Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
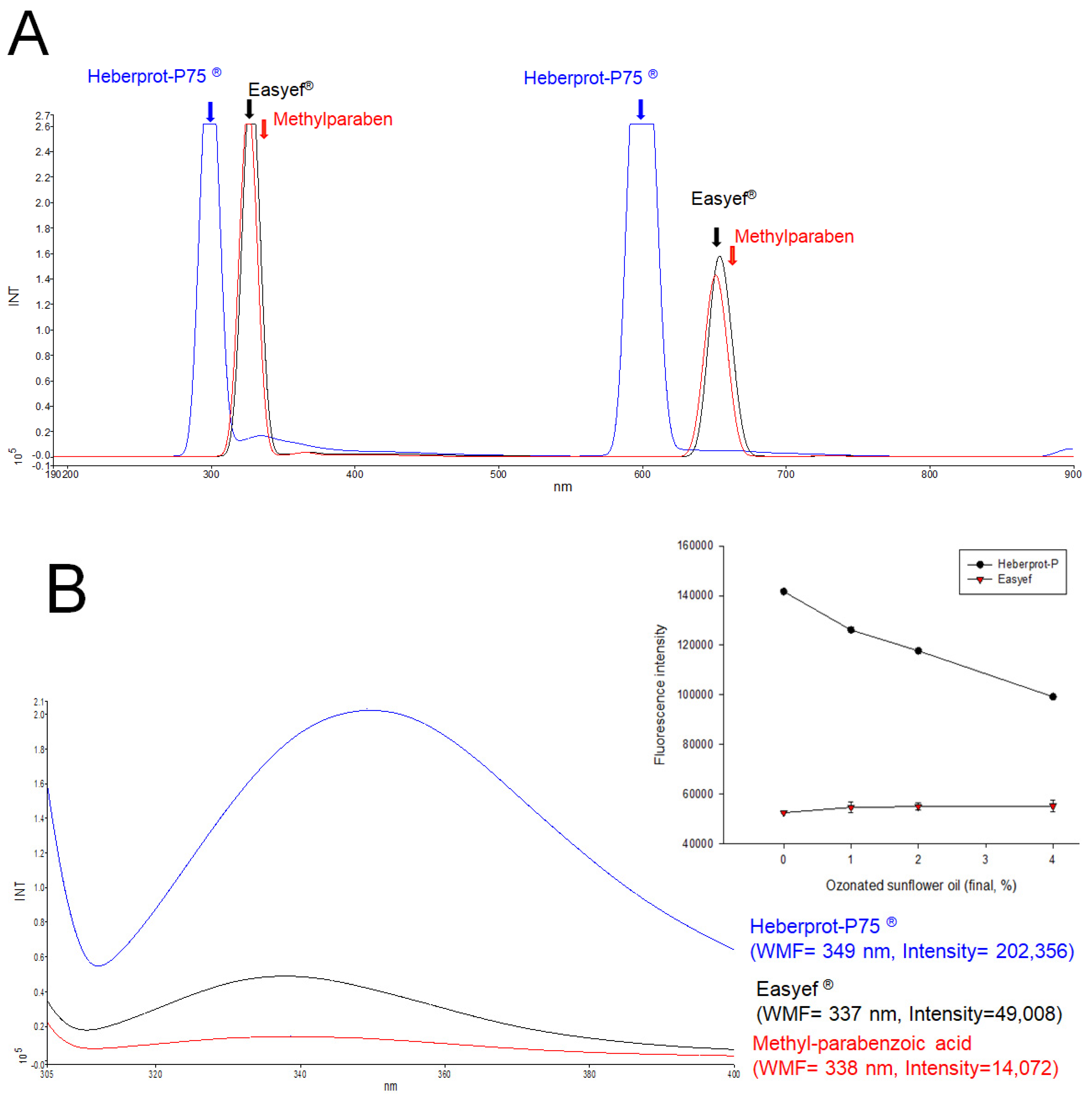
2.2. Fluorescence Spectroscopy
2.3. Zebrafish and Embryo
2.4. Water Exposure of Zebrafish Embryos
2.5. Microinjection of Zebrafish Embryos
2.6. Tail Fin Regeneration
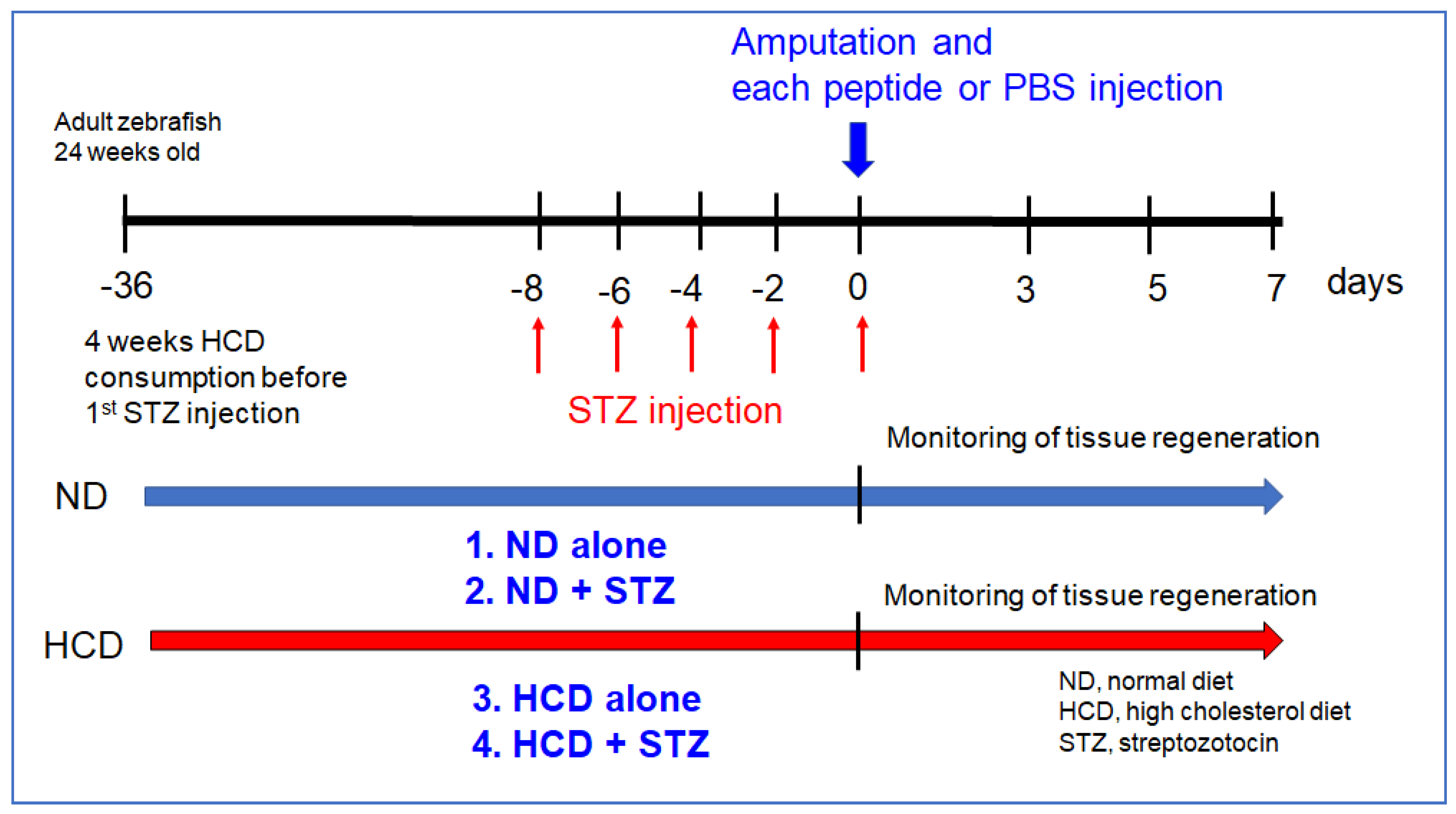
2.7. Experimental Design of Tail Fin Regeneration
2.8. Statistical Analysis
3. Results and Discussion
3.1. Comparison of the Fluorescence Emission Spectra
3.2. Developmental Speed after Water Exposure
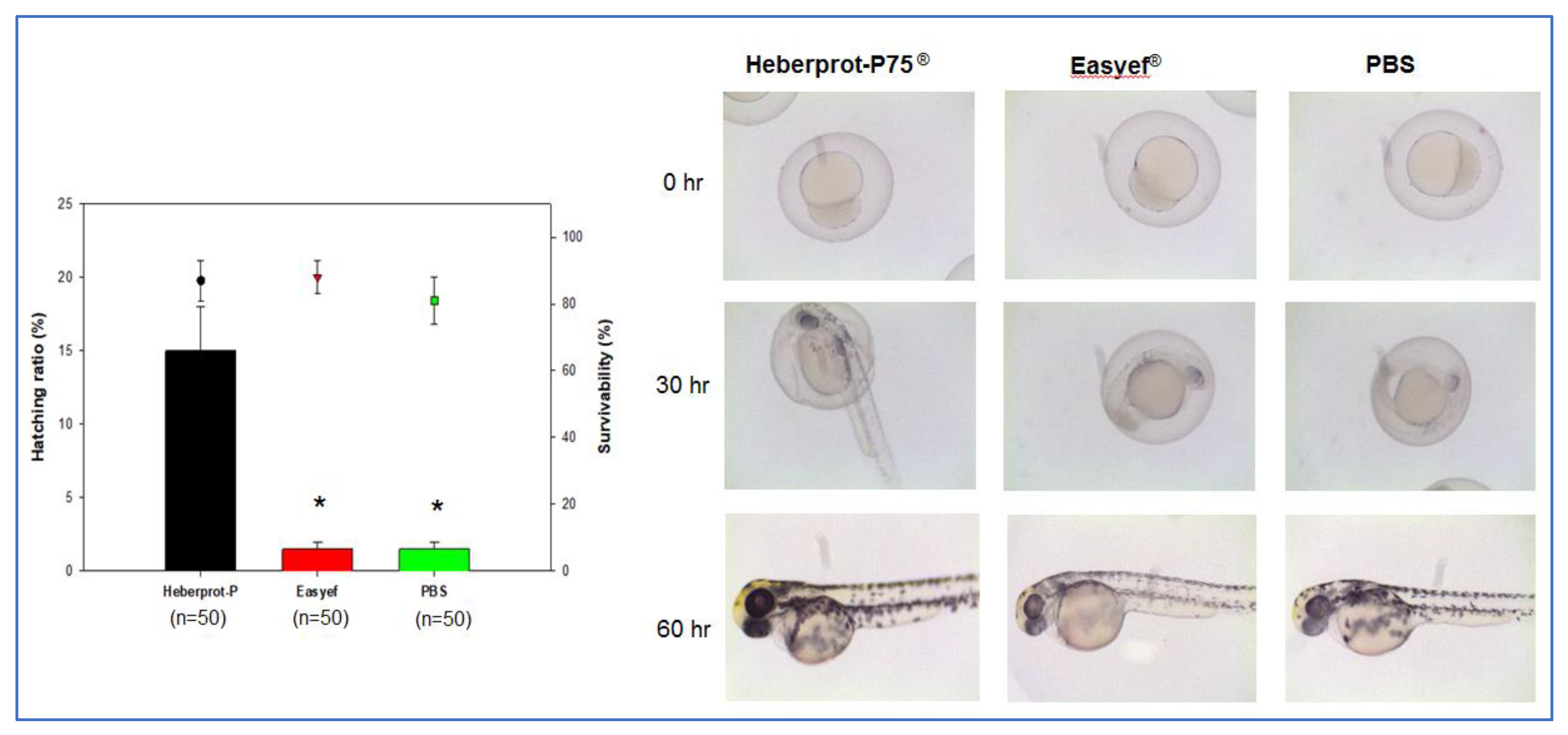
3.3. Embryo Development after Microinjection
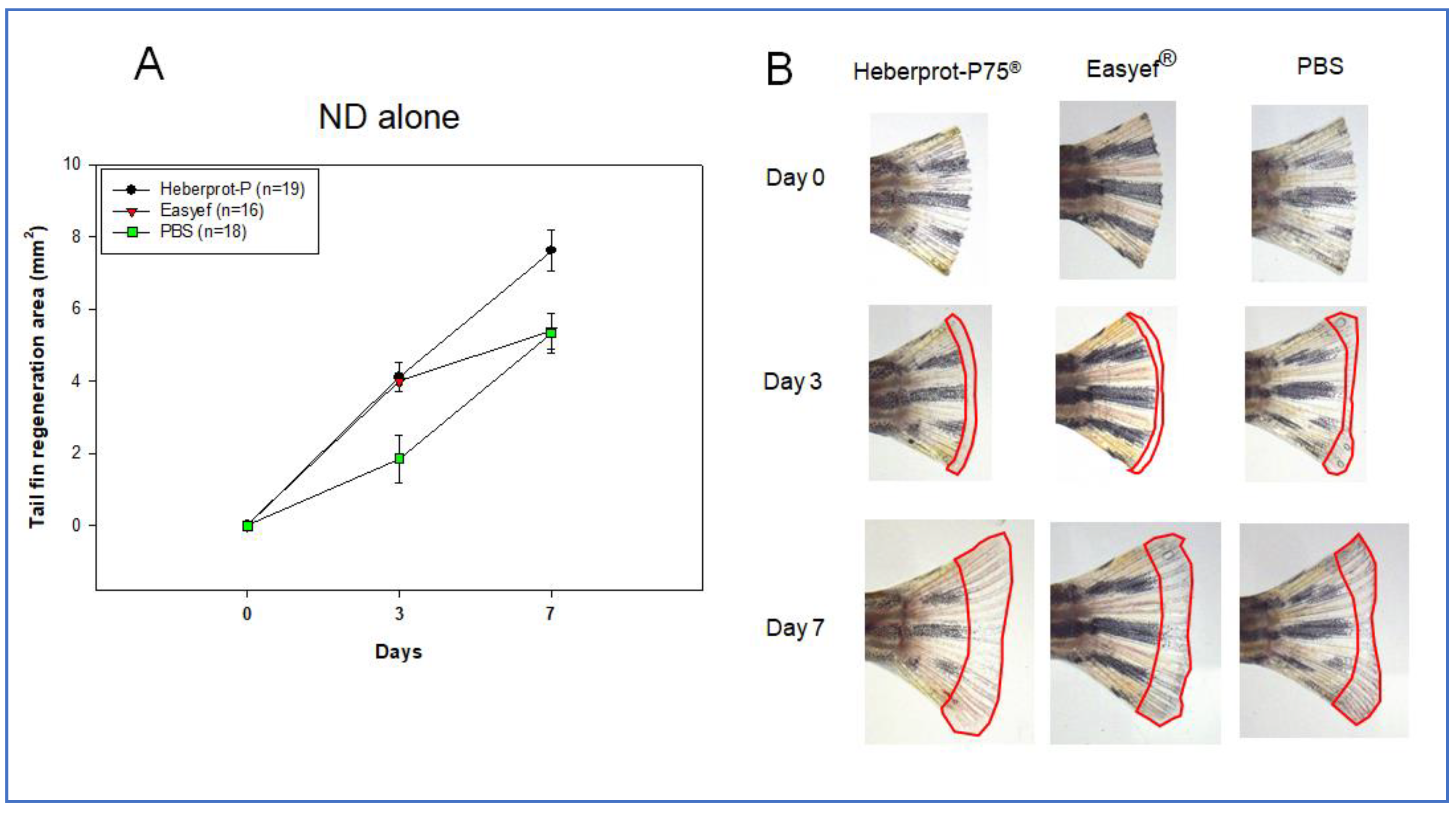
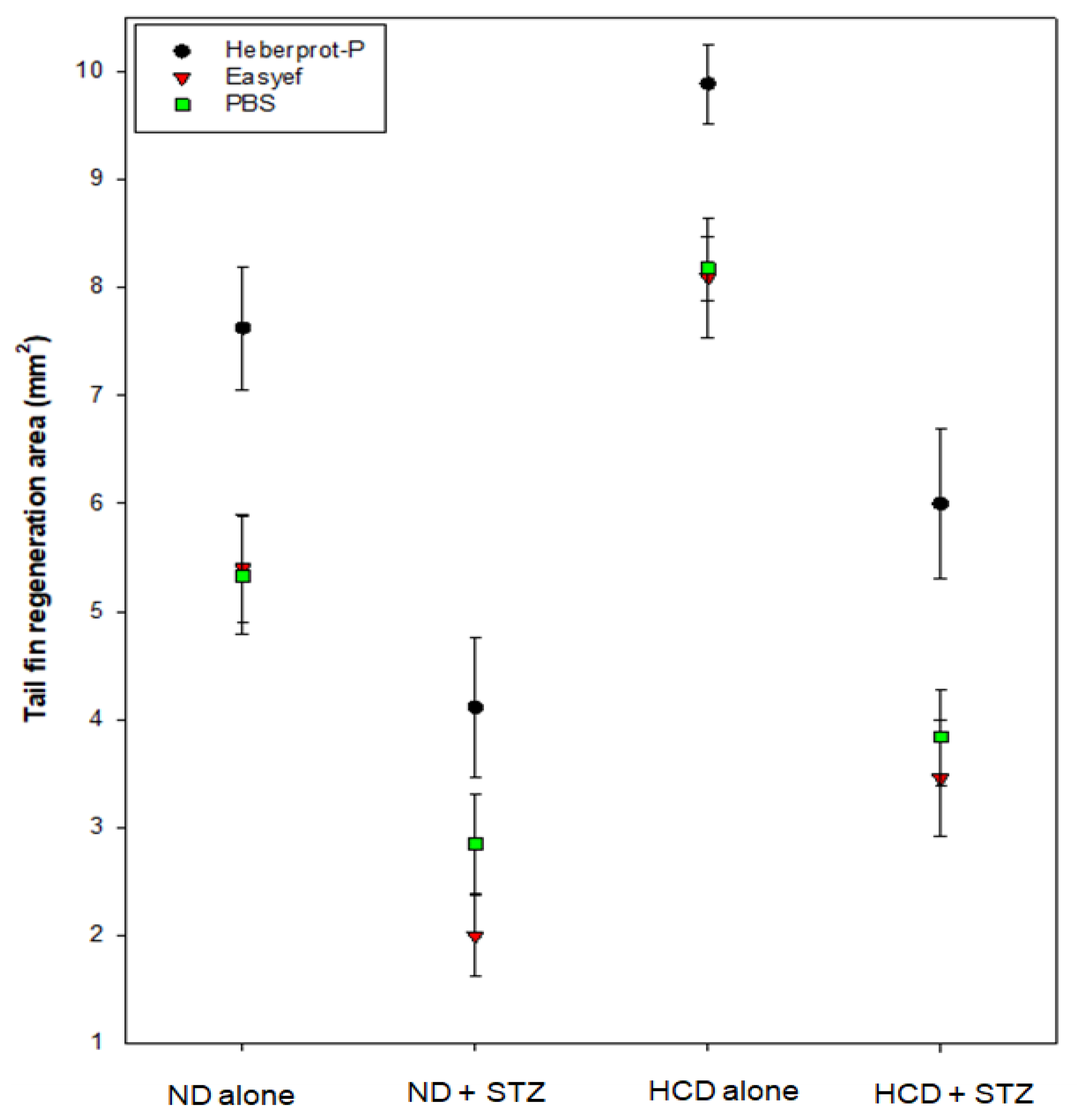
3.4. Tail Fin Regeneration under ND Consumption Alone
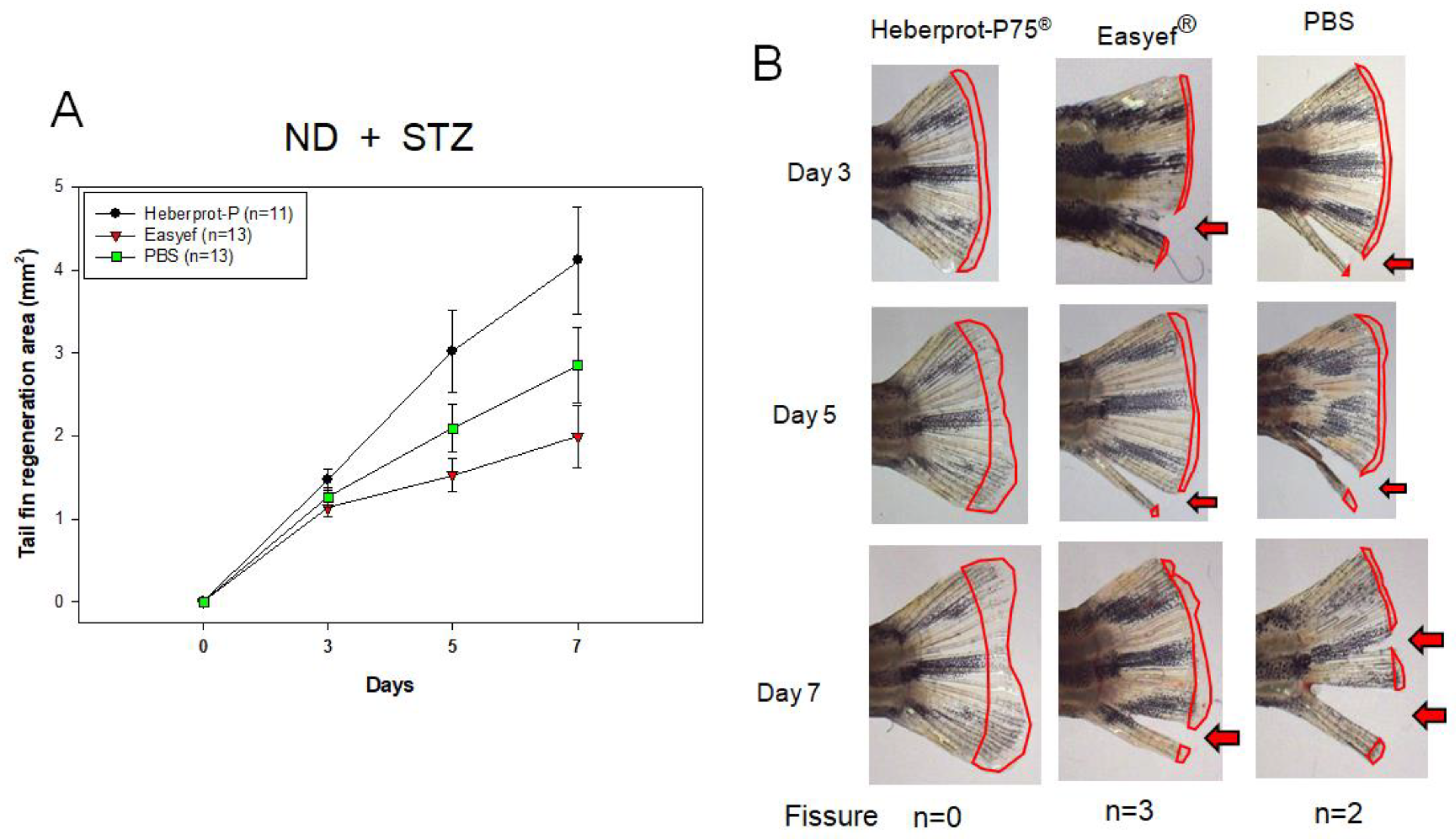
3.5. Tail Fin Regeneration under Diabetic Condition and ND Consumption
3.6. Tail Fin Regeneration under HCD Consumption Alone
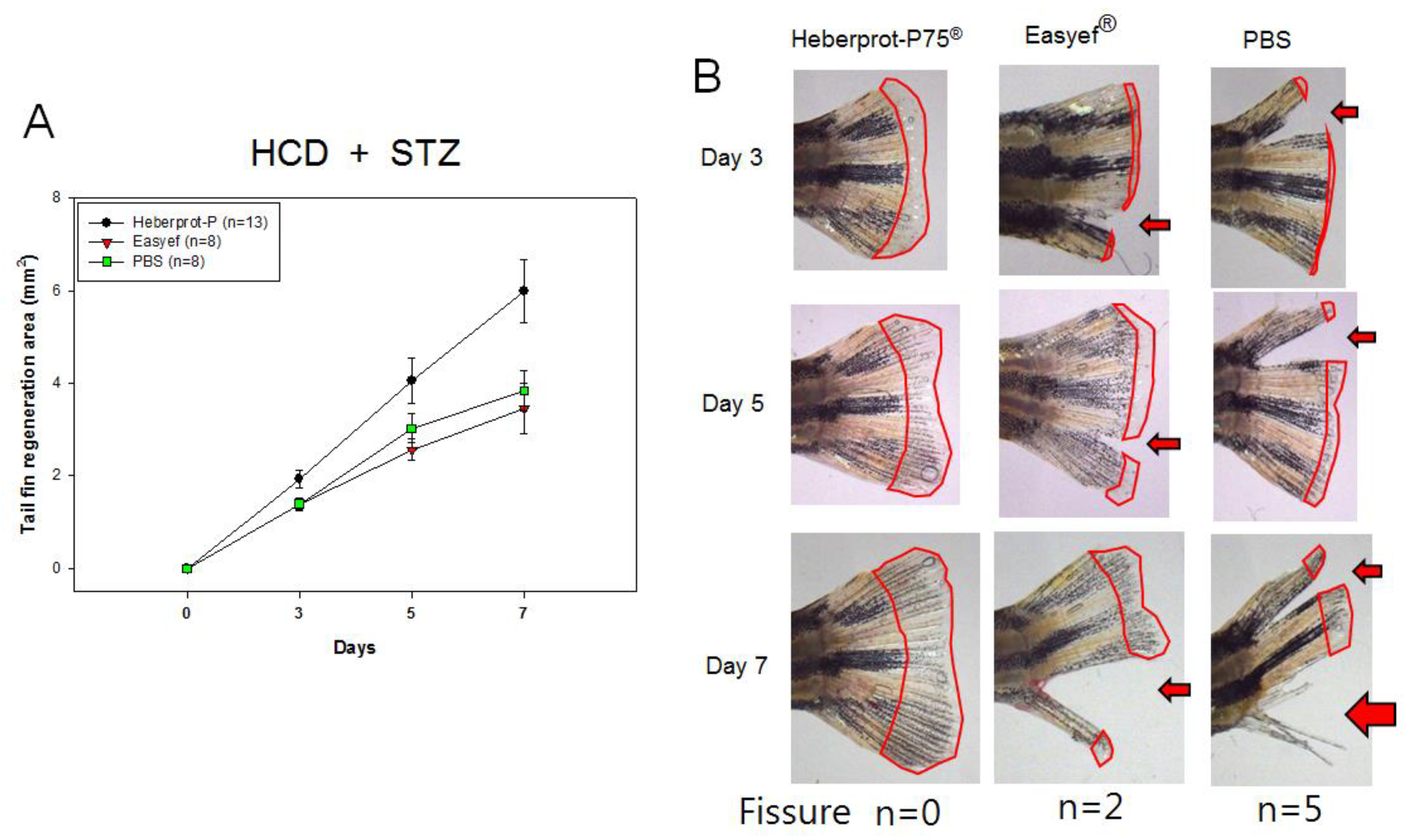
