1. Introduction
Several factors may contribute to an older individual suffering from nutrient deficiencies and other imbalances. For example, sensory disturbances, functional disabilities, and social isolation that typically accompany aging all increase the likelihood that a person will develop unhealthy eating habits. What is more, the mere fact of being female is one of the risk factors for developing these habits [
1,
2,
3,
4,
5], which may lead to nutrient deficiencies or other imbalances that ultimately result in physical manifestations such as altered body composition and body cell mass. These physical changes cause (i) diminished physical and mental function, (ii) alterations in the immune system, (iii) worsening of the underlying disease, (iv) longer hospital stays and readmissions, and (v) a lower quality of life (QoL) [
6,
7,
8,
9,
10,
11]. As presented in [
12], malnutrition among the elderly is overlooked and under-diagnosed in the United States, affecting up to 60% of hospitalized patients aged 65 and older. Additionally, Goates et al. [
13] have demonstrated the financial impact malnutrition may have on several pathologies when considering the cost of medical care. Therefore, as reported by the European Society of Parenteral and Enteral Nutrition (ESPEN), systematic screening for malnutrition should be carried out using a validated tool to substantiate the diagnosis of malnutrition and as a basis for the definition of individual treatment goals and the development of a comprehensive nutritional care plan [
14].
Several scientific societies as well as national and international organizations call for the early detection of malnutrition, enabling the health system to mitigate negative associated health consequences by proactively implementing corrective measures to address the patient’s nutrient deficiency. In Spain, some studies, which focused on the elderly population, show the nutritional status to which this population is exposed both living at home as well as in the institutional environment. The PREDyCES study (Prevalence of hospital malnutrition and associated costs in Spain) is an example of the observations made regarding patients’ nutritional status [
15]. This ground-breaking study showed that 57% of hospitalized elderly patients ran the risk of being malnourished. Hence, the SEGG-SENPE consensus document on nutritional assessment in the elderly indicates that “malnutrition in the elderly could be partly avoided if all those maneuvers aimed at preventing the development of malnutrition or treating it early were carried out” [
16].Therefore, it is imperative to consider innovative multidisciplinary approaches, such as personalized nutritional guidance systems carried out by our research group [
17] for the prevention of malnutrition in hospitalized multimorbid older patients, and the use of nutritional formulas to solve malnutrition as it is a highly prevalent problem that carries significant costs for the public health system.
Currently, nutritional questionnaires are used to detect malnutrition in older adults. The most widely used for this population is the Mini Nutritional Assessment (MNA) questionnaire [
8]. However, it can be time consuming for caregivers to complete this type of survey each time a patient is admitted to the hospital. Moreover, some studies have shown that women may have a higher risk of malnutrition [
1,
2,
3,
4,
5], and it is necessary to have a special focus on that. Thus, identifying the factors that contribute most to malnutrition, checking the factors that may have higher impacts on older women and developing new tools, such as predictive models for assessing the nutritional status, facilitates the identification of malnourished populations and simplifies the health professionals’ work.
It is essential to consider the most suitable variables to design an efficient predictive model for the risk of malnutrition. Some studies have reported that several physiological changes associated with age, socio-economic status, and neuropsychological factors may contribute to insufficient dietary intake [
18,
19,
20]. Regarding physiological factors, slower gastric emptying, altered hormonal responses, decreased basal metabolic rate, and altered taste and smell may also contribute to lowered energy intake [
10,
20,
21]. In terms of socio-economic and neurophysiological aspects, other factors like marital status, social isolation, cognitive impairment, depression, and education level may be associated with malnutrition [
18]. Additionally, O’Keeffe et al. [
22] present a systematic review of potentially modifiable determinants of malnutrition, which involve seven domains (oral, psychosocial, medication and care, health, physical function, lifestyle, and eating). Besides, gender inequality in nutrition is also studied [
1,
2,
3,
4,
5], showing that poverty, education, lack of awareness and marital status may contribute to malnutrition in women.
However, these studies have some limitations: (i) lack of sufficient data for a representative sample, (ii) focus on a specific population with a particular disease or condition, (iii) usage of non-validated measures to determine malnutrition, (iv) ignore other potential factors, such as living conditions, or (v) focus on developing countries.
In addition, although some studies have been carried out in the last years to develop models for the prediction of malnutrition, few studies focus on older hospitalized people, and do not focus on women cases, and the approaches centered in this population have limitations regarding their predictive capacity. For instance, in the case of the models developed by Muñoz et al. [
23], a nutritional screening standard was considered for the selection of variables, which makes the predictive capacity of these models questionable.
To overcome these limitations, the current observational study aimed at identifying the key factors that contribute to malnutrition in the older adults, further studying the situation with women, and using those key factors to develop an efficient model for predicting the risk of malnutrition in this population. Furthermore, a statistical analysis was carried out to assess the effect of malnutrition on hospital admission, such as length of stay (LOS) and readmission.
