Outcomes of COVID-19-Associated Hospitalizations in Geriatric Patients with Dementia in the United States: A Propensity Score Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics and Baseline Comorbidities
3.2. In-Hospital Mortality
3.3. In-Hospital Complications
3.4. Predictors of Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabaan, A.A.; Smajlović, S.; Tombuloglu, H.; Ćordić, S.; Hajdarević, A.; Kudić, N.; Al Mutai, A.; Turkistani, S.A.; Al-Ahmed, S.H.; Al-Zaki, N.A.; et al. SARS-CoV-2 infection and multi-organ system damage: A review. Biomol. Biomed. 2023, 23, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Numbers, K.; Brodaty, H. The effects of the COVID-19 pandemic on people with dementia. Nat. Rev. Neurol. 2021, 17, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Di Fiorino, M.; Cammisuli, D.M. Dementia in the era of COVID-19. Some considerations and ethical issues. Psychogeriatrics 2022, 22, 132–136. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dementia: Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 14 October 2023).
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, J.; Wang, X.; Zhao, M.; Huang, Q.; Li, H. The Impact of Dementia on the Clinical Outcome of COVID-19: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 78, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Wilke, V.; Sulyok, M.; Stefanou, M.I.; Richter, V.; Bender, B.; Ernemann, U.; Ziemann, U.; Malek, N.; Kienzle, K.; Klein, C.; et al. Delirium in hospitalized COVID-19 patients: Predictors and implications for patient outcome. PLoS ONE. 2022, 17, e0278214. [Google Scholar] [CrossRef]
- Toniolo, S.; Scarioni, M.; Di Lorenzo, F.; Hort, J.; Georges, J.; Tomic, S.; Nobili, F.; Frederiksen, K.S. Dementia and COVID-19, a bidirectional liaison: Risk factors, biomarkers, and optimal health care. J. Alzheimer’s Dis. 2021, 82, 883–898. [Google Scholar] [CrossRef]
- Veronese, N.; Barbagallo, M. Specific approaches to patients affected by dementia and covid-19 in nursing homes: The role of the geriatrician. Ageing Res. Rev. 2021, 69, 101373. [Google Scholar] [CrossRef]
- Healthcare Cost and Utilization Project. NIS Database Documentation. Available online: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp (accessed on 23 July 2023).
- U.S. Department of Health and Human Services. Federal Policy for the Protection of Human Subjects (‘Common Rule’). Available online: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html (accessed on 23 July 2023).
- Burns, A.; Lobo, A.; Rikkert, M.O.; Robert, P.; Sartorius, N.; Semrau, M.; Stoppe, G. COVID-19 and dementia: Experience from six European countries. Int. J. Geriatr. Psychiatry 2021, 36, 943–949. [Google Scholar] [CrossRef]
- Ghaffari, M.; Ansari, H.; Beladimoghadam, N.; Aghamiri, S.H.; Haghighi, M.; Nabavi, M.; Mansouri, B.; Mehrpour, M.; Assarzadegan, F.; Hesami, O.; et al. Neurological features and outcome in COVID-19: Dementia can predict severe disease. J. Neurovirol. 2021, 27, 86–93. [Google Scholar] [CrossRef]