3.7. Tail Fin Regeneration under Diabetic Condition and HCD Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bui, T.Q.; Bui, Q.V.P.; Németh, D.; Hegyi, P.; Szakács, Z.; Rumbus, Z.; Tóth, B.; Emri, G.; Párniczky, A.; Sarlós, P.; et al. Epidermal growth factor is effective in the treatment of diabetic foot ulcers: Meta-analysis and systematic review. Int. J. Environ. Res. Public Health 2019, 16, 2584. [Google Scholar] [CrossRef] [Green Version]
- Eskens, O.; Amin, S. Challenges and effective routes for formulating and delivery of epidermal growth factors in skin care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Miller-Kobisher, B.; Suárez-Vega, D.V.; Velazco de Maldonado, G.J. Epidermal growth factor in aesthetics and regenerative medicine: Systematic review. J. Cutan. Aesthet. Surg. 2021, 14, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Braunagel, S.; Rosenblum, B.I. Growth factors in wound healing: The present and the future? Clin. Podiatr. Med. Surg. 2015, 32, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhou, Q.; Zeng, W.; Wu, L.; Zhao, S.; Chen, W.; Luo, C.; Shen, M.; Zhang, J.; Tang, C.E. Growth factors in the pathogenesis of diabetic foot ulcers. Front. Biosci. 2018, 23, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhang, Y.; Yin, H.; Lu, Y. Topical recombinant human epidermal growth factor for diabetic foot ulcers: A meta-analysis of randomized controlled clinical trials. Ann. Vasc. Surg. 2020, 62, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Lim, J.Z.; Ng, N.S.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Boulton, A.J. The pathway to foot ulceration in diabetes. Med. Clin. N. Am. 2013, 97, 775–790. [Google Scholar] [CrossRef]
- Dumantepe, M.; Fazliogullari, O.; Seren, M.; Uyar, I.; Basar, F. Efficacy of intralesional recombinant human epidermal growth factor in chronic diabetic foot ulcers. Growth Factors 2015, 33, 128–132. