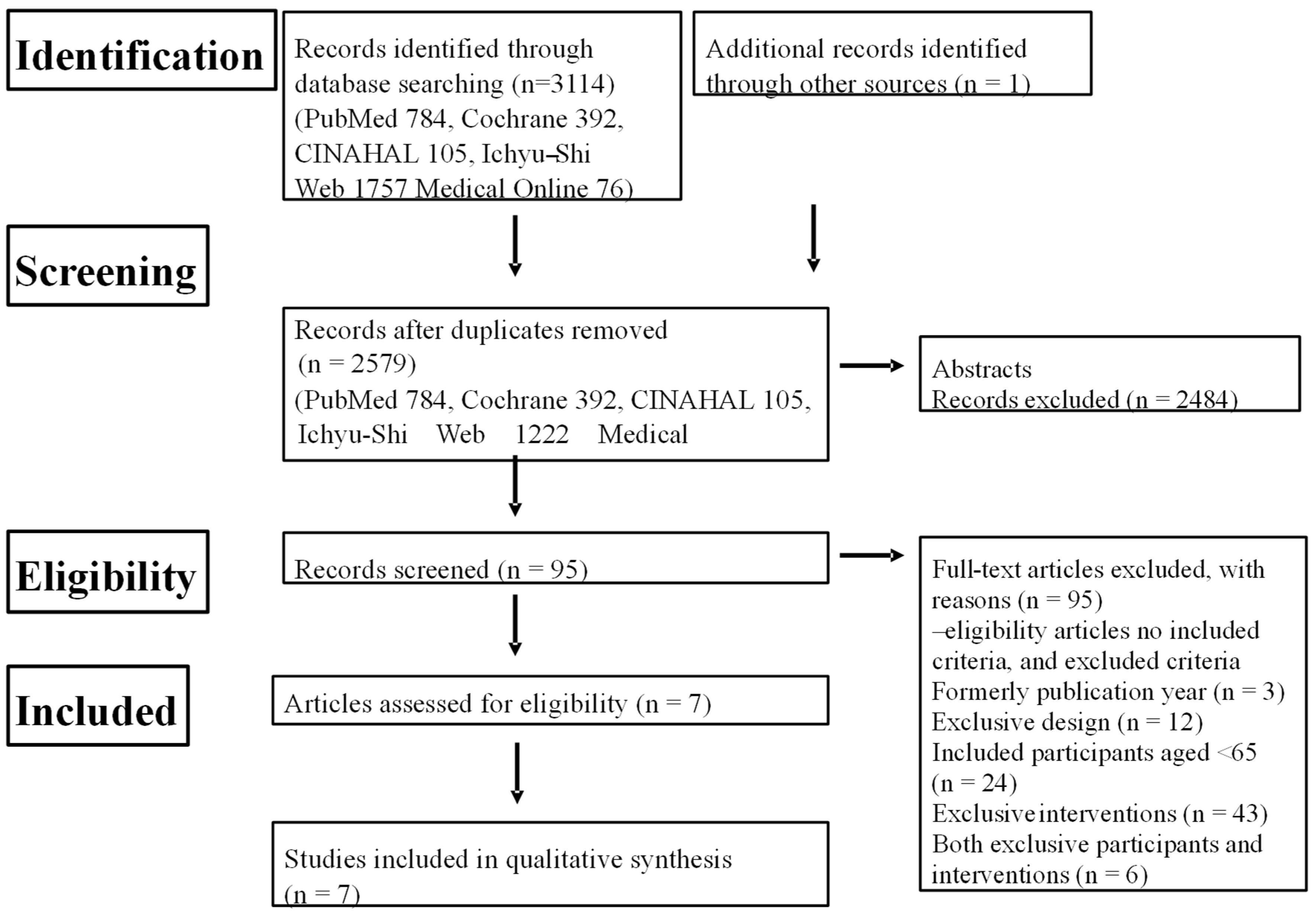
We implemented this systematic review following standard procedures [17] and in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [18]. Our systematic search and the selection process for eligible studies are outlined in Figure 1, as detailed in our protocol.
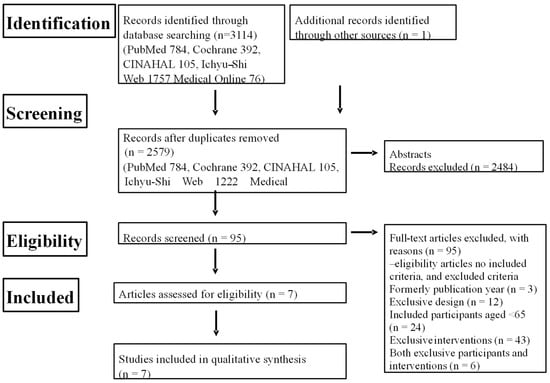
Figure 1.
Flow chart of systematic review.
CINAHL: Cumulative Index to Nursing and Allied Health Literature.
2.2. Review Questions
The review question addressed in this study was “What is the efficacy and methodology of DEMI for community-dwelling older adults in Japan?”
2.3. Aim of This Review
The aim of this review is to clarify issues concerning the efficacy of DEMI for the older adults in Japan as follows: the level of evidence provided by the previous studies, specific programs and interventions implemented, and the efficacy of these interventions.
2.4. Research Strategy
The literature search, encompassing previously published research relevant to our study questions, was conducted through a combination of electronic and manual searching, led by a single author (T.M.). After excluding duplicate and noneligible publications, the remaining literature was evaluated and critically reviewed by all authors (T.M., H.B., and H.F.). An assessment of the quality and certainty of the included studies was performed by all authors (T.M., H.B., and H.F.). The meta-analysis was done by one author (T.M.), and the results of both the systematic review and meta-analysis were discussed and reviewed by all authors (T.M., H.B., and H.F.).
2.5. Searching Methods
The literature search involved the use of several electronic databases, including Medical online [19], Ichu-shi web [20], PubMed [21], Cumulative Index to Nursing and Allied Health Literature (CINAHL) [22], and Cochrane [23]. In addition, a manual search was conducted from selected literature sources. A combination of Medical Subject Headings (MeSH) terms and keywords, in both English and Japanese, were used for the search either singularly or in combination. In English, the terms included “old”, “elderly people”, “nutrition”, “diet”, “food”, “eating”, “physical”, “exercise”, “function”, “muscle”, “mobility”, “activity”, “gait”, “walking”, “balance”, “community”, “district”, “dwelling”, “living”, “house”, “resident”. In Japanese, synonymous keywords were “Nippon”, “Koureisha”, “Chiiki-Zaizyu”, “Eiyou”, “Syokumotsu”, “Shoku-Ji”, “Tairyoku”, “Undou”, “Tiiki”, and “Zaitaku”.
2.6. Protocol for Selecting Eligible Studies
We initiated our search by querying a database. In the first step, one author, T. F., conducted an initial selection of literature matching the specified keywords and excluded any duplicate abstracts. Next, the inclusion and exclusion criteria were applied to the selected abstracts by all authors (T.M., H.B., and H.F.). The process of moving from the initial pool of studies to the final selection of studies was facilitated using the literature management software, Rayyan (PC ver.) [24], which enables seamless collaboration among multiple reviewers. This matching and discussion phase continued until a consensus was reached among the authors. In the third step, all authors collectively assessed the selected literature based on their abstracts. Finally, the authors examined the full texts of all studies, and the selection of eligible studies was made after a critical review conducted by each of the three reviewers.
2.7. Inclusion and Exclusion Criteria
All authors selected literature that met the eligibility criteria based on the following inclusion and exclusion criteria for the type of study, participants, and interventions.
The review on the efficacy of DEMI for community-dwelling older adults in Japan included the following criteria:
Inclusion criteria were as follows: (1) full-text literature; (2) in IMRAD format (Introduction, Methods, Results, and Discussion); (3) randomized controlled trial (RCT) study design; (4) case-comparison group comparison studies between intervention and non-intervention or placebo-control groups; (5) with English or Japanese as the language of publication; (6) study participants aged 65 years and older; (7) residing in community dwellings in Japan; (8) receipt of combined diet, food intake, and exercise interventions; and (9) published between April 2000 and January 2021, since LTC for older adults in Japan was started on 1 April 2000.
In this review, the intervention programs referred to as “DEMI” encompass a combination of interventions targeting eating behavior and exercise among community-dwelling older individuals in Japan. Eating behavior, in this context, means not only food and meals but also the entire process including meal preparation, cooking, eating, and meal cleanup, and includes instruction, consultations, and group-based practical activities related to eating behavior. Additionally, exercise interventions are provided, which can be either self-guided or instructor-led. The manner of intervention for exercise is limited to face-to-face interactions, such as personal or group exercise sessions. This excludes indirect intervention methods conducted at home, such as using telephone support, written communication (letters), electronic devices, or other remote communication tools.