4. Discussion
In the present population-based cohort study of older adults aged ≥65, 44.4% of the studied population is well nourished, whereas 41.8% is at risk of malnutrition and 13.8% has malnutrition based on the MNA-SF screening test. This picture comes out worse when it comes to women with 36.1% of women well nourished, 47.5% at risk and 16.4% with malnutrition, as also presented in [
1].
In this observational study we examined the association between risk of malnutrition and a wide range of variables including demographic, clinical (chronic diseases) and laboratory data. From the demographic point of view, our findings suggested that older age, female gender, having assistance at home, and being dependent (via Barthel index) are all factors associated with malnutrition. However, it is important to note that the MNA-SF screening test applied in our study makes use of BMI, and asks for mobility, which is related with BARTHEL and assisted living factors of our study. Therefore, we cannot say that BMI is a key indicator of malnutrition, and BARTHEL and assisted living factors may also be influenced by the MNA-SF mobility question.
Regarding architectural barriers, although the absence of elevator does not have
p < 0.001, is close to it with a value of
p = 0.007, and hence, we should consider it since it may contribute patients to be more isolated [
23], and hence, with less access to food.
Note that from the clinical point of view, some of the studied patient factors are causative risk factors, such as age, sex and assisted, and others are consequences of malnutrition, such as BMI value. However, other factors may not be clear, such as the dependency situation (i.e., BARTHEL index), since they could be both causative and consequence factors of malnutrition or the case of albumin, as both (albumin level and malnutrition) are related to inflammation which can describe the association but not the causality between them as described by Evans et al. [
33].
Considering the threshold of
p < 0.001, only patients with pressure ulcers seem to have a higher risk of malnutrition, but multimorbidity shows a
p = 0.003, which is close to the value, aligned with the literature [
34]. However, diabetes, heart failure (HF), or chronic obstructive pulmonary disease (COPD) single diseases do not seem to be associated with malnutrition. In addition, upon examining obtained laboratory data, only serum albumin presents a high correlation with malnutrition.
Regarding hospitalization factors, the present study demonstrates the high impact of the type of admission on malnutrition. The hospital unit factor, which is highly related with the patient diagnosis, shows that patients admitted in cardiology, neurology, traumatology, and urology have a lower prevalence of malnourished patients, while in internal medicine units, the number is higher. This way, internal medicine seems to be a unit with higher risk of malnourishment. DRG severity is another factor that is highly related to malnutrition, where more severe patients show a higher risk of malnutrition. Besides, medical admissions are also highly correlated with malnutrition, since they are more related to medical conditions that affect the patient as a whole, while surgical admissions are inversely related, since they used to be programmed admissions.
To the best of our knowledge, this is the first study in the Basque Region to evaluate the risk of malnutrition in the elderly population when admitted to the hospital. Other studies in Spain examining the risk of malnutrition [
30,
32] suggest that undernutrition rates are over 40% using the CONUT and SGA screening tests. On the other hand, other Spanish studies performed using the MNA screening test obtain similar results to ours, with a low prevalence of malnutrition (around 7% in the study population), whereas the risk of malnutrition reaches nearly half of the study population (49%) [
35,
36,
37].
As expected, the Barthel index, which determines the dependency level of a person, is associated with malnutrition. However, chronic diseases do not seem to influence the risk of malnutrition in the elderly population. In addition, increased age correlates with malnutrition. This could also be related to cognitive functioning, as suggested by Katsas et al. [
19], since increased age may cause a decline in cognitive functioning, particularly in memory. Memory is associated with many aspects of daily life, such as eating, and consequently can lead to an increased risk of malnutrition [
38,
39].
Aligned with other studies, we found that women had a higher risk of malnutrition than men [
2,
3,
4,
5]. Therefore, further analysis was performed to identify the main differences that may exist between both groups (see
Table 2 and
Table A1 in
Appendix A). We noted that marital status in
Table 1 seems to be a relevant factor. However, when analyzing men and women separately, it appears that marital status does not have such a great impact. The reason behind this is that the number of widowed women is much higher than the number of widowed men (i.e., there is a high correlation between gender and marital status,
p < 0.001). On the other hand, age seem to have a higher impact on men (
p < 0.001) than women, although for women it is close to
p < 0.001 (
p = 0.002). For this reason, we conclude that age and sex are the primary factors that contribute to malnutrition, but not marital status in and of itself. In addition, we analyzed the distribution of the MNA variable for both sex factors (woman and man), finding worse nutritional conditions for the female gender. This higher prevalence of malnutrition risk among women may be related to social issues related to social and financial living as suggested in [
4], or depressive states being more common in women as found in [
2,
3].
Additionally, the results show that malnutrition is associated with longer hospital LOS, aligned with [
10,
11,
40]. This extended LOS leads to a significant increase in hospital costs [
11]. However, there is a high correlation between LOS and DRG severity, and hence, the association between LOS and malnutrition is not clearly demonstrated.
