Inhalation Therapy with Nebulized Capsaicin in a Patient with Oropharyngeal Dysphagia Post Stroke: A Clinical Case Report
Abstract
:1. Introduction
2. Case Presentation
- Severity of dysphagia according to Bogenhausener Dysphagie Score (BODS) [27]: 6 points (moderate severity of dysphagia);
- Modified diet, recommending modified food texture at International Dysphagia Diet Standardization Initiative (IDDSI) [28] level 4 and thickened drinks at IDDSI level 2;
- Nutritional intake had initially been managed via nasogastric tube. Weaning from tube feeding had been carried out prior to discharge from the acute hospital;
- Dysphagia management included therapy according to Facial-Oral Tract Therapy (FOTT) [29], modified diet and assistance with feeding.
3. Investigations
Initial Speech and Language Therapy Assessment
- Reduced tongue motor skills with tongue deviation to the left.
- Velum asymmetry (reduced tone on the left) with rhinolalia during speech production.
- Weak/ineffective voluntary cough.
- Ineffective voluntary throat clearing.
- Continuous wet voice without effective expectoration.
- Patient unaware of wet voice and ineffective expectoration.
- Negative modified (IDDSI 2) 3-oz water swallow test [35].
- Central facial nerve palsy (left) with residual oral branch weakness.
- Dysarthrophonia.
- ROS 3 (laryngeal penetrations of secretion).
- PAS 5 for thickened water (IDDSI 2).
- PAS 3 for apple sauce (IDDSI 4).
- Sensory testing [36]: no patient reaction to light touch of the scope to the epiglottis, no pharyngeal constriction.
4. Dysphagia Management and Rehabilitation
4.1. Dysphagia Management
4.2. Rehabilitation of Facial Weakness and Dysphagia
4.3. Monitoring for Aspiration
- ROS 2 (up to 25% pooling of secretion in the valleculae and sinus piriformes).
- PAS 5 for thickened water (IDDSI 2).
- PAS 8 for thickened water (IDDSI 1).
- Sensory testing: no patient reaction to light touch of the scope to the epiglottis, minimal pharyngeal constriction.
- Highly inflamed/reddened mucous membrane and edema between arytenoid cartilage and vocal folds—possibly as a result of coughing and excessive throat clearing.
5. Inhalation Therapy with Nebulized Capsaicin
5.1. Indication
5.2. Preparation and Delivery
5.3. Observations
- Self-rated patient comfort (feeling thermometer [42]).
- Oxygen saturation (pulse oximetry).
- Sputum swallowing frequency per minute (SLT observation).
- Collection of intraoral saliva status (SLT observation).
- Quality of voice, e.g., wet voice (SLT observation).
6. Follow-Up and Outcomes
6.1. Clinician and Patient-Assessed Outcomes
6.2. Follow-Up Diagnostics
- ROS 0.
- PAS 1 for thickened water IDDSI 2.
- PAS 1 for thickened water IDDSI 1.
- PAS 3 for thin water IDDSI 0.
- Sensory testing: normal response to light touch of the scope to the epiglottis, prompt coughing.
- Mucous membranes unremarkable.