Exclusion criteria were as follows: (1) case reports, qualitative studies, government or institution reports, conference reports, doctoral theses, or book chapters; (2) with severe disability impeding participant’s independence in any daily activities; (3) studies without a combination of interventions, or those without any form of intervention; and (4) studies involving one group analysis without a comparison control or placebo group.
2.8. Data Extraction
One author (T.M.) was responsible for data extraction from the eligible studies and subsequently presented the results in a single table. This table includes key information such as the authors of the study, publication year, study design, participant details, descriptions of interventions, and the reported outcomes.
2.9. Assessment of Quality and Risk of Bias of the Included Studies
The quality assessment of the included studies was conducted by all three authors (T.M., T.F., and H.B.) using the “Risk of Bias ver 2” (RoB 2) [25,26] tool developed by Cochrane. The RoB 2 is recognized for its reliability and validity in assessing the quality of studies. It evaluates six key components, namely the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias. The ratings for each of these components were categorized into three grades, namely low risk of bias, unclear risk of bias, and high risk of bias. In instances where there were discrepancies in the risk of bias assessments among the three reviewers, these discrepancies were resolved through discussion and consensus until an agreement was reached.
2.10. Primary and Secondary Outcomes
We established that primary outcomes were directly affected, while the secondary outcomes were influenced by the changes in the primary outcomes or through synergistic effects. The primary outcomes primarily focused on nutrition and physical elements. However, considering the contents and mechanism of DEMI, we further assumed that DEMI could also impact secondary outcomes, which included functional mobilities, activities, and psychosocial changes.
All outcomes were categorized into the following groups: “frailty”, “physical functions” (Phy F), “psychosocial functions” (Psy F), “nutritional status” (Nu), “food intake” (F), “behavior (frequency, duration)” (B), and “other outcome factors” (O).
2.11. Effect Measures
To assess the significant effects of DEMI interventions, we compared the results of outcome variables in the reviewed studies and identified significant changes. The effects of DEMI were measured by comparing the mean differences (MDs) of outcomes between pre- and post-interventions using meta-analysis if two or more comparable variable values were available. The results were visualized using forest plots. A random-effects model was utilized, and heterogeneity was provided with I2.
2.12. Synthesis Methods
In the eligible studies that satisfied the inclusion criteria, we employed meta-analysis as the synthesis method for outcomes. A meta-analysis was conducted when pre- and post-intervention data were extractable. In instances where the compared data were not explicitly reported, they were tabulated, and a qualitative descriptive analysis was conducted as part of the systematic review. Only ordinary scale data and interval scale data were extracted for analysis, excluding nominal data.
Regarding statistical methods, the number of eligible studies was determined using the PRISMA checklist [27]. To structure our analysis, we followed the “patient/population, intervention, comparison and outcomes” (PICO) framework, which helped to systematically examine and discuss the key characteristics and outcomes of the included studies. Statistical analysis was performed using the statistical software EZR [28]. The effect size was represented by the weighted mean difference and its corresponding 95% confidence interval (CI). Forest plots were used to visualize the results of the meta-analysis for the available comparable variables. Imprecision was assessed using forest plot, optimal information size, and the range of CI.
2.13. Reporting Bias Assessment
Reporting bias was assessed using a funnel plot to visualize potential asymmetry in the distribution of study results. However, due to the limited number of comparable studies (less than 10), a statistical assessment of the funnel plot was not performed.
2.14. Certainty Assessment
Assessing the certainty of evidence was conducted by evaluating the quality of evidence in five domains, namely study design, risk of bias, inconsistency, indirectness, and imprecision. Reporting bias was also considered using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) certainty assessment of evidence [29]. The evidence was categorized into four grades, namely high, moderate, low, and very low. The GRADE tool from Cochrane demonstrated the RoB 2 through a graph providing a visual representation of the certainty in the body of evidence. All authors independently estimated the GRADE certainty and made rating adjustments by downgrading one grade (serious concern) or two grades (very serious concern) for reasons like risk of bias, inconsistency, indirectness, imprecision, and publication bias [30]. Six domains of the GRADE certainty were assessed by two authors, and any discrepancies were discussed until a consensus was reached on the final results.
3. Results
3.1. Search Results
Number of Retrieved Studies
The search of the databases initially yielded 3114 literature items matching the keywords. After the removal of the duplicate abstracts by a single author (T.F.), a total of 2579 studies remained for further consideration (Figure 1).
In the initial stage, all authors collectively selected studies based on their abstracts, resulting in 95 eligible studies. Next, all authors examined the full text of all of these studies. Finally, after critical review, a total of seven eligible studies were selected for inclusion in the review [31,32,33,34,35,36,37].
3.2. General Information of Searched Studies
Quality of the Included Studies (Methodological Quality)
Results of the RoB score and a summary of the quality of the eligible studies are presented in Table S1 (Cochrane). Additionally, the RoB score summary is presented in Figure S1 and the RoB score graph is shown in Figure S2, both using the GRADE approach.
Regarding domain 1, one study [37] (Sakurai et al.) was rated as having “some concerns”, while the other six studies had a “low” RoB score. For domains 2 and 4, all studies were judged as having a “low” RoB score. Concerning domain 3, two studies [32,37] (Kwon et al., Sakurai et al.) were rated as “high”, whereas the remaining five studies were judged to have a “low” RoB score. As for domain 5, one study [33] (Kawabata et al.) received a rating of “high” RoB score, while the other six studies were judged as “low”. Finally, considering the overall RoB score, three studies [32,33,37] (Kwon et al., Kawabata et al., Sakurai et al.) were categorized “high” on the RoB 2, while the other four studies were rated as “low”.
The results of the certainty assessment are presented in Table 1. The RoB 2 was categorized as either low or high. The inconsistency and indirectness of all outcomes were assessed and were found to have no serious concerns. However, due to the small sample sizes, imprecision was rated as a “serious risk” for all outcomes. The certainty of each outcome varied from very low to moderate.

Table 1.
GRADE Certainty Assessment.
3.3. Descriptive Results after Systematic Review
Descriptive results of this review, organized according to the PICO framework, are as follows: participant characteristics (Table 2), interventions (Table 2), comparative manners (Table 3), outcomes (Table 3), and impacts of interventions (Table 3). A summary of the efficiency categories comparing between the intervention and control groups and between the beginning and end of the intervention showed good results. Specifically, all outcome categories of each study individually demonstrated improvement, except for the “Frailty” outcome category.

Table 2.
Participants (Characteristics of Participants Included in Study) and Intervention.

Table 3.
Comparison, Outcome, and Impact (Measures of Frailty and Outcomes of Study).
3.4. Participants
The extracted participant characteristics are presented in Table 2. Four of the included studies focused on individuals who were either prefrail or frail in terms of their frailty status [31,32,33,35].
Among the included studies, four studies identified participants as either frail or prefrail based on Fried’s criteria [38,39] or commonly used frailty criteria in Japan [38,39,40]. The other three studies [34,36,37] did not record the frailty status of participants. Additionally, three studies did not involve participants with frailty conditions.
3.5. Interventions (Table 2)
All seven studies [31,32,33,34,35,36,37] included in the review encompassed both DEMI and additional interventions, which can be categorized into group activities (GA), health education (HE), and community participation.
The interventions examined in our review were categorized into three main categories: “Food and diet intake”, “Exercise”, and “Other”. The following sections provide an overview of the primary outcomes observed in each category, presented in sequential order.
3.5.1. “Food and Diet Intake” (Table 2)
We categorized interventions into lecture (Lc), practical exercise, GA, and consulting, instruction, and guidance. These categories were determined based on the form of intervention delivery and how these interventions were implemented, including whether they were offered by professionals to participants, conducted in a group setting, self-directed, involving Lcs or instructions, GA, home programs, or active learning (AL).
3.5.2. “Exercise” (Table 2)
Types of “Exercise” interventions
Programs of “exercise” interventions were mainly categorized as follows: light exercise, muscle strengthening exercise (MS), balance exercise (BE), functional exercise (FE), functional activities (or activities of daily living [ADL]), gait exercise (GE), GA, health class, AL, and special anti-aging program (AP). One study had no access to detailed records (35). The contents of the exercise programs were as follows.
3.6. Special Anti-Aging Program
These exercises are widely and comprehensively recognized as being beneficial for older individuals. Their primary objectives include reducing the risk of falls and improving or preventing functional impairments. Interventions focused on fall prevention exercises were also implemented, as described in one of the studies [33].
“Other” (Table 2)
In addition to diet, food, and mixed interventions, other interventions also included activities such as cooking sessions, social engagement, AL, and various forms of GA.
Six studies [31,33,34,35,36,37], except one [32], incorporated not only food and exercise interventions but also added different forms of interventions. These interventions fell into the “Other” category and encompassed a diverse range of GA. Some examples of these activities included group discussions, shared experiences, community outings, information exchange, HE, AL, self-health checks, and visiting hot springs.
