Long-Term Survival Prediction Model for Elderly Community Members Using a Deep Learning Method
Abstract
1. Introduction
2. Methods
2.1. Data and Study Design
2.2. Study Population
2.3. Variables
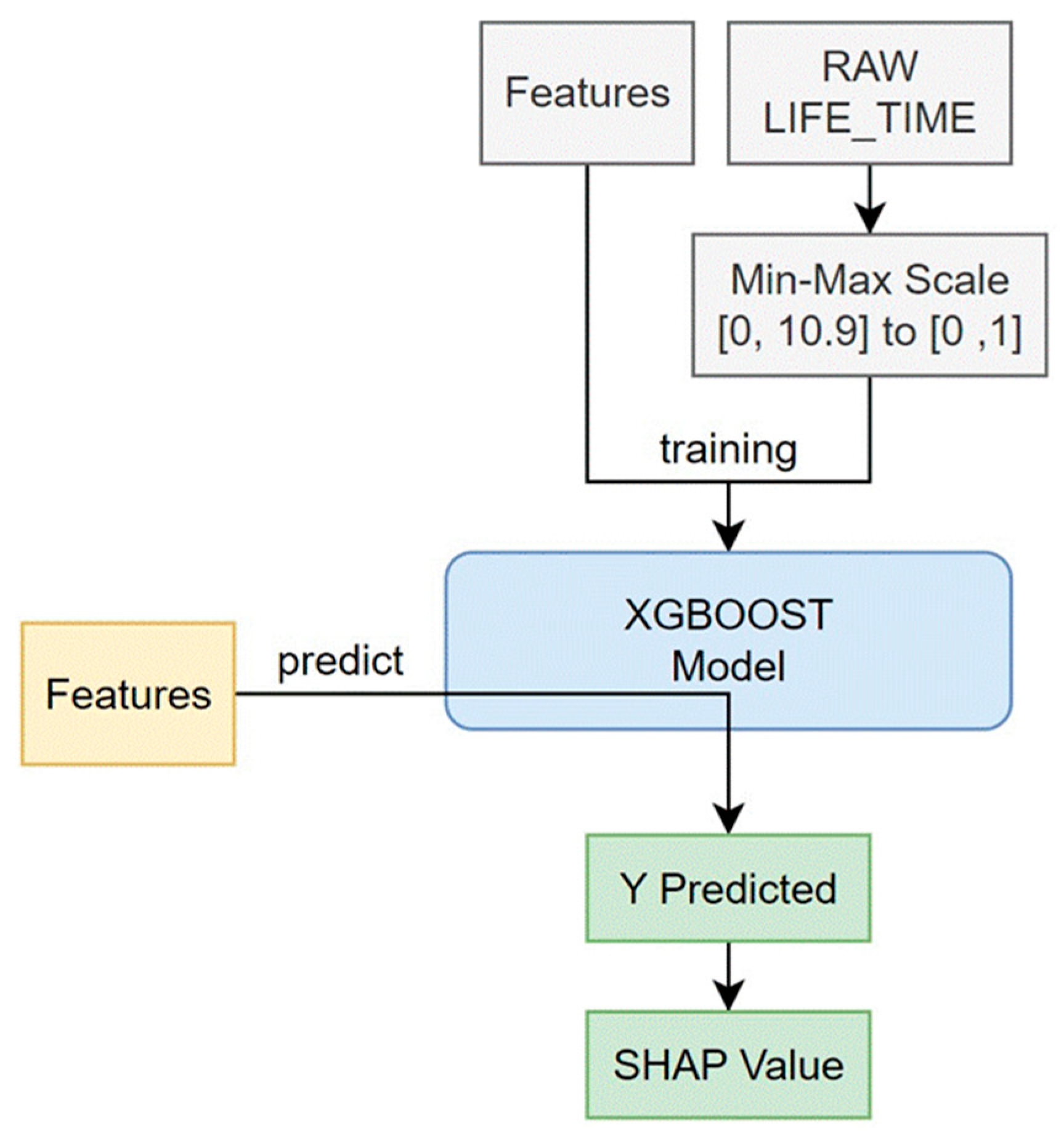
2.4. Statistical Analysis