6.3. Intervention Adherence and Tolerability
6.4. Adverse and Unanticipated Events
6.5. Remaining Rehabilitation Stay and Discharge from Rehabilitation
7. Discussion
7.1. Scientific Rationale
7.2. Relevant Literature
7.3. Strengths and Limitations of This Report
7.4. Conclusions
8. Patient Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD. 2015 Risk Factors Collaborators Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R. Oropharyngeal Dysphagia. Gastroenterol. Hepatol. (N. Y.) 2006, 2, 633–634. [Google Scholar]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef]
- Jones, C.A.; Colletti, C.M.; Ding, M.-C. Post-Stroke Dysphagia: Recent Insights and Unanswered Questions. Curr. Neurol. Neurosci. Rep. 2020, 20, 61. [Google Scholar] [CrossRef]
- Eltringham, S.A.; Kilner, K.; Gee, M.; Sage, K.; Bray, B.D.; Smith, C.J.; Pownall, S. Factors Associated with Risk of Stroke-Associated Pneumonia in Patients with Dysphagia: A Systematic Review. Dysphagia 2020, 35, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after Stroke: Incidence, Diagnosis, and Pulmonary Complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [Green Version]
- Eltringham, S.A.; Kilner, K.; Gee, M.; Sage, K.; Bray, B.D.; Pownall, S.; Smith, C.J. Impact of Dysphagia Assessment and Management on Risk of Stroke-Associated Pneumonia: A Systematic Review. Cerebrovasc. Dis. 2018, 46, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Daniels, S.K.; Anderson, J.A.; Willson, P.C. Valid items for screening dysphagia risk in patients with stroke: A systematic review. Stroke 2012, 43, 892–897. [Google Scholar] [CrossRef]
- Toscano, M.; Viganò, A.; Rea, A. Sapienza Global Bedside Evaluation of Swallowing after Stroke: The GLOBE-3S study. Eur. J. Neurol. 2019, 26, 596–602. [Google Scholar] [CrossRef]
- Martino, R.; Silver, F.; Teasell, R. The Toronto Bedside Swallowing Screening Test (TOR-BSST): Development and validation of a dysphagia screening tool for patients with stroke. Stroke 2009, 40, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Suntrup-Krueger, S.; Minnerup, J.; Muhle, P. The Effect of Improved Dysphagia Care on Outcome in Patients with Acute Stroke: Trends from 8-Year Data of a Large Stroke Register. Cerebrovasc. Dis. 2018, 45, 101–108. [Google Scholar] [CrossRef]
- Jannini, T.B.; Ruggiero, M.; Viganò, A. The role of the Sapienza GLObal Bedside Evaluation of Swallowing after Stroke (GLOBE-3S) in the prevention of stroke-associated pneumonia (SAP). Neurol. Sci. 2022, 43, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, Y.; Hannawi, B.; Rao, C.P.V.; Suarez, J.I.; Bershad, E.M. Stroke-Associated Pneumonia: Major Advances and Obstacles. Cerebrovasc. Dis. 2013, 35, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, J.G.; Addington, W.R.; Fontana, G.A.; Stephens, R.E. Voluntary and Reflex Cough and the Expiration Reflex; Implications for Aspiration after Stroke. Pulm. Pharmacol. Ther. 2011, 24, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.; Rao, P.; Reilly, C.C.; Rafferty, G.F.; Polkey, M.I.; Kalra, L.; Moxham, J. Poor Cough Flow in Acute Stroke Patients Is Associated with Reduced Functional Residual Capacity and Low Cough Inspired Volume. BMJ Open Respir. Res. 2017, 4, e000230. [Google Scholar] [CrossRef] [PubMed]
- Addington, W.R.; Stephens, R.E.; Widdicombe, J.G.; Rekab, K. Effect of Stroke Location on the Laryngeal Cough Reflex and Pneumonia Risk. Cough 2005, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Kulnik, S.T.; Birring, S.S.; Hodsoll, J.; Moxham, J.; Rafferty, G.F.; Kalra, L. Higher Cough Flow Is Associated with Lower Risk of Pneumonia in Acute Stroke. Thorax 2016, 71, 474–475. [Google Scholar] [CrossRef] [Green Version]
- Masiero, S.; Pierobon, R.; Previato, C.; Gomiero, E. Pneumonia in Stroke Patients with Oropharyngeal Dysphagia: A Six-Month Follow-up Study. Neurol. Sci. 2008, 29, 139–145. [Google Scholar] [CrossRef]
- PubChem Capsaicin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1548943 (accessed on 21 January 2022).
- Mazzone, S.B. An Overview of the Sensory Receptors Regulating Cough. Cough 2005, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Morice, A.H. Inhalation Cough Challenge in the Investigation of the Cough Reflex and Antitussives. Pulm. Pharmacol. 1996, 9, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V. Experimentally Induced Cough. Pulm. Pharmacol. Ther. 2007, 20, 319–324. [Google Scholar] [CrossRef]
- Morice, A.H.; Fontana, G.A.; Belvisi, M.G.; Birring, S.S.; Chung, K.F.; Dicpinigaitis, P.V.; Kastelik, J.A.; McGarvey, L.P.; Smith, J.A.; Tatar, M.; et al. ERS Guidelines on the Assessment of Cough. Eur. Respir. J. 2007, 29, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V.; Alva, R.V. Safety of Capsaicin Cough Challenge Testing. Chest 2005, 128, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.J.; Kishore, A.K.; Vail, A.; Chamorro, A.; Garau, J.; Hopkins, S.J.; Di Napoli, M.; Kalra, L.; Langhorne, P.; Montaner, J.; et al. Diagnosis of Stroke-Associated Pneumonia: Recommendations From the Pneumonia in Stroke Consensus Group. Stroke 2015, 46, 2335–2340. [Google Scholar] [CrossRef]
- Bartolome, G.; Schröter-Morasch, H. Bogenhausener Untersuchungsprotokoll Für Die Klinische Schluckuntersuchung (KSU). In Schluckstörungen—Diagnostik und Rehabilitation; Elsevier: Munich, Germany, 2014; pp. 155–168. [Google Scholar]
- IDDSI. IDDSI Framework. Available online: https://iddsi.org/framework/ (accessed on 21 January 2022).