The programs under the “Other” category intervention were quite diverse. They extend beyond indoor settings and often involve going outdoors. Examples include group meetings centered around hobbies and interests [31], communal lunch sessions [34], and activities like HE and AL, aimed at promoting well-being.
3.7. Outcome (Table 3)
The variables of outcomes in each article, described in order, are shown in Table 3. We categorized those variables as follows: frailty, physical function (“Phy F”), psychological function (“Psy F”), food and dietary factors (“F”), nutritional factors (“Nu”), behavior factors like daily activities, frequency, or duration (“B”), and other factors including quality of life (QOL) (“O”). The variables for each criterion are shown in Table 3.
Outcomes Comparison by Meta-Analysis
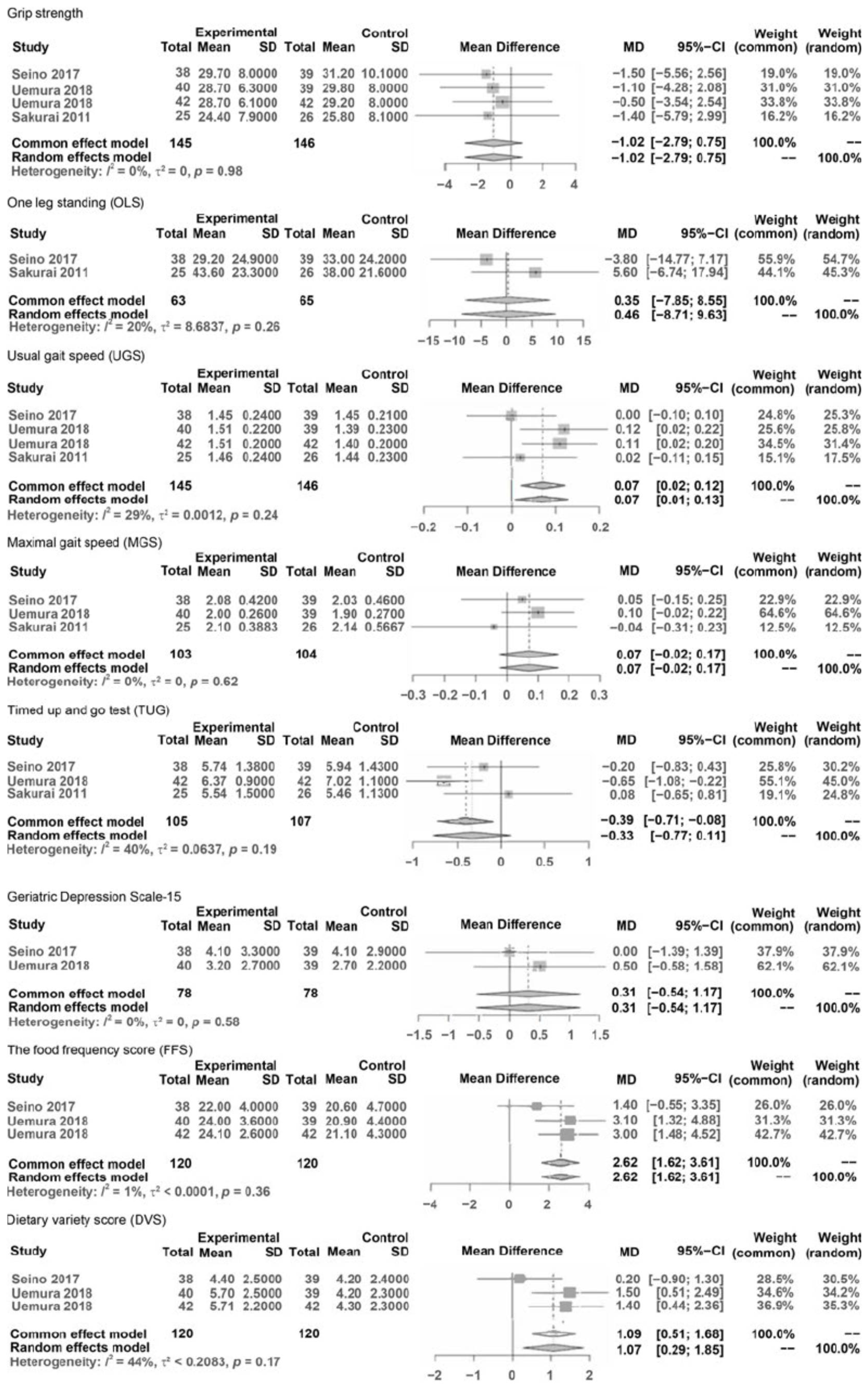
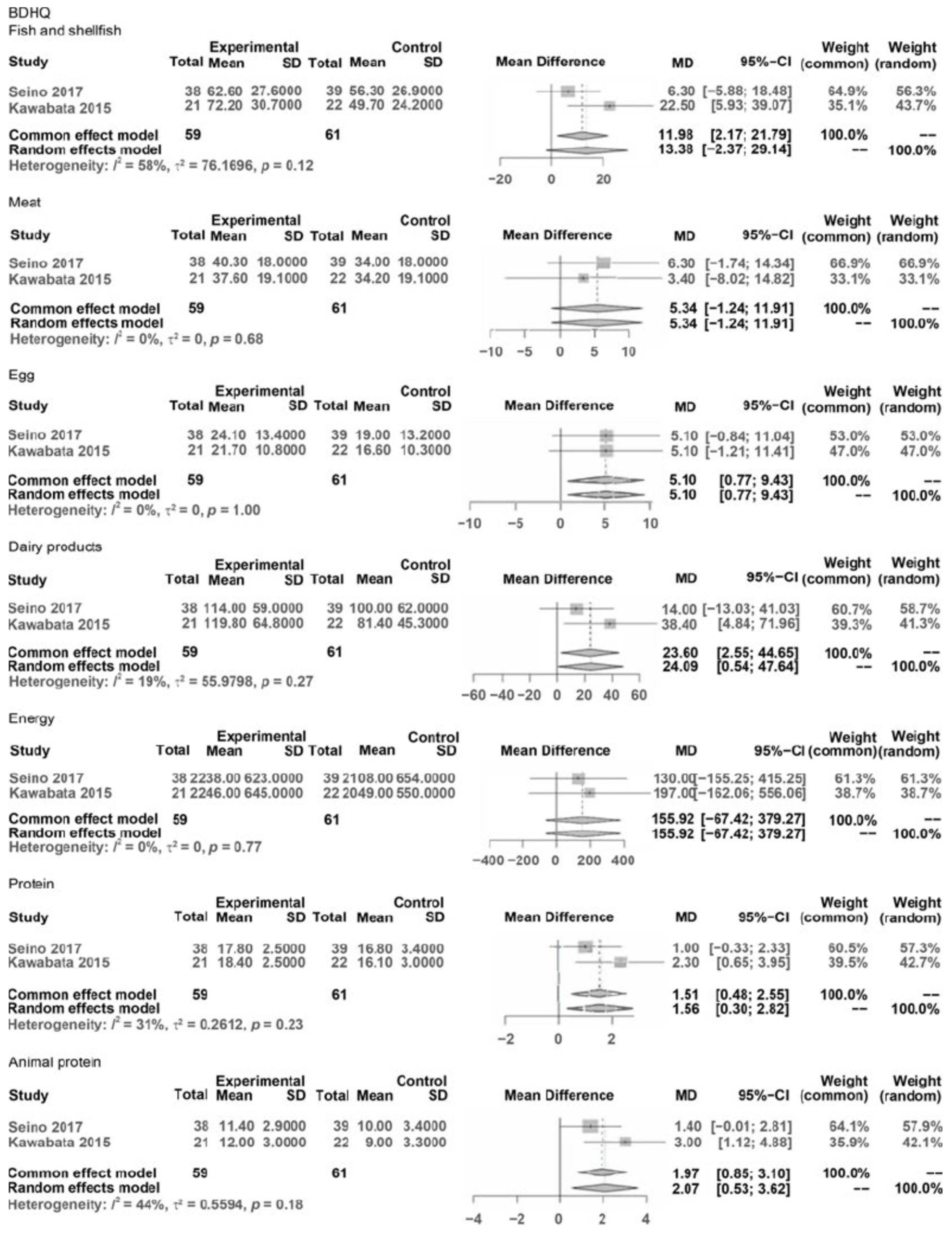
The results pre- and post-interventions were synthesized by meta-analysis and are presented in Figure 2. A meta-analysis was conducted only when the number of reported outcome data from eligible studies was more than two and when data at pre- and post-intervention could be extracted. The synthesized and compared outcomes were described as follows:
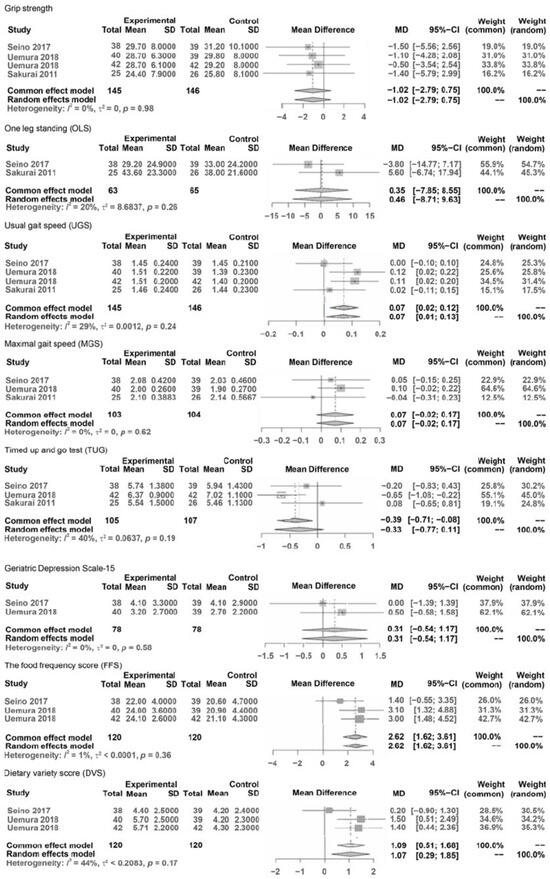
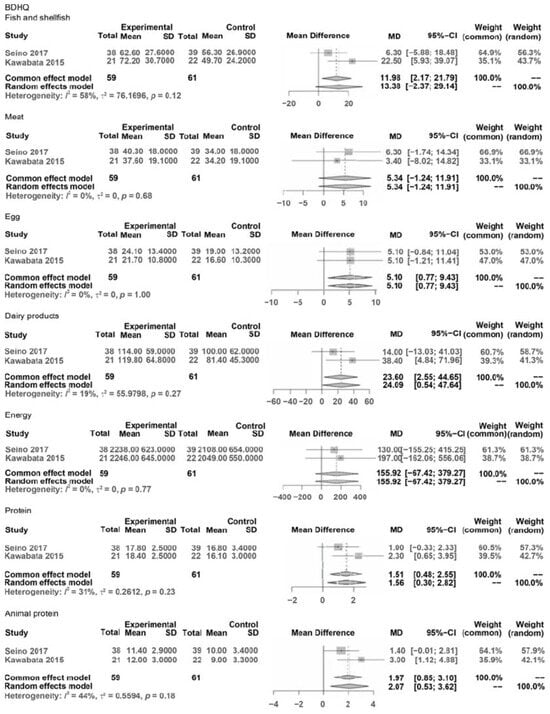
Figure 2.
Forest plots of pooled mean differences.
Phys F: grip strength, one leg standing (OLS), usual gait speed (UGS), maximal gait speed (MGS), and Timed Up and Go test (TUG).
Psy F: Geriatric Depression Scale (GDS)
Food: Food Frequency Questionnaire (FFS), Diet Variety Score (DVS), and Brief Self-
Administered Diet History Questionnaire (BDSQ)
BDSQ: BDSQ fish and shellfish, BDSQ meat, BDHQ egg, BDHQ dairy products, BDHQ energy, BDHQ protein, and BDHQ Animal protein.
In Physical Function (Phy F), variables were hand grip strength, balance (OLS with eyes open, TUG test), and gait (usual and maximal gait speeds).
In Psychological Function (Psy), the variable was the GDS score.
In Food (F) and Nutrition (N), the variables were FFS, DVS, and BDHQ (meat, eggs, dairy products, energy, protein, and animal protein).
In B and O, comparable extracted data did not exist.
The results of the meta-analysis comparing variables of outcomes were as follows (Figure 2). The results of the meta-analysis showed that significant differences were considered under 0.05 and characteristic statistical data were demonstrated as MDs, 95% CIs, Z values of overall effects, and p values of overall effects between pre- and post-interventions.
MD in outcomes of Phys F: grip strength was NS (−1.02, CI: −2.79–0.758, Z = −1.13, p = 0.26), OLS was NS (MD = 0.46, CI: −8.71; 9.63, Z = 0.10, p = 0.92), UGS significantly improved (MD = 0.07, CI: 0.01; 0.13, Z = 2.22, p = 0.027), MGS was NS (MD = 0.07, CI: −0.02; 0.17, Z = 1.48, p = 0.139), TUG was NS (MD = −0.33, CI: −0.77; 0.11, Z = −1.48, p = 0.14).
MD in outcomes of Psy F: GDS was NS (MD = 0.31, CI: −0.54; 1.17, Z = 0.71, p = 0.477),
MD in outcomes of Food: FFS improved significantly (MD = 2.62, CI: 1.62; 3.61, Z = 5.16, p < 0.0001), and DVS improved significantly (MD = 35.78, CI: 33.58; 37.98, Z = 31.84, p < 0.001).
In the outcomes of subitems in the BDSQ: BDSQ fish and shellfish was NS (MD = 13.38, CI: −2.37; 29.14, Z = 1.67, p < 0.096), BDSQ meat was NS (MD = 5.34, CI: −1.24; 11.91, Z = 1.59, p = 0.112), BDSQ egg significantly improved (MD = 5.10, CI: 0.77; 9.43, Z = 2.31, p = 0.021), BDSQ dairy products significantly improved (MD = 24.09, CI: 0.54; 47.64, Z = 2.00, p = 0.045), BDSQ energy was NS (MD = 155.92, CI: −67.42; 379.27, Z = 1.37, p = 0.171), BDHQ protein significantly improved (MD = 1.56, CI: 0.30; 2.82, Z = 2.42, p = 0.016), and BDSQ animal protein significantly improved (MD = 2.07, CI: 0.53; 3.62, Z = 2.62, p = 0.009).