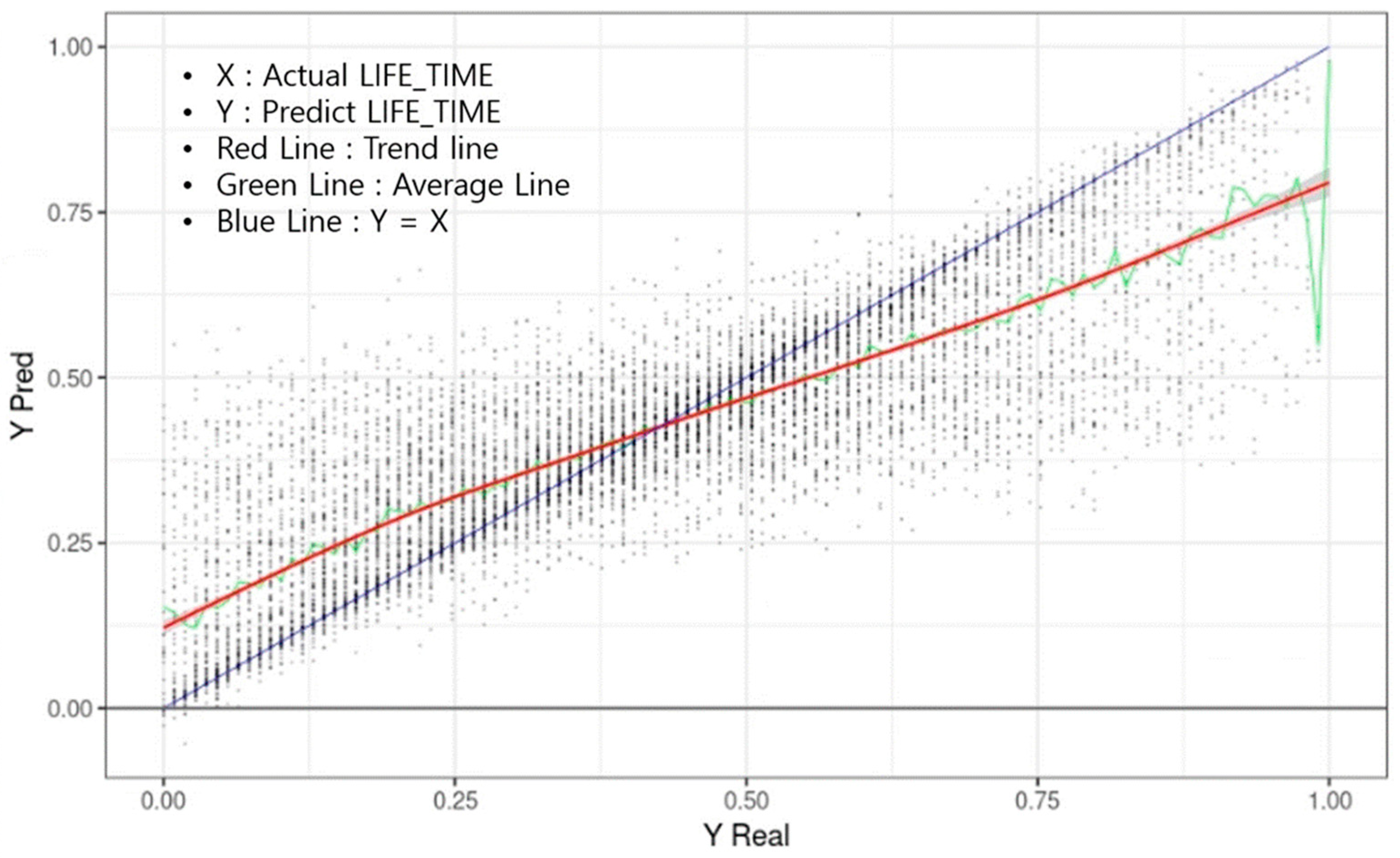
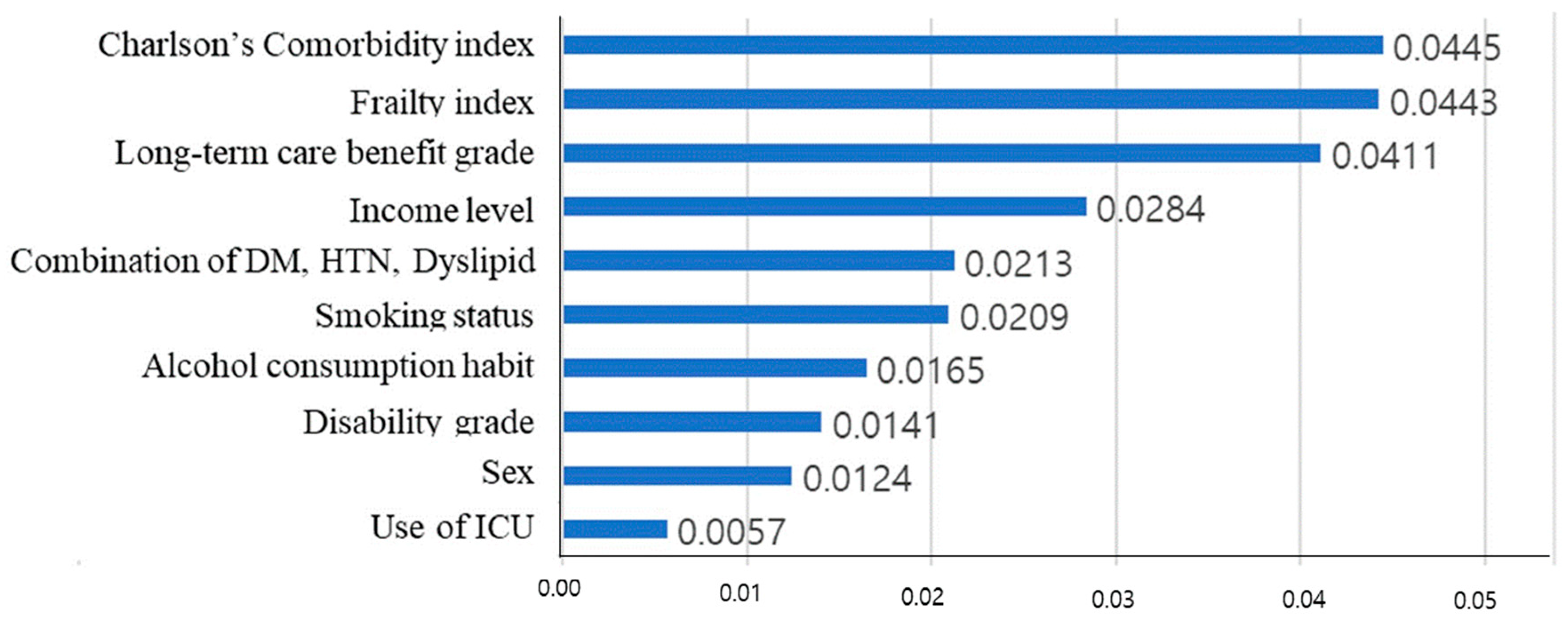
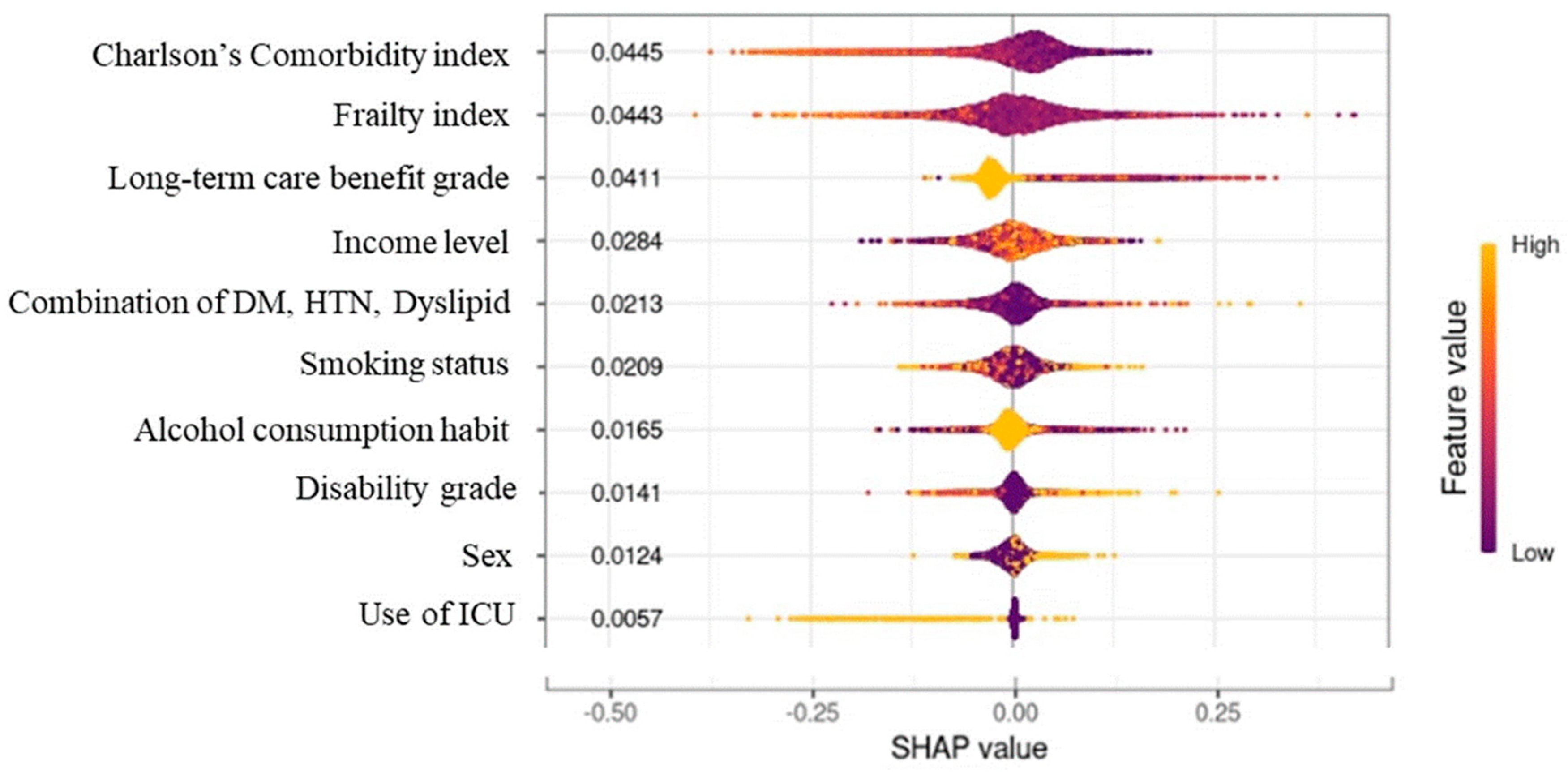
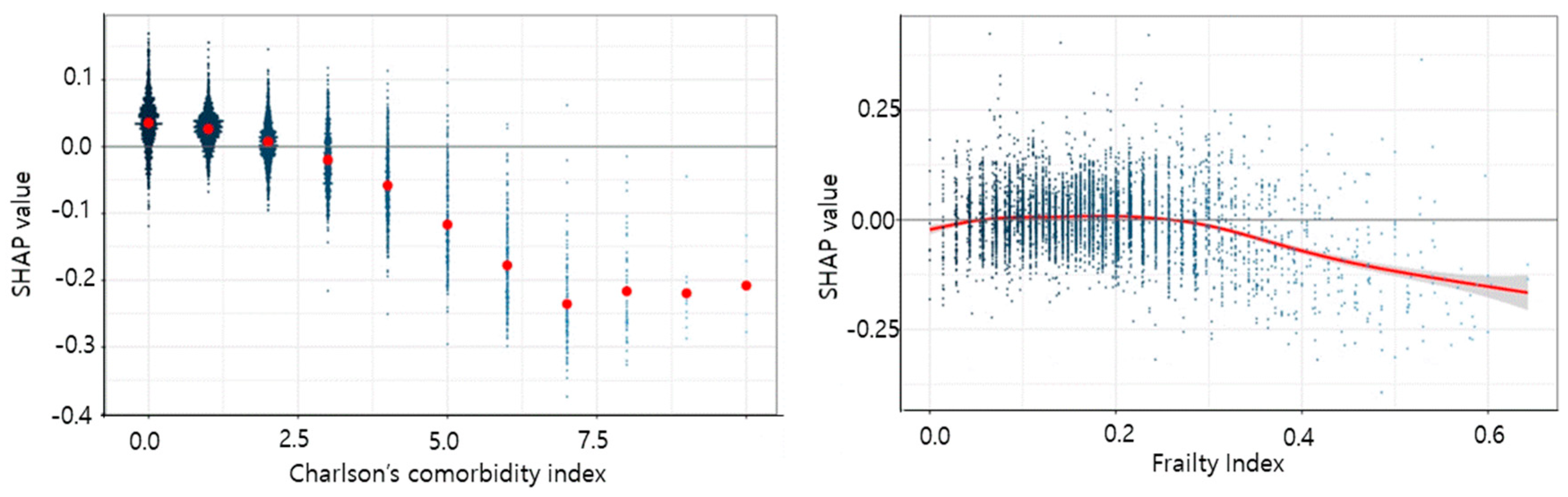