Regarding readmissions, in contrast with [
10], this study does not present significantly higher readmission rates in patients with malnutrition or those who are at risk of malnutrition, compared to those who are well-nourished.
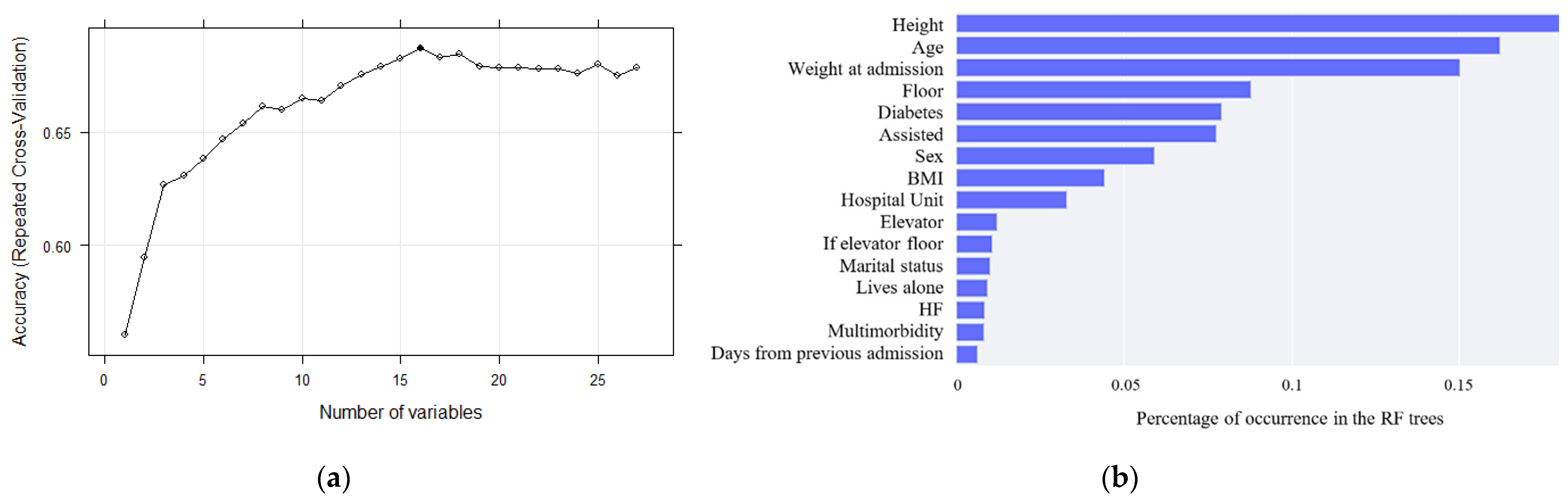
Finally, identifying all these risk factors has led to the development of a model to predict the risk of malnutrition in hospitalized older adults. Agreeing with [
41], fewer features can allow machine learning algorithms to run more efficiently (less space or time complexity) and be more effective, as some algorithms can be misled by irrelevant input features, resulting in worse predictive performance. Thus, after the application of a recursive feature elimination (RFE) algorithm, sixteen variables were selected for the development of the models. After comparing the ROC-AUC values of all the designed models, the random forest and gradient boosting algorithms obtained the highest accuracy values. However, concurring with [
42], discrimination is assumed to be useful if AUC ≥ 0.75, and hence the obtained models present fair predictive ability. This assumption can be confirmed by the interpretation of the confusion matrix, which shows that the model is able to predict correctly with an accuracy of 51.6%. Although this result is not as expected, we have almost no extreme errors and, considering that it is a three-way classification, it may be suitable for its use.
Among the limitations of this study is the lack of availability of each separate MNA-SF response to examine answers independently. Information regarding cognitive functioning, education level, and socioeconomic status has not been collected, which could also be of interest to the current study. Throughout the study, information regarding complications, such as surgical wound dehiscence, nosocomial infection, and ulcer complications upon admission was collected to determine the impact of malnutrition on patients. Nevertheless, since the number of patients that suffered these complications was too small, no conclusions could be drawn, and consequently, these results have not been presented in the study.
Regarding the strengths of the study, we used a representative sample of older adults admitted to the hospital, with almost 50% of them women, in contrast with other studies which used specific subgroups of older people. Additionally, the results show a clear correlation of the factors with a higher impact on malnutrition, and we also examined the primary reason why, since the sample data may influence the results.
The findings of the present research emphasize the urgent need for physicians and clinical institutions to be aware of the high prevalence of malnutrition in elderly patients, with a greater focus on women, and the influence of the admission information in malnutrition. Therefore, health experts should perform nutritional screening for all older adults as part of secondary prevention, with a special focus on more vulnerable populations, such as widowed elderly women who require assistance at home, those who are considered dependent as categorized by the Barthel index, or patients admitted in internal medicine with high DRG severity. To those who are more vulnerable, nutrition counseling and support should be offered.