Reporting bias was not analyzed in detail in our review. Although we discussed visualizing the funnel plot graph for each outcome, the analysis was not adequately conducted due to insufficient information on concentration and the small size of the studies.
4. Discussion
In this review, we discussed the effects of DEMI to some extent, and the meta-analysis could verify the effects of limited variables, with the certainty of evidence ranging from very low to moderate. Although the narrative review in this manuscript solely demonstrated positive results in each study for the effects of DEMI on community-dwelling older adults in Japan, the results of the meta-analysis were limited. However, through the meta-analysis, significant improvements were observed in variables such as UGS, FFS, DVS, and the consumption of egg, dairy products, protein, and animal protein based on the BDHQ. According to the results of this review, we obtained some insight into implications and considerable issues.
4.1. Results of Meta-Analysis
The outcomes of “Phys” slightly improved, while those of “Food” were improved in many variables.
In the outcome of “Phys”, an improvement of UGS was often reported regarding the effects of various interventions on older adults. Previous meta-analyses reported that exercise intervention improved gait speed [72], muscle strength, TUG [73], and Short Physical Performance Battery (SPPB) [74] but yielded uncertain results regarding functional performance or ADL [74]. Similarly, interventions combining nutrition and exercise were found to improve UGS; however, their impact on functional performance remained uncertain [8]. A qualitative analysis through systematic review [3] also reported uncertain results but did not include meta-analysis.
One of the reasons for the uncertain results yielded by these studies was due to the variations in participants’ frailty or functional condition and the small sample size. UGS is a representative symptom, making it easier to detect improvements. However, functional performance encompasses various physical basic functions, making it more challenging to observe significant changes in overall physical performance.
Another reason might be that the duration of interventions was too short to observe sufficient substantial effects. UGS represents a basic function for the older adults; however, improvements in functional mobilities or ADL might require a more extended period of intervention. Previous studies [75,76] reported that muscle strength improved after 12 weeks of intervention, but the duration was not sufficient to show significant changes in muscle mass. However, after 24 weeks, positive results in functional mobility were observed, prompting discussions on the potential limitation of short intervention durations in achieving significant recovery.
In the outcome of “Psy”, the improvement of the GDS score showed variable results. Hsieh et al.’s study [77], which involved home-based exercise, reported no change in depression symptoms. However, studies conducted by Singh et al. [78] and Blumenthal et al. [79] demonstrated improvement in depression syndrome among older adults was noted following home-based exercise. Many studies have reported that mental function is closely related to social functioning [80,81,82,83]. Regarding behavior or social aspects, our review could not verify the effects associated with “Psy”. Therefore, the effects of social functioning should be further researched to gain a comprehensive understanding.