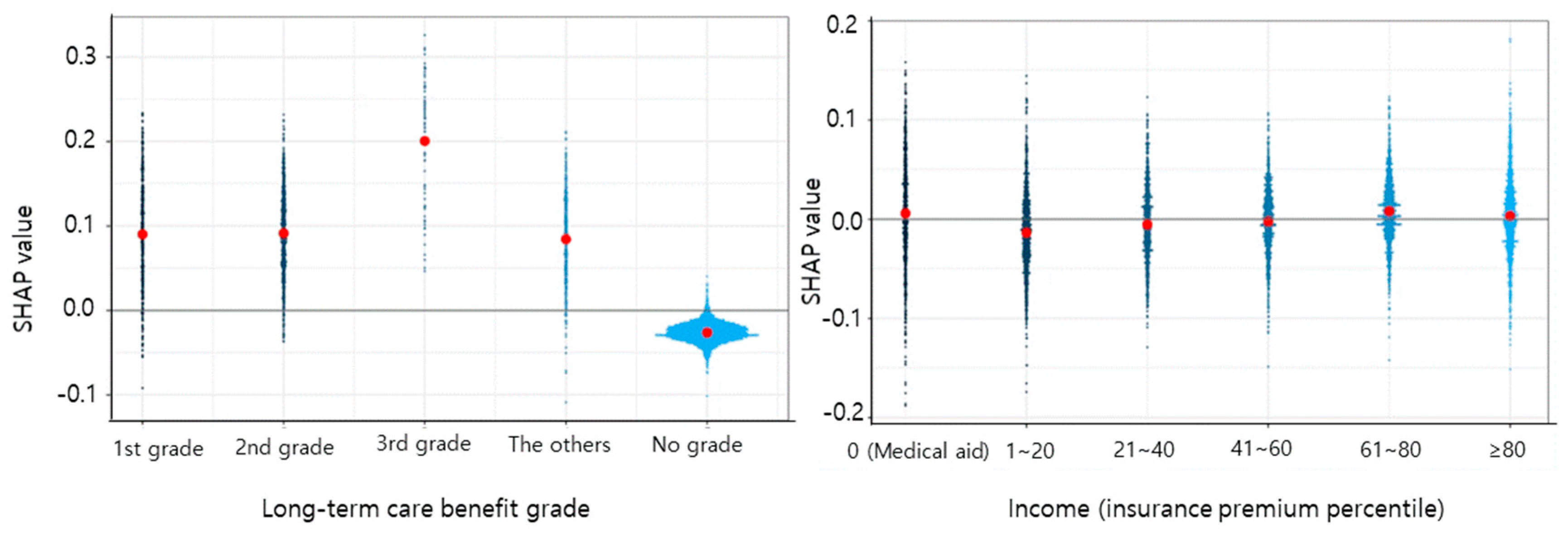
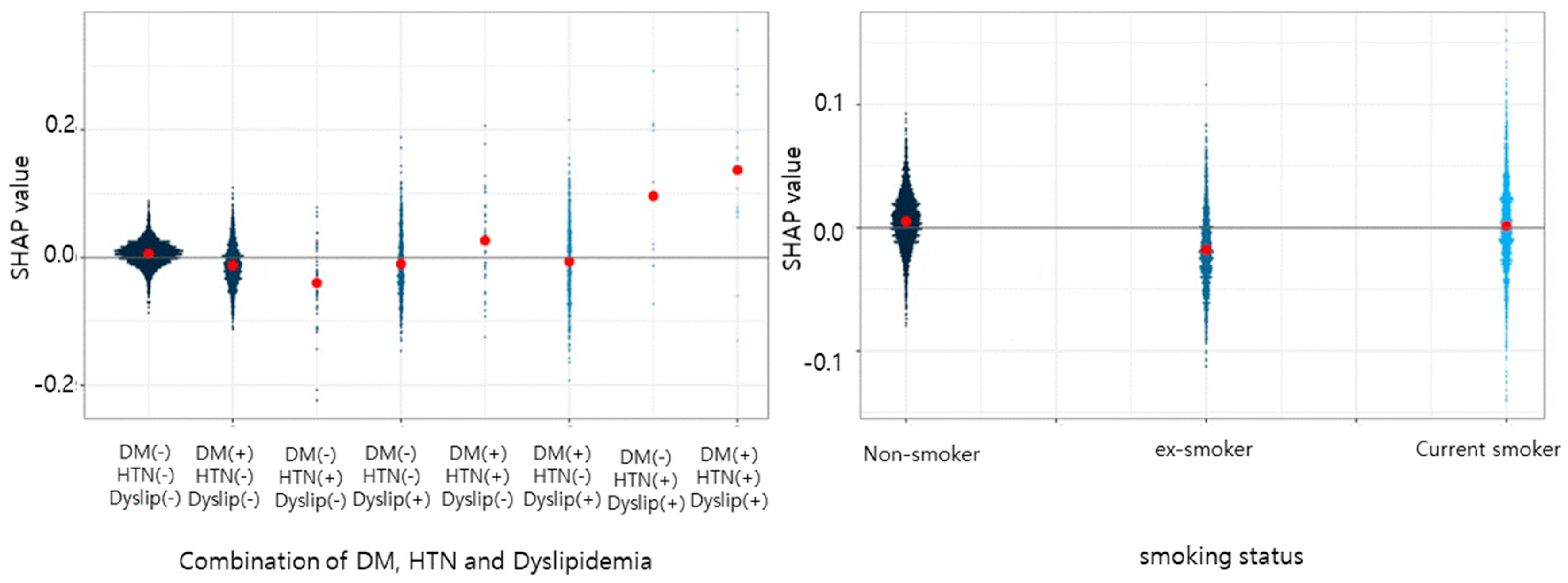
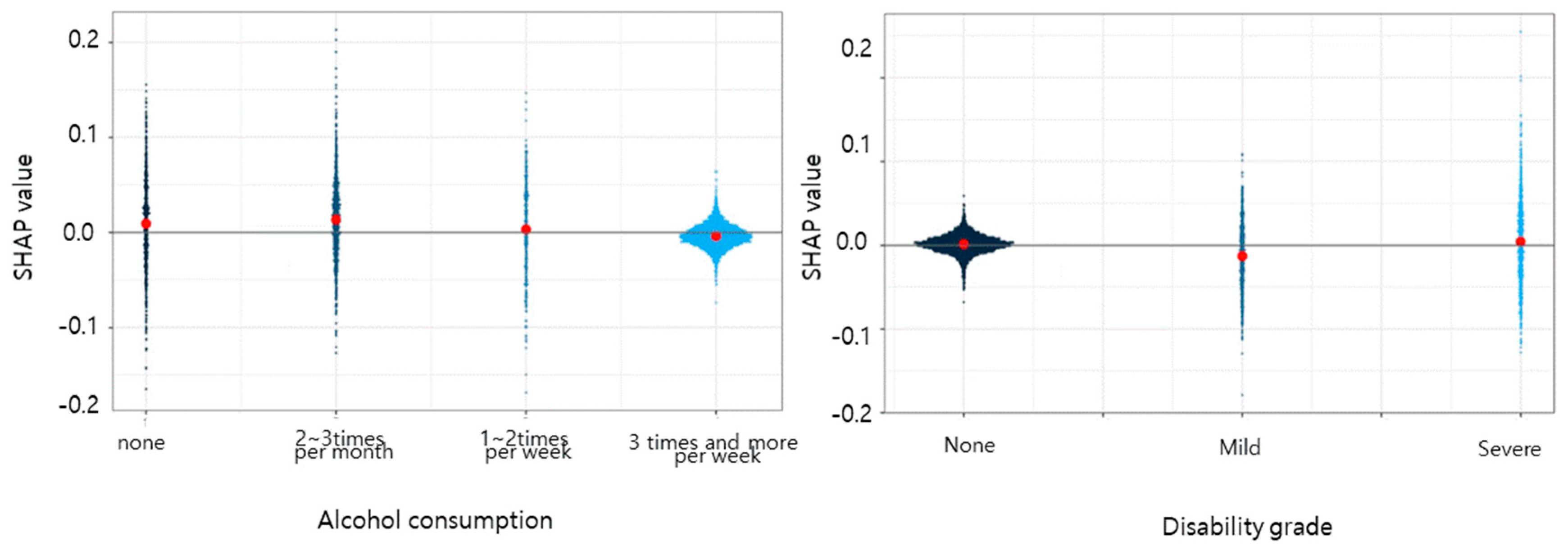