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Fernández-Montequín, J.; Valdés-Pérez, C.; Savigne-Gutiérrez, W.; Mendoza-Marí, Y.; García-Ojalvo, A.; Falcón-Cama, V.; García Del Barco-Herrera, D.; Fernández-Mayola, M.; Pérez-Saad, H.; et al. Diabetic foot ulcers and epidermal growth factor: Revisiting the local delivery route for a successful outcome. BioMed Res. Int. 2017, 2017, 2923759. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Han, S.H.; Hong, J.P.; Han, S.K.; Lee, D.H.; Kim, B.S.; Ahn, J.H.; Lee, J.W. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res. Clin. Pract. 2018, 142, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Tuyet, H.L.; Nguyen Quynh, T.T.; Vo Hoang Minh, H.; Thi Bich, D.N.; Do Dinh, T.; Le Tan, D.; Van, H.L.; Le Huy, T.; Doan Huu, H.; Tran Trong, T.N. The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: The preliminary results. Int. Wound J. 2009, 6, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tiaka, E.K.; Papanas, N.; Manolakis, A.C.; Georgiadis, G.S. Epidermal growth factor in the treatment of diabetic foot ulcers: An update. Perspect. Vasc. Surg. Endovasc. Ther. 2012, 24, 37–44. [Google Scholar] [CrossRef]