Regarding the outcome of “Food” in DEMI, FFS, DVS, and some outcomes of the BDHQ demonstrated significant improvements. The interventions related to “Food” included instructions on eating behaviors, food habits, and recommended food or ingredients, provided through instruction or counseling by nutritionists, or through information exchange and communal eating among participants. These intervention contents are related to daily activities about “Food” and are commonly practiced in clinical settings, involving aspects like increasing protein intake, diversifying food choices, and providing nutritional guidance on shopping and eating behaviors. However, the specific reasons for the improvements in the outcome of “Food” remained uncertain. Previous studies [43,84,85] have reported that nutritional status is related to social interaction. Nevertheless, we could not definitively determine whether the interventions improved dietary nutrient intake or the underlying reasons and mechanisms behind these improvements.
4.2. Quality of Retrieving Eligible Studies
Previous systematic reviews on mixed nutrition and exercise interventions for the older adults have reported poor quality in some studies [3,8]. The quality of the eligible studies in our review varied from very low to moderate. Several issues remain to be addressed when designing future research studies, including concerns about blinding and the adequacy of sample sizes.
4.3. General Information on Interventions Extracted from the Narrative Review
Previous studies have not yet reached a conclusive determination regarding the appropriate optimal duration of intervention. Regarding exercise interventions, a previous systematic review demonstrated that durations of around 12 weeks were insufficient for achieving outcomes [73]. In our review, all seven relevant individual studies discussed showed positive outcomes. However, only two studies had a duration of more than approximately 12 weeks [35,36]. We could not definitively ascertain whether the longer durations of some studies in this review consistently yielded better outcomes. Therefore, further research to determine the appropriate duration of DEMI is desirable.
4.4. Characteristics of Intervention Extracted from the Narrative Review
We discussed three categories of interventions: “Food and diet intake”, “Exercise”, and “Other”.
4.4.1. Food and Diet Intake
The interventions related to “Food and diet intake” were predominantly implemented through GA in our review. These GA often involved practical tasks that encompassed various stages of the eating process, for instance, cooking, shopping, preparation, and tidying up. Additionally, community participation was encouraged through these practical activities within this domain. Previous studies highlighted that eating meals alone among community-dwelling older individuals aged 65 years and older in Japan can lead to issues like obesity and malnutrition [86,87]. Therefore, practical activities related to daily living, especially those centered around food and diet, hold potential benefits for the older adult population.
Our review emphasized interventions that focused more on specific aspects of food and dietary interventions than broader nutritional considerations like supplementation. The rationale behind this emphasis is rooted in the idea that incorporating food and diet practices into daily activities is highly relevant to the wellbeing of community-dwelling older individuals.
4.4.2. Exercise
We obtained similar findings to those reported in previous studies, which widely implemented similar programs.
Previous studies targeting very older adults over the age of 75 years suggested notable improvements in muscle strength [76,88]. In Grgic et al.’s meta-analysis, the muscle strength of the lower leg increased and hand grip strength was not significantly changed [88]. In Stewart et al.’s systematic review [76], three out of the four eligible studies [89] demonstrated enhancements in muscle strength through muscle and high-intensity physical training. Similarly, another systematic review and meta-analysis [72] and an RCT [90] provided evidence for the efficacy of exercises with high- or middle-to-high-load intensity exercise. Other previous studies [91,92] examined light-intensity MS exercises. Watanabe et al.’s study [91] demonstrated the efficacy of light and slow exercises using body weight for older adults aged 60–77 years [92]. Kanda et al. [93] investigated the effects of low-intensity bodyweight training with slow movements on older individuals aged 66–93 years [92]. These studies attributed the validated effects to the differentiated exercise load, which was influenced by the characteristics of the participants, especially their age. In our review, the majority of interventions focused on middle- to low-intensity training, with one exception [31]. We assumed that exercise intensity can prove effective not only at a high level but also within the middle- to low-intensity ranges, particularly for the older adult population.
Our review highlighted a unique set of exercise programs aimed at facilitating the implementation and efficacy of exercise. These programs, which formed a part of HE, FE, and ADL, were also designed to enable exercises to be carried out at home, similar to the exercises implemented in home settings [32,34].
For community-dwelling older individuals, we recommend FE and ADL interventions. These programs are tailored to participants’ daily lives, thereby promoting increased activity levels for the older adults within their own homes [93]. A study conducted in Japan demonstrated the positive impact of exercise habits adopted in middle age on the wellbeing of older adults through interviews [94]. Within the context of FE and ADL, our review encompassed BE, FE, ADL, and GE. For older adults rehabilitation, interventions that are adapted to individuals’ ADL or daily exercise habits [94] are pivotal, given the close relationship between physical functions and ADL [74,95,96]. One study examined behavior within the context of ADL [33], while another focused on FEs such as kneeling and chair stands [32]. Moreover, another study introduced GE [97], which was grounded in both exercise and nutritional interventions. The FEs within this study were adapted to each participant’s specific goals, thereby emphasizing the significance of tailoring activities to individual movements within daily life [97].
Another crucial facet of interventions within the “Exercise” domain was their incorporation of community participation, GA, HE, exercises at home, and community participation.
4.4.3. Others
The majority of the eligible studies (four of seven) focused on interventions categorized as “Other” programs [32,34,36,37]. GA was used to foster engagement and motivation through various shared events. These events provided participants with opportunities to interact, discuss, and share their experiences, ultimately promoting group cohesion. Additionally, other diverse programs within this category aimed to enhance multiple aspects, such as social participation and physical activities, as outlined in Table 2.
HE programs were also implemented, including interventions like lifestyle change, AL, and communication facilitated through GA. Although the specifics of these programs were not extensively detailed in our review, they align with the broader “Other” category. The studies that incorporated these programs reported positive outcomes in each case. However, due to limitations in synthesizing the data for meta-analysis, a conclusive assessment of the efficacy of HE interventions remains challenging.
The defining features of interventions within the “Other” category aimed to enhance behavioral changes and facilitate participant interactions. The synergistic effects of these interventions were likely derived from the interactions participants had with one another during the shared activities. The efficacy of these interactions within various activities in the “Other” interventions warrants further discussion and exploration.
4.4.4. Frailty
Four studies [31,33,35,36] that included participants who were either frail or pre-frail indicated improvements in frailty or pre-frailty status. However, the synthesis of these results does not definitively establish whether interventions can consistently lead to improvements in frailty. This uncertainty arises from the lack of comparative studies between individuals with frailty and those without. It is possible that the participants categorized as frail or pre-frail might have been present across all of the retrieved studies. This situation makes it challenging to comprehensively assess changes in frail or pre-frail participants and to determine the extent of their improvement.
4.4.5. Behavior Changes
Furthermore, another critical aspect that deserves attention is the scarcity of studies focusing on the health behavior aspect of this review’s thesis, which pertains to the promotion of a healthy lifestyle among older adults. Although the number of such studies was limited, these investigations remain crucial for addressing the overall wellbeing of the older adult population.
We assumed that DEMI plays a pivotal role in enhancing the lives of the older adults. Another pivotal area for investigation pertains to behavior changes related to dietary habits. Direct measurement of outcomes about behavior changes, such as transitions through various stages of behavior change [52], was not readily apparent. However, certain outcomes indirectly demonstrated the effects of behavior changes, such as improvements in ADL, Instrumental Activities of Daily Living (IADL), QOL, and mental function.
A particularly remarkable contribution to the field was Uemura et al.’s two studies [35,36], both of which centered on participants’ health literacy, a factor that significantly influences health behavior. The AL program implemented in these studies is notably practical for integration as a preventative care initiative or as part of voluntary community health activities. Its cost-effectiveness and absence of specific equipment requirements make it easily implementable.
Throughout this review, the enhancement of the nutritional and functional status of the older adults was identified as being partly achieved through changes in their behavior. However, it is essential to note that behavior change needs prolonged and sustained treatment, often spanning at least 6 months [52]. The absence of studies that were implemented over such an extended duration is noteworthy. Additionally, interventions involving mentoring or counseling were not identified in our findings. This necessitates meticulous research into long-term studies exceeding a 6-month duration and exploring the potential benefits of mentoring or counseling interventions.
4.5. Novelty of This Review
Our review discusses the efficacy of DEMI for community-dwelling older adults in Japan. The novelty lies in selecting well-designed RCTs in Japan that have not been adequately discussed in previous studies. Previous studies on mixed interventions encompassing exercise and food for community-dwelling older adults in Japan were often hindered by poor designs, including single-cohort and non-RCT designs, lacking comparative analyses. Only a few studies managed to verify the efficacy, primarily through appropriate methods like meta-analysis [8]. These studies explored the impact of exercise and nutritional mixed interventions on daily eating behavior, physical activities, and healthy food habits. Some studies specifically examined the benefits of adopting a Mediterranean diet [98] or focusing on healthy food for specific purposes [42]. Studies from other countries on diet and exercise interventions have shown improvements in physical function and QOL, focusing on areas such as daily eating behavior and maintaining physical activities. However, most of these studies suffered from small sample sizes and uncertain effects. Past systematic reviews or meta-analysis [3,8] also struggled to provide clear evidence due to the scarcity of well-designed studies.