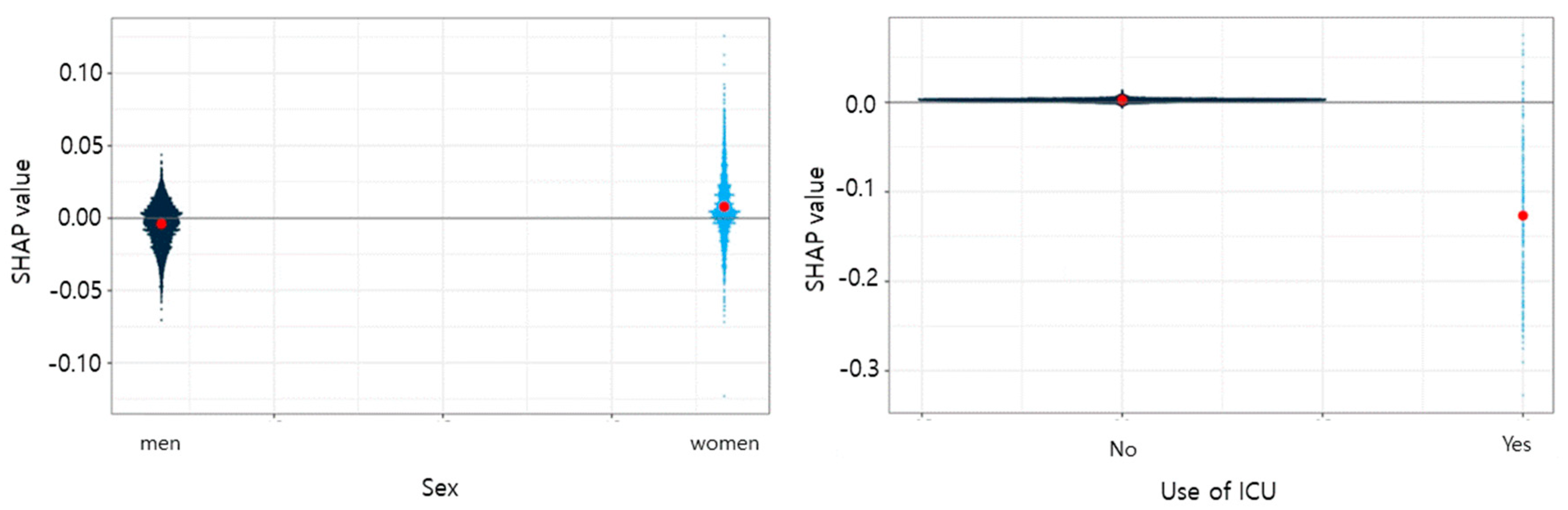
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Korea. Population Projection for Korea (2020~2070). 2021. Available online: https://kosis.kr/edu/visualStats/detail.do?ixId=161&ctgryId=&menuId=M_05 (accessed on 25 April 2023).