- Harb, A.A.; Chen, R.J.; Chase, H.S.; Natarajan, K.; Noble, J.M. Clinical Features and Outcomes of Patients with Dementia Compared to an Aging Cohort Hospitalized during the Initial New York City COVID-19 Wave. J. Alzheimer’s Dis. 2021, 81, 679–690. [Google Scholar] [CrossRef]
- Barnato, A.E.; Birkmeyer, J.D.; Skinner, J.S.; O’Malley, A.J.; Birkmeyer, N.J.O. Treatment intensity and mortality among COVID-19 patients with dementia: A retrospective observational study. J. Am. Geriatr. Soc. 2022, 70, 40–48. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sahebari, S.S.; Naseri, A. Dementia and covid-19: Complications of managing a pandemic during another pandemic. Dement. Neuropsychol. 2020, 14, 438–439. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Putri, C.; Arisa, J.; Situmeang, R.F.V.; Kurniawan, A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2021, 93, 104299. [Google Scholar] [CrossRef]
- Chen, B.; Wang, M.; He, Q.; Wang, Y.; Lai, X.; Chen, H.; Li, M. Impact of frailty, mild cognitive impairment and cognitive frailty on adverse health outcomes among community-dwelling older adults: A systematic review and meta-analysis. Front. Med. 2022, 9, 1009794. [Google Scholar] [CrossRef]
- Emmerton, D.; Abdelhafiz, A.H. Care for Older People with Dementia During COVID-19 Pandemic. SN Compr. Clin. Med. 2021, 3, 437–443. [Google Scholar] [CrossRef]
- Acanfora, D.; Ciccone, M.M.; Scicchitano, P.; Acanfora, C.; Casucci, G. Neprilysin inhibitor-angiotensin II receptor blocker combination (sacubitril/valsartan): Rationale for adoption in SARS-CoV-2 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 135–136. [Google Scholar] [CrossRef]
- De Matteis, G.; Burzo, M.L.; Della Polla, D.A.; Serra, A.; Russo, A.; Landi, F.; Gasbarrini, A.; Gambassi, G.; Franceschi, F.; Covino, M. Outcomes and Predictors of In-Hospital Mortality among Older Patients with Dementia. J. Clin. Med. 2023, 12, 59. [Google Scholar] [CrossRef]
- Tanaka, S.; Okusa, M.D. Crosstalk between the nervous system and the kidney. Kidney Int. 2020, 97, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Priest, N.; Anderson, N.B. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016, 35, 407–411. [Google Scholar] [CrossRef]
- Kharroubi, S.A.; Diab-El-Harake, M. Sex-differences in COVID-19 diagnosis, risk factors and disease comorbidities: A large US-based cohort study. Front. Public Health 2022, 10, 1029190. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Chen, R.; Charpignon, M.-L.; Raquib, R.V.; Wang, J.; Meza, E.; Aschmann, H.E.; DeVost, M.A.; Mooney, A.; Bibbins-Domingo, K.; Riley, A.R.; et al. Excess Mortality With Alzheimer Disease and Related Dementias as an Underlying or Contributing Cause During the COVID-19 Pandemic in the US. JAMA Neurol. 2023, 80, 919–928. [Google Scholar] [CrossRef]
| Characteristics | COVID and Dementia− | COVID and Dementia+ | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| n = 816,960 | 636,115 | 77.86 | 180,845 | 22.14 | -- |
| Gender (%) | n | % | n | % | <0.001 |
| Female | 298,580 | 46.94 | 100,555 | 55.6 | |
| Male | 337,535 | 53.06 | 80,290 | 44.4 | |