Our review differs from previous systematic reviews on the older adults in Japan; interventions of previous studies have been designed around a combination of nutrition and exercise intervention and focused on nutritional supplementation. However, for the nutrition status of community-dwelling older adults, it is essential to consider nutrition intake behaviors in daily life. These behaviors include aspects like eating, cooking, preparing, and tidying up after meals; shopping; and relevant series of daily eating. Surprisingly, very few studies have evaluated specific areas of food and eating behaviors in this context.
In this review, we have obtained novel results, particularly regarding outcome measurements that were not commonly explored in previous studies. In studies investigating DEMI, outcomes related to behavior change were not measured in earlier research. According to Yoshimura et al.’s systematic review [8], participants with sarcopenia did not receive “food” interventions and did not engage in discussions related to behavior change.
From a quality assessment perspective, we demonstrated good quality in the included studies; however, previous studies conducted in Japan did not assess the quality of research adequately. Most reviews in Japan did not assess the quality of the literature. Only one review [8] focused on a relevant area, discussed the possibility of conducting an RCT, and assessed studies using the GRADE system. Studies included in Yoshimura et al.’s review included participants from hospitals or facilities, not solely community-dwelling older individuals [8]. Moreover, their focus was primarily on nutrition, particularly supplementation, rather than a broader exploration of food-related interventions, as in our review.
4.6. Limitations
This review has some limitations. First, the process of retrieving eligible studies for our review might have been subject to selection bias for several reasons. One major challenge we encountered was limiting the search to specific languages, which introduced difficulties during the selection process. This could potentially lead to literature selection bias. During the study selection process, the restriction of searching for studies both in Japanese and English might have introduced a language bias. Despite these limitations, we made efforts to minimize bias by conducting searches in both Japanese and English, as it was essential for our targeted research in Japan, i.e., focusing on community-dwelling older adults. Additionally, age was another factor that influenced participant selection and could potentially introduce selection bias. The characteristics of participants were not compared in detail, such as between different age groups. In our review, the age of participants was inclusive, with individuals aged over 65 or 70 years old. The mean age of participants ranged from 72.1 to 76.8 years. Moreover, we did not conduct a comparison between young-old and old-old age groups in all of the studies. This lack of differentiation in age groups contributes to uncertainty regarding the outcomes, such as the relationship between outcomes for the young-old and old-old populations. Issues related to the old-old age group were not researched in all studies.
Second, in each study, all participants had different statuses. One systematic review and meta-analysis [74] analyzed studies that included different functional statuses, such as health or frailty status. As a result, these studies might have estimated different functional statuses at the research baseline for their respective participants.
Third, we recognize that our selection of only seven studies may have limited the evidence level of our review due to the small number of included studies.
Finally, a critical issue in researching older adults revolves around their lifestyle, which has a significant impact on their status of ADL and IADL, food and eating habits, and exercise behaviors. These factors are unique and may vary among countries, emphasizing the importance of considering cultural differences in any study involving older adult populations. In fact, the Japanese population has Japanese-style daily living activities, food, cooking, eating style, and daily physical activities. Certainly, it is essential to recognize that differences exist among various communities in Japan, including suburban, urban, or rural areas. This review treated Japan in contradistinction to foreign countries.
The older adult population in Japan exhibits diverse lifestyles and is marked by complexity. Furthermore, previous studies have not sufficiently addressed important issues related to functional status, household situations, and the very old older adult population. In the present day, it is crucial to recognize that many older individuals, including a significant portion of the very old adults, continue to be active in the workforce.
5. Conclusions
We demonstrated the efficacy of DEMI for community-dwelling older adults in Japan. Based on our findings, we recommend the implementation of practical projects that include DEMI, with a particular focus on areas that need further research. Additional research is needed to provide deeper insight. Finally, longitudinal studies are warranted, and discussions regarding the relationship of behavior change should be central to the research agenda for community-dwelling older adults in Japan. To advance the care system for older individuals in Japan, it is imperative to conduct better-designed studies that contribute to a more sophisticated and comprehensive understanding of their needs and the interventions that can best serve them.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geriatrics9020032/s1, Table S1: Version 2 of the Cochrane risk-of-bias assessment tool for randomized trials: bias domains, signaling questions, response options, and risk-of-bias judgments. Figure S1: Risk of Bias Summary. Figure S2: Risk of Bias Graph.
Author Contributions
All authors conceptualized and designed the systematic review. All authors conducted the systematic review. T.M. wrote the first draft of the manuscript with the support of T.F. and H.B. Conceptualization, T.M., T.F. and H.B.; Data curation, T.M. and T.F.; Format analysis, T.M. and T.F.; Funding acquisition, T.M.; Investigation, T.M., T.F. and H.B.; Methodology, T.M., T.F. and H.B.; Project administration, T.M., T.F. and H.B.; Resources, T.M., T.F. and H.B.; Software, T.M. and T.F.; Validation, T.M., T.F. and H.B.; Visualization, T.M. and T.F.; Writing—original draft preparation, T.M.; Writing—review & editing, T.M., T.F. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank Hiroki Saito (Tokyo University of Technology, Department of Rehabilitation) for careful and precise mentorship in statistical analysis methods.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ADL | activities of daily living |
| AL | active learning |
| B | behavior (frequency, duration) |
| BDHQ | brief self-administered diet history questionnaire |
| BE | balance exercise |
| CI | confidence interval |
| CINAHL | cumulative index to nursing and allied health literature |
| DEMI | diet, food intake, and exercise mixed intervention |
| DVS | diet variety score |
| F | food intake |
| FE | functional exercise |
| FFS | food frequency questionnaire |
| GA | group activities |
| GDS | geriatric depression scale |
| GE | gait exercise |
| GRADE | grading of recommendations, assessment, development, and evaluation |
| HE | health education |
| IADL | Instrumental Activities of Daily Living |
| IMRAD | Introduction, Methods, Results, and Discussion |
| Lc | lecture |
| LTC | long-term care insurance system |
| MDs | mean differences |
| MeSH | medical subject headings |
| MGS | maximal gait speed |
| MS | muscle strengthening exercise |
| NS | not significant |
| Nu | nutritional status |
| O | other outcome factors |
| OLS | one leg standing |
| PE | physical exercise |
| Phy F | physical function |
| PICO | patient/population, intervention, comparison, and outcomes |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| Psy F | psychosocial function |
| QOL | quality of life |
| RCT | randomized control trial |
| RoB | risk of bias |
| RoB 2 | risk of bias version 2 |
| SPPB | short physical performance battery |
| TUG | timed up and go test |
| UGS | usual gait speed |
References
- Cruz-Jentoft, A.J.; Landi, F.; Stéphane, M.S.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]
- iLibrary: Health at a Glance: Asia/ Pacific 2022: Measuring Progress towards Universal Health Coverage. Life Expectancy at Birth and Survival Rate to Age. Available online: https://www.oecd-ilibrary.org/sites/7ab6cf3b-en/index.html?itemId=/content/component/7ab6cf3b-en (accessed on 18 September 2023).
- Smart Life Project. Available online: https://www.smartlife.mhlw.go.jp/ (accessed on 18 September 2023).
- Aoki, K.; Sakuma, M.; Endo, N. The impact of exercise and vitamin D supplementation on physical function in community-dwelling elderly individuals: A randomized trial. J. Orthop. Sci. 2018, 23, 682–687. [Google Scholar] [CrossRef]
- Kim, H.; Won, C.W.; Kim, M.; Kojima, N.; Fujino, K.; Osuka, Y.; Hosoi, E.; Suzuki, T. The effects of exercise and milk-fat globule membrane (MFGM) on walking parameters in community-dwelling elderly Japanese women with declines in walking ability: A randomized placebo controlled trial. Arch. Gerontol. Geriatr. 2019, 83, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Aizawa, J.; Nagasawa, H.; Gomi, I.; Kugota, H.; Nanjo, K.; Jinno, T.; Masuda, T.; Morita, S. Effects and feasibility of exercise therapy combined with branched-chain amino acid supplementation on muscle strengthening in frail and pre-frail elderly people requiring long-term care: A crossover trial. Appl. Physiol. Nutr. Metab. 2016, 41, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suzuki, T.; Kim, M.; Kojima, N.; Ota, N.; Shimotoyodome, A.; Hase, T.; Hosoi, E.; Yoshida, H. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: A randomized double blind, placebo-controlled, follow-up trial. PLoS ONE 2015, 10, e0116256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tokuda, Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: A randomized controlled trial. Geriatr. Gerontol. Int. 2018, 18, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yagyu, S.; Hata, A.; Nirengi, S.; Kotani, K.; Moritani, T.; Sakane, N. Maslinic acid derived from olive fruit in combination with resistance training improves muscle mass and mobility functions in the elderly. J. Clin. Biochem. Nutr. 2019, 64, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Osuka, Y.; Kojima, N.; Wakaba, K.; Miyauchi, D.; Tanaka, K.; Kim, H. Effects of resistance training and/or beta-hydroxy-beta-methylbutyrate supplementation on muscle mass, muscle strength and physical performance in older women with reduced muscle mass: Protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open 2019, 9, e025723. [Google Scholar] [CrossRef] [PubMed]
- Eiyou Kaizen Manual Kaitei-Ban. Available online: https://www.mhlw.go.jp/topics/2009/05/dl/tp0501-1e.pdf (accessed on 2 January 2022). (In Japanese).