- Statistics Korea (Press Release). Population Status and Prospects of the World and Korea Reflecting Future Population Projections in 2021. 5 September 2022. Available online: https://kostat.go.kr/board.es?mid=a10301010000&bid=207&act=view&list_no=420361&tag=&nPage=1&ref_bid= (accessed on 25 April 2023).
- Gong, Y.H.; Jo, M.W. Cost estimation of productivity loss of elderly over 70 due to premature mortality reflecting elderly employment in an aged society. Korean Assoc. Health Technol. Assess. 2017, 5, 89–94. [Google Scholar]
- WHO. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Barbat-Artigas, S.; Pion, C.H.; Leduc-Gaudet, J.P.; Rolland, Y.; Aubertin-Leheudre, M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J. Am. Med. Dir. Assoc. 2014, 15, 303.e13–303.e20. [Google Scholar] [CrossRef]
- Rattan, S.I. Aging is not a disease: Implications for intervention. Aging Dis. 2014, 5, 196–202. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, D.W.; Lee, W.C.; Kim, Y.T.; Cho, B. National screening program for transitional ages in Korea: A new screening for strengthening primary prevention and follow-up care. J. Korean Med. Sci. 2012, 27, S70–S75. [Google Scholar] [CrossRef]
- Pedone, C.; Scarlata, S.; Forastiere, F.; Bellia, V.; Antonelli Incalzi, R. BODE index or geriatric multidimensional assessment for the prediction of very-long-term mortality in elderly patients with chronic obstructive pulmonary disease? A prospective cohort study. Age Ageing 2014, 43, 553–558. [Google Scholar] [CrossRef]
- Jang, I.; Jung, H.; Shin, J.; Kim, D.H. Assessment of fraity index at 66 years of age and association with age-related diseases, disability, and death over 10 years in Korea. JAMA Netw. Open. 2023, 6, e2248995. [Google Scholar] [CrossRef]
- Suemoto, C.K.; Ueda, P.; Beltrán-Sánchez, H.; Lebrão, M.L.; Duarte, Y.A.; Wong, R.; Danaei, G. Development and Validation of a 10-Year Mortality Prediction Model: Meta-Analysis of Individual Participant Data from Five Cohorts of Older Adults in Developed and Developing Countries. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Porter, B.; Maynard, C.; Evans, G.; Bryson, C.; Sun, H.; Lowy, E.; McDonell, M.; Frisbee, K.; Nielson, C.; et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administrarion. Med. Care 2013, 51, 368–373. [Google Scholar] [CrossRef] [PubMed]
- van de Vorst, I.E.; Golüke, N.M.S.; Vaartjes, I.; Bots, M.L.; Koek, H.L. A prediction model for one- and three-year mortality in dementia: Results from a nationwide hospital-based cohort of 50,993 patients in the Netherlands. Age Ageing 2020, 49, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, D.; Kurichi, J.E.; Streim, J.; Zhang, G.; Stineman, M.G. Mortality predictive indexes for the community-dwelling elderly US population. J. Gen. Intern. Med. 2012, 27, 901–910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tirandi, A.; Arboscello, E.; Ministrini, S.; Liberale, L.; Bonaventura, A.; Vecchié, A.; Bertolotto, M.; Giacobbe, D.R.; Castellani, L.; Mirabella, M.; et al. Early sclerostin assessment in frail elderly patients with sepsis: Insights on short- and long-term mortality prediction. Intern. Emerg. Med. 2023, 18, 1509–1519. [Google Scholar] [CrossRef]
- National Health Insurance Sharing Service. Elderly Cohort. Available online: https://nhiss.nhis.or.kr/bd/ab/bdaba001cv.do (accessed on 25 April 2023).