| Mean Age Years (SD) | Mean | SD | Mean | SD | |
| Female | 7616 | 7.54 | 8284 | 6.73 | |
| AGE Groups (%) | n | % | n | % | <0.001 |
| 65–69 | 158,270 | 24.88 | 10,645 | 5.89 | |
| 70–74 | 155,240 | 24.4 | 19,820 | 10.96 | |
| 75–79 | 128,120 | 20.14 | 31,240 | 17.27 | |
| ≥80 | 194,485 | 30.57 | 119,140 | 65.88 | |
| RACE (%) | n | % | n | % | <0.001 |
| Asian or Pacific Islander | 19,775 | 3.11 | 5295 | 2.93 | |
| Black | 99,140 | 15.59 | 30,195 | 16.7 | |
| Hispanic | 93,935 | 14.77 | 20,920 | 11.57 | |
| Native American | 4530 | 0.71 | 665 | 0.37 | |
| Other | 21,905 | 3.44 | 5690 | 3.15 | |
| White | 396,830 | 62.38 | 118,080 | 65.29 | |
| MEDIAN HOUSEHOLD INCOME (%) | n | % | n | % | <0.001 |
| ≤49,999 | 208,790 | 32.82 | 55,130 | 30.48 | |
| 50K–64,999 | 179,450 | 28.21 | 46,710 | 25.83 | |
| 65K–85,999 | 142,335 | 22.38 | 40,745 | 22.53 | |
| ≥86k | 105,540 | 16.59 | 38,260 | 21.16 | |
| INSURANCE STATUS (%) | n | % | n | % | <0.001 |
| Medicaid | 18,710 | 2.94 | 3970 | 2.2 | |
| Medicare | 529,345 | 83.22 | 161,940 | 89.55 | |
| No charge | 690 | 0.11 | 150 | 0.08 | |
| Other | 17,215 | 2.71 | 4030 | 2.23 | |
| Private insurance | 64,095 | 10.08 | 9860 | 5.45 | |
| Self-pay | 6060 | 0.95 | 895 | 0.49 | |
| HOSPITAL DIVISION (%) | n | % | n | % | <0.001 |
| East North Central | 110,515 | 17.37 | 30,240 | 16.72 | |
| East South Central | 46,700 | 7.34 | 12,530 | 6.93 | |
| Middle Atlantic | 98,055 | 15.41 | 28,225 | 15.61 | |
| Mountain | 40,065 | 6.3 | 7420 | 4.1 | |
| New England | 24,960 | 3.92 | 9575 | 5.29 | |
| Pacific | 60,885 | 9.57 | 19,790 | 10.94 | |
| South Atlantic | 124,210 | 19.53 | 40,210 | 22.23 | |
| West North Central | 45,810 | 7.2 | 10,450 | 5.78 | |
| West South Central | 84,915 | 13.35 | 22,405 | 12.39 | |
| HOSPITAL BEDSIZE (%) | n | % | n | % | <0.001 |
| Large | 288,785 | 45.4 | 78,770 | 43.56 | |
| Medium | 184,900 | 29.07 | 56,265 | 31.11 | |
| Small | 162,430 | 25.53 | 45,810 | 25.33 | |
| HOSPITAL TEACHING STATUS (%) | n | % | n | % | <0.001 |
| Rural | 76,190 | 11.98 | 17,730 | 9.8 | |
| Urban nonteaching | 123,675 | 19.44 | 36,115 | 19.97 | |
| Urban teaching | 436,250 | 68.58 | 127,000 | 70.23 | |
| COMORBIDITIES (%) | n | % | n | % | |
| Coronary artery disease | 172,895 | 27.18 | 48,970 | 27.08 | 0.392 |
| Myocardial infarction | 39,655 | 6.23 | 9425 | 5.21 | <0.001 |
| Essential hypertension | 502,555 | 79 | 144,645 | 79.98 | <0.001 |
| Diabetes | 286,660 | 45.06 | 69,910 | 38.66 | <0.001 |
| Cancer | 39,265 | 6.17 | 6805 | 3.76 | <0.001 |
| Obesity | 133,510 | 20.99 | 15,165 | 8.39 | <0.001 |
| Drug use | 5105 | 0.8 | 795 | 0.44 | <0.001 |
| Smoking | 195,245 | 30.69 | 41,325 | 22.85 | <0.001 |
| Alcohol | 8695 | 1.37 | 1815 | 1 | <0.001 |
| Chronic pulmonary disease | 169,840 | 26.7 | 40,125 | 22.19 | <0.001 |
| Peripheral vascular disease | 38,480 | 6.05 | 12,125 | 6.7 | <0.001 |
| Chronic kidney disease | 118,010 | 18.55 | 37,260 | 20.6 | <0.001 |
| Hypothyroidism | 108,635 | 17.08 | 36,955 | 20.43 | <0.001 |
| Autoimmune | 24,180 | 3.8 | 4805 | 2.66 | <0.001 |