- Watanabe, Y.; Yamada, Y.; Yokoyama, K.; Yoshida, T.; Yoshinaka, Y.; Yoshimoto, M.; Tanaka, Y.; Itoi, A.; Yamagata, E.; Ebine, N.; et al. Comprehensive geriatric intervention program with and without weekly class-style exercise: Research protocol of a cluster randomized controlled trial in Kyoto-Kameoka Study. Clin. Interv. Aging 2018, 13, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Machi, L.A.; McEvoy, B.T. The Literature Review Six Steps to Success; Corwin, A., Ed.; SAGE Publications Company: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Medical Online. Available online: https://www.medicalonline.jp/ (accessed on 5 May 2020).
- Ichu-Shi. Available online: https://search.jamas.or.jp/ (accessed on 5 May 2020).
- Pub Med. Available online: https://pubmed.ncbi.nlm.nih.gov/?otool=ijputmlib (accessed on 5 June 2020).
- CINAHL. Available online: https://web.a.ebscohost.com/ehost/search/advanced?vid=0&sid=5d776eb8-32b2-4555-b64b-9d1771b9918c%40sessionmgr4008 (accessed on 5 June 2020).
- Cochrane Library. Available online: https://www.cochranelibrary.com/search?cookiesEnabled (accessed on 12 June 2020).
- Rayyan. Available online: https://www.rayyan.ai/ (accessed on 5 May 2020).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). Available online: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view (accessed on 27 August 2022).
- PRISMA Checklist. Available online: http://prisma-statement.org/PRISMAstatement/checklist.aspx (accessed on 27 August 2022).
- EZR Version 1.61. Available online: https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statm.ed.html (accessed on 27 August 2022).
- Training. Cochrane Handbook for Systematic Reviews Interventions Version 6.2, 2021. Available online: https://training.cochrane.org/handbook/archive/v6. (accessed on 27 August 2022).
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Nishi, M.; Murayama, H.; Narita, M.; Yokoyama, Y.; Nofuji, Y.; Taniguchi, Y.; Amano, H.; Kitamura, A.; Shinkai, S. Effects of a multifactorial intervention comprising resistance exercise, nutritional and psychosocial programs on frailty and functional health in community-dwelling older adults: A randomized, controlled, cross-over trial. Geriatr. Gerontol. Int. 2017, 17, 2034–2045. [Google Scholar] [CrossRef]
- Kwon, J.; Yoshida, Y.; Yoshida, H.; Kim, H.; Suzuki, T.; Lee, Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 263.e1–263.e8. [Google Scholar] [CrossRef]
- Kawabata, T.; Takemi, Y.; Murayama, H.; Nishi, M.; Shimizu, Y.; Narita, M.; Kim, M.J.; Shinkai, S. Effects of an intervention program for community-dwelling elderly to improve frailty and dietary habits. Nihon Koshu Eisei Zasshi 2015, 62, 169–181. (In Japanese) [Google Scholar] [CrossRef]
- Takai, I. Influence of comprehensive intervention composed of nutrition and exercise on the development of exercise habits and self-perceived health among community-dwelling elderly individuals. Nihon Ronen Igakkai Zasshi 2013, 50, 522–527. [Google Scholar] [CrossRef][Green Version]
- Uemura, K.; Yamada, M.; Okamoto, H. Effects of health education intervention through active learning for preventing frailty in older adults: A randomized controlled trial. Rigakuryouhougaku 2018, 45, 209–217. (In Japanese) [Google Scholar] [CrossRef]
- Uemura, K.; Yamada, M.; Okamoto, H. Effects of active learning on health literacy and behavior in older adults: A randomized controlled trial. Am. Geratri. Soc. 2018, 66, 1721–1729. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Kim, H.; Saito, K.; Yasunaga, M.; Nonaka, K.; Kobayashi, K.; Ogawa, K.; Yoshida, H.; Tanaka, C.; et al. A randomized controlled trial of the effects of a comprehensive intervention program for community-dwelling older adults. Nihon Ronen Igakkai Zasshi 2011, 48, 352–360. [Google Scholar] [CrossRef][Green Version]
- Shinkai, S.; Watanabe, N.; Yoshida, H.; Fujiwara, Y.; Amano, H.; Lee, S.; Nishi, M.; Tsuchiya, Y. Research on screening for frailty: Development of “the Kaigo-Yobo Checklist”. Nihon Koshu Eisei Zasshi 2010, 57, 345–354. (In Japanese) [Google Scholar] [CrossRef]
- Shinkai, S.; Watanabe, N.; Yoshida, H.; Fujiwara, Y.; Nishi, M.; Fukaya, T.; Lee, S.; Kim, M.J.; Ogawa, K.; Murayama, H.; et al. Validity of the “Kaigo-Yobo Check-List” as a frailty index. Nihon Koshu Eisei Zasshi 2013, 60, 262–274. (In Japanese) [Google Scholar] [CrossRef]
- Shinkai, S.; Yoshida, H.; Taniguchi, Y.; Murayama, H.; Nishi, M.; Amano, H.; Nofuji, Y.; Seino, S.; Fujiwara, Y. Public health approach to preventing frailty in the community and its effect on healthy aging in Japan. Geriatr. Gerontol. Int. 2016, 16 (Suppl. S1), 87–97. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, C.; Bowling, A.; Casè, A.; Nicolini, P.; Mauri, S.; Castelli, M.; Vergani, C.; Castelli, M.; Vergani, C. Dimensions and correlates of quality of life according to frailty status: A cross-sectional study on community-dwelling older adults referred to an outpatient geriatric service in Italy. Health Qual. Life Outcomes 2010, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Watanabe, S.; Shibata, H.; Amano, H.; Fujiwara, Y.; Shinkai, S.; Yoshida, H.; Suzuki, T.; Yukawa, H.; Yasumura, S.; et al. Effects of dietary variety on declines in high-level functional capacity in elderly people living in a community. Nihon Koshu Eisei Zasshi 2003, 50, 1117–1124. (In Japanese) [Google Scholar] [CrossRef]
- Kimura, M.; Moriyasu, A.; Kumagai, S.; Furuna, T.; Akita, S.; Kimura, S.; Suzuki, T. Community-based intervention to improve dietary habits and promote physical activity among older adults: A cluster randomized trial. BMC Geriatr. 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Burke, W.J.; Roccaforte, W.H.; Wengel, S.P. The short form of the Geriatric Depression Scale: A comparison with the 30-item form. J. Geriatr. Psychiatry Neurol. 1991, 4, 173–178. [Google Scholar] [CrossRef]
- Takahashi, M.; Shibazaki, S.; Hashimoto, S.; Kawakami, N.; Tamakoshi, A.; Ojima, T.; Nagai, M. Evaluation of social activities of the elderly in 27 regions with use of the “check list for vivid social activities”. Nihon Koshu Eisei Zasshi 2000, 47, 936–944. [Google Scholar] [PubMed]
- Kwon, J.; Suzuki, T.; Kumagai, S.; Shinkai, S.; Yukawa, H. Risk factors for dietary variety decline among Japanese elderly in a rural community: A 8-year follow-up study from TMIG-LISA. Eur. J. Clin. Nutr. 2006, 60, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Oka, K. Stages of change for exercise behavior and self-efficacy for exercise among middle-aged adults. Nihon Koshu Eisei Zasshi 2003, 50, 208–215. (In Japanese) [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Reference values for the five-repetition sit-to-stand test: A descriptive meta-analysis of data from elders. Percept. Mot. Skills 2006, 103, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Fedoroff, J.P.; Price, T.R.; Leiguarda, R.; Robinson, R.G. Apathy following cerebrovascular lesions. Stroke 1993, 24, 1625–1630. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yokoyama, K.; Noriyasu, R.; Osaki, T.; Adachi, T.; Itoi, A.; Naito, Y.; Morimoto, T.; Kimura, M.; Oda, S. Light-intensity activities are important for estimating physical activity energy expenditure using uniaxial and triaxial accelerometers. Eur. J. Appl. Physiol. 2009, 105, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Moriyasu, A.; Kumagai, S.; Furuna, T. Evaluation of the comprehensive health program “Sumida TAKE10!” for community-dwelling older adults, which aims to prevent or delay the need for long-term nursing care. Nihon Koshu Eisei Zasshi 2016, 63, 682–693. (In Japanese) [Google Scholar] [CrossRef]