- Marques, I.J.; Lupi, E.; Mercader, N. Model systems for regeneration: Zebrafish. Development 2019, 146, dev167692. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Cho, K.H. A point mutant of apolipoprotein A-I (V156K) showed enhancement of cellular insulin secretion and potent activity of facultative regeneration in zebrafish. Rejuvenation Res. 2012, 15, 313–321. [Google Scholar] [CrossRef]
- Lee, E.Y.; Yoo, J.A.; Lim, S.M.; Cho, K.H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Cho, K.H. Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food Chem. Toxicol. 2011, 49, 1521–1529. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, S.J.; Kim, J.Y.; Kim, J.R.; Cho, K.H. Breast milk from smokers contains less cholesterol and protein and smaller size of apolipoprotein A-I resulting in lower zebrafish embryo survivability. Breastfeed. Med. 2017, 12, 365–372. [Google Scholar] [CrossRef]
- Park, K.H.; Cho, K.H. A zebrafish model for the rapid evaluation of pro-oxidative and inflammatory death by lipopolysaccharide, oxidized low-density lipoproteins, and glycated high-density lipoproteins. Fish Shellfish. Immunol. 2011, 31, 904–910. [Google Scholar] [CrossRef]
- Cohen, S.; Carpenter, G.; Lembach, K.J. Interaction of epidermal growth factor (EGF) with cultured fibroblasts. Adv. Metab. Disord. 1975, 8, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kang, D.J.; Nam, H.S.; Kim, J.H.; Kim, S.Y.; Lee, J.O.; Kim, B.J. Ozonated sunflower oil exerted protective effect for embryo and cell survival via potent reduction power and antioxidant activity in HDL with strong antimicrobial activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Davies, M. Free radicals, oxidants and protein damage. Aust. Biochem. 2012, 43, 8–12. [Google Scholar]