| Depression | 68,370 | 10.75 | 32,900 | 18.19 | <0.001 |
| AIDS | 1510 | 0.24 | 295 | 0.16 | <0.001 |
| Characteristics | COVID and Dementia− | COVID and Dementia+ | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| n = 816,960 | 636,115 | 77.86 | 180,845 | 22.14 | -- |
| Disposition (%) | n | % | n | % | <0.001 |
| Against medical advice | 4155 | 0.65 | 430 | 0.24 | |
| Discharged alive unknown destination | 525 | 0.08 | 330 | 0.18 | |
| Home health care | 116,605 | 18.33 | 25,010 | 13.83 | |
| Routine | 239,630 | 37.67 | 17,845 | 9.87 | |
| Transfer other | 136,925 | 21.53 | 91,450 | 50.57 | |
| Transfer to short-term hospital | 19,965 | 3.14 | 3945 | 2.18 | |
| COMORBIDITIES (%) | n | % | n | % | |
| Acute liver failure | 7480 | 1.18 | 1150 | 0.64 | <0.001 |
| Adjusted odds ratio * = 0.66 (95% CI 0.57–0.77) | |||||
| Sudden cardiac arrest | 20,805 | 3.27 | 4770 | 2.64 | <0.001 |
| Adjusted odds ratio * = 0.87 (95% CI 0.81–0.94) | |||||
| Mean total hospitalization charge (USD) | USD 94,274.62 | USD 74,896.89 | <0.001 | ||
| Adjusted total charge * = USD 6075.18 lower for dementia+ | |||||
| Mean length of stay (days) | 8.43 | 8.50 | <0.001 | ||
| Adjusted length of stay * = 0.64 days higher for dementia+ | |||||
| In-hospital mortality (n = 160,145) | 118,310 | 18.6 | 41,835 | 23.13 | <0.001 |
| Adjusted odds ratio * = 1.17 (95% CI 1.13–1.2) | |||||
| Vasopressor use | 20,260 | 3.18 | 3725 | 2.06 | <0.001 |
| Adjusted odds ratio * = 0.81 (95% CI 0.75–0.88) | |||||
| AKI | 226,915 | 35.67 | 74,950 | 41.44 | <0.001 |
| Adjusted odds ratio * = 1.22 (95% CI 1.19–1.26) | |||||
| VTE | 31,725 | 4.99 | 7395 | 4.09 | 0,001 |
| Adjusted odds ratio * = 0.90 (95% CI 0.84–0.95) | |||||
| Hemodialysis | 35,945 | 5.65 | 5290 | 2.93 | <0.001 |
| Adjusted odds ratio * = 0.67 (95% CI 0.62–0.72) | |||||
| Invasive mechanical ventilation | 94,190 | 14.81 | 14,565 | 8.05 | <0.001 |
| Adjusted odds ratio * = 0.68 (95% CI 0.66–0.72) | |||||
| Non-invasive mechanical ventilation | 45,535 | 7.16 | 9315 | 5.15 | <0.001 |
| Adjusted odds ratio * = 0.80 (95% CI 0.75–0.84) | |||||
| CVA | 12,850 | 2.02 | 3660 | 2.02 | 0.598 |
| Adjusted odds ratio * = 1.02 (95% CI 0.94–1.12) | |||||
| Tracheostomy | 6680 | 1.05 | 480 | 0.27 | <0.001 |
| Adjusted odds ratio * = 0.43 (95% CI 0.35–0.54) | |||||
| AMI | 16,625 | 2.61 | 4755 | 2.63 | 0.105 |
| Adjusted odds ratio * = 0.94 (95% CI 0.87–1.01) | |||||
| Characteristics | COVID and Dementia− | COVID and Dementia+ | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| n = 361,690 | 180,845 | 50.00 | 180,845 | 50.00 | -- |
| Disposition (%) | n | % | n | % | <0.001 |
| Against medical advice | 835 | 0.46 | 430 | 0.24 | |
| Discharged alive unknown destination | 240 | 0.13 | 330 | 0.18 | |
| Home health care | 37,070 | 20.5 | 25,010 | 13.83 | |
| Routine | 49,300 | 27.26 | 17,845 | 9.87 | |
| Transfer other | 50,480 | 27.91 | 91,450 | 50.57 | |
| Transfer to short-term hospital | 4745 | 2.62 | 3945 | 2.18 | |
| COMORBIDITIES (%) | n | % | n | % | |
| Acute liver failure | 1680 | 0.93 | 1150 | 0.64 | <0.001 |