- Yokokawa, Y.; Kai, I.; Nakajima, T. Development of a “self efficacy for health promotion scale” in community-dwelling elderly. Nihon Koshu Eisei Zasshi 1999, 46, 103–112. (In Japanese) [Google Scholar] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Suka, M.; Odajima, T.; Kasai, M.; Igarashi, A.; Ishikawa, H.; Kusama, M.; Nakayama, T.; Sumitani, M.; Sugimori, H. The 14-item health literacy scale for Japanese adults (HLS-14). Environ. Health Prev. Med. 2013, 18, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Osaka, W.; Togari, T.; Ishikawa, H.; Yonekura, Y.; Sekido, A.; Matsumoto, M. Comprehensive health literacy in Japan is lower than in Europe: A validated Japaneselanguage assessment of health literacy. BMC Public Health 2015, 15, 505. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, K.; Van den Broucke, S.; Pelikan, J.M.; Fullam, J.; Doyle, G.; Slonska, Z.; Kondilis, B.; Stoffels, V.; Osborne, R.H.; Brand, H. Measuring health literacy ill populations: Illuminating the design and development process of the European Health Literacy Survey Questionnaire (HLs-Eu-Q). BMC Public Health 2013, 13, 948. [Google Scholar] [CrossRef]
- Tiller, D.; Herzog, B.; Kluttig, A.; Haerting, J. Health literacy in an urban elderly East-German population-results from the population-based CARLA study. BMC Public Health 2015, 15, 883. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation Limited: London, UK, 1997. [Google Scholar] [CrossRef]
- Benton, A.L. Differential behavioral effects in frontal lobe disease. Neuropsychologia 1968, 6, 53–60. [Google Scholar] [CrossRef]
- Takechi, H.; Dodge, H.H. Scenery Picture Memory Test: A new type of quick and effective screening test to detect early stage Alzheimer’s disease patients. Geriatr. Gerontol. Int. 2010, 10, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Turner-Bowker, D.M.; Bayliss, M.S.; Ware, J.E., Jr.; Kosinski, M. Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual. Life Res. 2003, 12, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, H.; Gondou, Y.; Masui, Y.; Inagaki, H.; Kawai, C.; Otsuka, R.; Ogawa, M.; Takayama, M.; Imuta, H.; Suzuki, T. Nihongo-ban WHO-5 seishinteki kennkouzyoutai hyou no shinraisei narabini datousei. J. Health Welf. Stat. 2007, 54, 48–55. (In Japanese) [Google Scholar]
- Suzuki, Y.; Sakihara, S. Measurement of psychological independence -scale validity and reliability. Minsoku Eisei 2003, 69, 47–56. (In Japanese) [Google Scholar]
- de Vries, N.M.; van Ravensberg, C.D.; Hobbelen, J.S.; Olde Rikkert, M.G.; Staal, J.B.; Nijhuis-van der Sanden, M.W. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: A meta-analysis. Ageing Res. Rev. 2012, 11, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef]
- Giné-Garriga, M.; Roqué-Fíguls, M.; Coll-Planas, L.; Sitjà-Rabert, M.; Salvà, A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 753–769.e3. [Google Scholar] [CrossRef] [PubMed]
- Churchwerd-Venne, T.A.; Tieland, M.; Verdijk, L.B.; Leenders, M.; Dirks, M.L.; de Groot, L.C.P.G.M.; van Loon, J.C. There are no nonresponders to resistance-type exercise training in older men and woman. J. Am. Dir. Assoc. 2015, 16, 400–411. [Google Scholar] [CrossRef]
- Stewart, V.H.; Saunders, D.H.; Greig, C.A. Responsiveness of muscle size and strength to physical training in very elderly people: A systematic review. Scand. J. Med. Sci. Sports 2014, 24, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Su, S.C.; Chen, C.W.; Kang, Y.W.; Hu, M.H.; Hsu, L.L.; Wu, S.Y.; Chen, L.; Chang, H.Y.; Chuang, S.Y.; et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Clements, K.M.; Fiatarone, M.A. A randomized controlled trial of progressive resistance training in depressed elders. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M27–M35. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Babyak, M.A.; Moore, K.A.; Craighead, W.E.; Herman, S.; Khatri, P.; Waugh, R.; Napolitano, M.A.; Forman, L.M.; Appelbaum, M.; et al. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999, 159, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Leveille, S.; Ferrucci, L.; van Eijk, J.T.; Guralnik, J.M. Exploring the effect of depression on physical disability: Longitudinal evidence from the established populations for epidemiologic studies of the elderly. Am. J. Public Health 1999, 89, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, V.; Arensman, E.; Spinhoven, P. Negative life events and depression in elderly persons: A meta-analysis. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P87–P94. [Google Scholar] [CrossRef] [PubMed]
- Greenglass, E.; Fiksenbaum, L.; Eaton, J. The relationship between coping, social support, functional disability and depression in the elderly. Anxiety Stress Coping 2006, 19, 15–31. [Google Scholar] [CrossRef]
- Vink, D.; Aartsen, M.J.; Schoevers, R.A. Risk factors for anxiety and depression in the elderly: A review. J. Affect. Disord. 2008, 106, 29–44. [Google Scholar] [CrossRef]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Social isolation and risk for malnutrition among older people. Geriatr. Gerontol. Int. 2017, 17, 286–294. [Google Scholar] [CrossRef]
- Aihara, Y. Social factors, diet and nutritional information, and dietary variety among older adults aged 75 years. J. Gerontol. 2012, 34, 394–402. (In Japanese) [Google Scholar] [CrossRef]
- Kushida, O.; Moon, J.S.; Matsumoto, D.; Yamasaki, N.; Takatori, K. Eating alone at each meal and associated health status among community-dwelling Japanese elderly living with others: A cross-sectional analysis of the KAGUYA study. Nutrients 2020, 12, 2805. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Wada, T.; Okumiya, K.; Ishimoto, Y.; Fukutomi, E.; Kasahara, Y.; Chen, W.; Sakamoto, R.; Fujisawa, M.; Otsuka, K.; et al. Eating alone among community-dwelling Japanese elderly: Association with depression and food diversity. J. Nutr. Health Aging 2012, 16, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of resistance training on muscle size and strength in very elderly adults: A systematic review and meta-analysis of randomized controlled trials. Sports Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, G.; Buonsenso, A.; Centorbi, M.; Calcagno, G.; Iuliano, E.; Angiolillo, A.; Ciccotelli, S.; di Cagno, A.; Di Costanzo, A. Long term physical activity improves quality of life perception, healthy nutrition, and daily life management in elderly: A randomized controlled trial. Nutrients 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tanimoto, M.; Oba, N.; Sanada, K.; Miyachi, M.; Ishii, N. Effect of resistance training using bodyweight in the elderly: Comparison of resistance exercise movement between slow and normal speed movement. Geriatr. Gerontol. Int. 2015, 15, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Yoda, T.; Suzuki, H.; Okabe, Y.; Mori, Y.; Yamasaki, K.; Kitano, H.; Kanda, A.; Hirao, T. Effects of low-intensity bodyweight training with slow movement on motor function in frail elderly patients: A prospective observational study. Environ. Health Prev. Med. 2018, 23, 4. [Google Scholar] [CrossRef]
- Maruya, K.; Asakawa, Y.; Ishibashi, H.; Fujita, H.; Arai, T.; Yamaguchi, H. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J. Phys. Ther. Sci. 2016, 28, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- Akune, T.; Muraki, S.; Oka, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Yoshimura, N. Exercise habits during middle age are associated with lower prevalence of sarcopenia: The ROAD study. Osteoporos. Int. 2014, 25, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Vanswearingen, J.M. Physical impairment and disability: Relationship to performance of activities of daily living in community-dwelling older men. Phys. Ther. 2002, 82, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; Neyens, J.C.; van Rossum, E.; Spreeuwenberg, M.D.; de Witte, L.P. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: A systematic review. BMC Geriatr. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Tsunematsu, M.; Kakehashi, M. A study on n long-term care prevention programs for community-dwelling frail elderly subjects: Comparison of the effects between a single program for physical function and a multipart program for physical function. J. Jpn. 2018, 69, 485–495. [Google Scholar] [CrossRef]
- Gené Huguet, L.; Navarro González, M.; Kostov, B.; Ortega Carmona, M.; Colungo Francia, C.; Carpallo Nieto, M.; Hervás Docón, A.; Vilarrasa Sauquet, R.; García Prado, R.; Sisó-Almirall, A. Pre Frail 80: Multifactorial intervention to prevent progression of pre-frailty to frailty in the elderly. J. Nutr. Health Aging 2018, 22, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).