- Vladislav Victorovich, K.; Tatyana Aleksandrovna, K.; Victor Vitoldovich, P.; Aleksander Nicolaevich, S.; Larisa Valentinovna, K.; Anastasia Aleksandrovna, A. Spectra of tryptophan fluorescence are the result of co-existence of certain most abundant stabilized excited state and certain most abundant destabilized excited state. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 257, 119784. [Google Scholar] [CrossRef] [PubMed]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sargent, I.L.; Martin, K.L.; Barlow, D.H. The use of recombinant growth factors to promote human embryo development in serum-free medium. Hum. Reprod. 1998, 13 (Suppl. 4), 239–248. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.P.; Park, S.W. The combined effect of recombinant human epidermal growth factor and erythropoietin on full-thickness wound healing in diabetic rat model. Int. Wound J. 2014, 11, 373–378. [Google Scholar] [CrossRef]
- Cinza, A.M.; Quintana, M.; Lombardero, J.; Poutou, R.; Perez, E.; Perez, L.C.; Mella, C.M.; Besada, V.; Padron, G.; Castellanos, L.; et al. A batch process for production of human epidermal growth factor in yeast. Product characterization. Biotecnol. Apl. 1991, 8, 166–174. [Google Scholar]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Kim, J.-H.; Nam, H.-S.; Kang, D.-J. Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients. Geriatrics 2022, 7, 45. https://doi.org/10.3390/geriatrics7020045
Cho K-H, Kim J-H, Nam H-S, Kang D-J. Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients. Geriatrics. 2022; 7(2):45. https://doi.org/10.3390/geriatrics7020045
Chicago/Turabian StyleCho, Kyung-Hyun, Ju-Hyun Kim, Hyo-Seon Nam, and Dae-Jin Kang. 2022. "Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients" Geriatrics 7, no. 2: 45. https://doi.org/10.3390/geriatrics7020045
APA StyleCho, K.-H., Kim, J.-H., Nam, H.-S., & Kang, D.-J. (2022). Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients. Geriatrics, 7(2), 45. https://doi.org/10.3390/geriatrics7020045