| Adjusted odds ratio * = 0.68 (95% CI 0.58–0.81) | |||||
| Sudden cardiac arrest | 5235 | 2.89 | 4770 | 2.64 | 0.006 |
| Adjusted odds ratio * = 0.88 (95% CI 0.8–0.96) | |||||
| Mean total hospitalization charge (USD) | USD 78,437.41 | USD 74,896.89 | <0.001 | ||
| Adjusted total charge * = USD 6291.82 lower for dementia+ | |||||
| Mean length of stay (days) | 7.93 | 8.50 | <0.001 | ||
| Adjusted length of stay * = 0.49 days higher for dementia+ | |||||
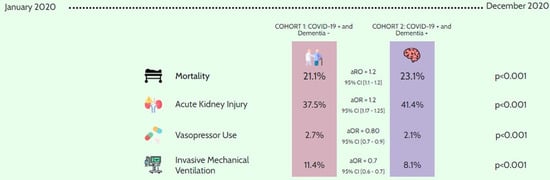
| In-hospital mortality (n = 80,010) | 38,175 | 21.11 | 41,835 | 23.13 | <0.001 |
| Adjusted odds ratio * = 1.16 (95% CI 1.12–1.2) | |||||
| Vasopressor use | 4930 | 2.73 | 3725 | 2.06 | <0.001 |
| Adjusted odds ratio * = 0.80 (95% CI 0.73–0.89) | |||||
| AKI | 67,750 | 37.46 | 74,950 | 41.44 | <0.001 |
| Adjusted odds ratio * = 1.21 (95% CI 1.17–1.25) | |||||
| VTE | 8270 | 4.57 | 7395 | 4.09 | 0.002 |
| Adjusted odds ratio * = 0.89 (95% CI 0.83–0.96) | |||||
| Hemodialysis | 7975 | 4.41 | 5290 | 2.93 | <0.001 |
| Adjusted odds ratio * = 0.66 (95% CI 0.6–0.71) | |||||
| Invasive mechanical ventilation | 20,690 | 11.44 | 14,565 | 8.05 | <0.001 |
| Adjusted odds ratio * = 0.68 (95% CI 0.64–0.71) | |||||
| Non-invasive mechanical ventilation | 11,450 | 6.33 | 9315 | 5.15 | <0.001 |
| Adjusted odds ratio * = 0.77 (95% CI 0.73–0.83) | |||||
| CVA | 3815 | 2.11 | 3660 | 2.02 | 0.299 |
| Adjusted odds ratio * = 0.95 (95% CI 0.85–1.05) | |||||
| Tracheostomy | 1240 | 0.69 | 480 | 0.27 | <0.001 |
| Adjusted odds ratio * = 0.41 (95% CI 0.32–0.52) | |||||
| AMI | 4890 | 2.7 | 4755 | 2.63 | 0.320 |
| Adjusted odds ratio * = 0.95 (95% CI 0.87–1.05) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar Gil, T.; Quazi, M.A.; Verma, T.; Sohail, A.H.; Ikram, H.A.; Nasrullah, A.; Gangu, K.; Farooq, A.; Sheikh, A.B. Outcomes of COVID-19-Associated Hospitalizations in Geriatric Patients with Dementia in the United States: A Propensity Score Matched Analysis. Geriatrics 2024, 9, 7. https://doi.org/10.3390/geriatrics9010007
Escobar Gil T, Quazi MA, Verma T, Sohail AH, Ikram HA, Nasrullah A, Gangu K, Farooq A, Sheikh AB. Outcomes of COVID-19-Associated Hospitalizations in Geriatric Patients with Dementia in the United States: A Propensity Score Matched Analysis. Geriatrics. 2024; 9(1):7. https://doi.org/10.3390/geriatrics9010007
Chicago/Turabian StyleEscobar Gil, Tomas, Mohammed A. Quazi, Tushita Verma, Amir H. Sohail, Hafiz Abdullah Ikram, Adeel Nasrullah, Karthik Gangu, Asif Farooq, and Abu Baker Sheikh. 2024. "Outcomes of COVID-19-Associated Hospitalizations in Geriatric Patients with Dementia in the United States: A Propensity Score Matched Analysis" Geriatrics 9, no. 1: 7. https://doi.org/10.3390/geriatrics9010007
APA StyleEscobar Gil, T., Quazi, M. A., Verma, T., Sohail, A. H., Ikram, H. A., Nasrullah, A., Gangu, K., Farooq, A., & Sheikh, A. B. (2024). Outcomes of COVID-19-Associated Hospitalizations in Geriatric Patients with Dementia in the United States: A Propensity Score Matched Analysis. Geriatrics, 9(1), 7. https://doi.org/10.3390/geriatrics9010007