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. 2001, 56, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Lee, S. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef] [PubMed]
- Zazzara, M.B.; Vetrano, D.L.; Carfì, A.; Onder, G. Frailty and chronic disease. Panminerva Med. 2019, 61, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef]
- Capurso, C. Increasing Our Understanding of How Dietary Components Can Affect Cellular Mechanisms That Regulate Aging and Slow the Onset of Frailty and Chronic Diseases. Nutrients 2023, 15, 2687. [Google Scholar] [CrossRef]
- Sugihara, T.; Harigai, M. Targeting Low Disease Activity in Elderly-Onset Rheumatoid Arthritis: Current and Future Roles of Biological Disease-Modifying Antirheumatic Drugs. Drugs Aging. 2016, 33, 97–107. [Google Scholar] [CrossRef]
- Fries, J.F. Frailty, heart disease, and stroke: The Compression of Morbidity paradigm. Am. J. Prev. Med. 2005, 29 (Suppl. S1), 164–168. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Vetrano, D.L.; Manes-Gravina, E.; Bernabei, R.; Onder, G.; Palmer, K. The Relationship Between COPD and Frailty: A Systematic Review and Meta-Analysis of Observational Studies. Chest 2018, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Cereda, E.; Stubbs, B.; Solmi, M.; Luchini, C.; Manzato, E.; Sergi, G.; Manu, P.; Harris, T.; Fontana, L.; et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res. Rev. 2017, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]


| (Feature Value) Individual Variables | ||
|---|---|---|
| Sex, n (%) | ||
| (1): Men | 6326 | (66.9) |
| (2): Women | 3136 | (33.1) |
| Income level, n (%) | ||
| (1): 0 percentile (Medical Aid) | 943 | (10.0) |
| (2): 1~20 Percentile | 1579 | (16.7) |
| (3:) 21~40 Percentile | 1219 | (12.9) |
| (4): 41~60 Percentile | 1542 | (16.3) |
| (5): 61~80 Percentile | 2115 | (22.4) |
| (6): 81~100 Percentile | 2064 | (21.8) |
| Charlson’s comorbidity index, n (%) | ||
| 0 | 2436 | (25.8) |
| 1 | 2791 | (29.5) |
| 2 | 1891 | (20.0) |
| 3 | 1119 | (11.8) |
| 4 | 541 | (5.7) |
| 5 | 289 | (3.1) |
| 6 | 220 | (2.3) |
| 7 | 111 | (1.2) |
| 8 | 48 | (0.5) |
| 9 | 11 | (0.1) |
| 10 or more | 5 | (0.1) |
| Frailty index, Mean (SD) | 0.1547 | (0.0824) |
| Long-term care benefit grade, n (%) | ||
| 1: 1st grade | 471 | (5.0) |
| 2: 2nd grade | 1020 | (10.8) |
| 3: 3~5th grade | 70 | (0.7) |
| 4: Out of grade | 463 | (4.9) |
| 5: Those who have not applied for a grade | 7438 | (78.6) |
| Disability grade, n (%) | ||
| (1): 1 None | 7384 | (77.7) |
| (2): Severe | 872 | (9.2) |
| (3): Mild | 1236 | (13.1) |
| Combination of DM, HTN, and dyslipidemia, n (%) | ||
| (0): DM(+), HTN(−), dyslipidemia(−) | 5660 | (59.8) |
| (1): DM(−), HTN(+), dyslipidemia(−) | 2385 | (25.2) |
| (2): DM(−), HTN(−), dyslipidemia(+) | 41 | (0.4) |
| (3): DM(+), HTN(+), dyslipidemia(−) | 778 | (8.2) |
| (4): DM(+), HTN(−), dyslipidemia(+) | 42 | (0.4) |
| (5): DM(−), HTN(+), dyslipidemia(+) | 528 | (5.6) |
| (6): DM(+), HTN(+), dyslipidemia(+) | 12 | (0.1) |
| (7): DM(−), HTN(−), dyslipidemia(−) | 16 | (0.2) |
| Smoking status, n (%) | ||
| (0): No smoking | 4910 | (52.0) |
| (1): Ex-smoking | 2166 | (22.9) |
| (2): Current smoking | 2374 | (25.1) |
| Alcohol consumption habit, n (%) | ||
| (0): No drinking | 870 | (9.2) |
| (1): 2~3 times per month | 1299 | (13.7) |
| (2): Once or twice per week | 454 | (4.8) |
| (3): More than 3 times per week | 6839 | (72.3) |
| Use of Intensive care unit, n (%) | ||
| (0): No | 9232 | (97.6) |
| (1): Yes | 230 | (2.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.H.; Paek, J.-M.; Ko, K.-M. Long-Term Survival Prediction Model for Elderly Community Members Using a Deep Learning Method. Geriatrics 2023, 8, 105. https://doi.org/10.3390/geriatrics8050105
Cho KH, Paek J-M, Ko K-M. Long-Term Survival Prediction Model for Elderly Community Members Using a Deep Learning Method. Geriatrics. 2023; 8(5):105. https://doi.org/10.3390/geriatrics8050105
Chicago/Turabian StyleCho, Kyoung Hee, Jong-Min Paek, and Kwang-Man Ko. 2023. "Long-Term Survival Prediction Model for Elderly Community Members Using a Deep Learning Method" Geriatrics 8, no. 5: 105. https://doi.org/10.3390/geriatrics8050105
APA StyleCho, K. H., Paek, J.-M., & Ko, K.-M. (2023). Long-Term Survival Prediction Model for Elderly Community Members Using a Deep Learning Method. Geriatrics, 8(5), 105. https://doi.org/10.3390/geriatrics8050105