- Nusser-Müller-Busch, R.; Gampp Lehmann, K. Facial-Oral Tract Therapy (F.O.T.T.); Springer: Cham, Switzerland, 2021. [Google Scholar]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef]
- Kidd, D.; Stewart, G.; Baldry, J.; Johnson, J.; Rossiter, D.; Petruckevitch, A.; Thompson, A.J. The Functional Independence Measure: A Comparative Validity and Reliability Study. Disabil. Rehabil. 1995, 17, 10–14. [Google Scholar] [CrossRef]
- Perry, L.; Love, C.P. Screening for Dysphagia and Aspiration in Acute Stroke: A Systematic Review. Dysphagia 2001, 16, 7–18. [Google Scholar] [CrossRef]
- Ickenstein, G.W.; Prosiegel, M.; Höhlig, C.; Bräuer, G.; Koch, H.; Müller, R.; Becker, U.; Riecker, A. Standardisierung des Untersuchungsablaufs bei neurogener oropharyngealer Dysphagie (NOD)—Evaluation des NOD-Stufenkonzeptes. Aktuelle Neurol. 2009, 36, P496. [Google Scholar] [CrossRef]
- Suiter, D.M.; Leder, S.B. Clinical Utility of the 3-Ounce Water Swallow Test. Dysphagia 2008, 23, 244–250. [Google Scholar] [CrossRef]
- Langmore, S.E. Endoscopic Evaluation and Treatment of Swallowing Disorders, 2nd ed.; Thieme Medical Publishers: New York, NY, USA, 2001. [Google Scholar]
- Pluschinski, P.; Zaretsky, Y.; Almahameed, A.; Koseki, J.-C.; Leinung, M.; Girth, L.; Wagenblast, J.; Sader, R.; Stöver, T.; Hey, C. Secretion scale by Murray et al. for FEES®: Comparison of reliability and validity of the German long and short version. Nervenarzt 2014, 85, 1582–1587. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A Penetration-Aspiration Scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Smedes, F.; Heidmann, M.; Schäfer, C.; Fischer, N.; Stępień, A. The Proprioceptive Neuromuscular Facilitation-Concept; the State of the Evidence, a Narrative Review. Phys. Ther. Rev. 2016, 21, 17–31. [Google Scholar] [CrossRef]
- Ozsoy, U.; Ogut, E.; Sekerci, R.; Hizay, A.; Rink, S.; Angelov, D.N. Effect of Pulsed and Continuous Ultrasound Therapy on the Degree of Collateral Axonal Branching at the Lesion Site, Polyinnervation of Motor End Plates, and Recovery of Motor Function after Facial Nerve Reconstruction. Anat. Rec. (Hoboken) 2019, 302, 1314–1324. [Google Scholar] [CrossRef]
- Becker, R.; Nieczaj, R.; Egge, K.; Moll, A.; Meinhardt, M.; Schulz, R.-J. Functional Dysphagia Therapy and PEG Treatment in a Clinical Geriatric Setting. Dysphagia 2011, 26, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Büsching, G. Den Gesundheitszustand einschätzen—Feeling Thermometer. Ergopraxis 2015, 8, 38–39. [Google Scholar] [CrossRef]
- Kulnik, S.T. Could Reflex Cough Induced through Nebulized Capsaicin Achieve Airway Clearance in Patients with Acute Retention of Lung Secretions? Med. Hypotheses 2018, 119, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.M. Perspective on the Human Cough Reflex. Cough 2011, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, G.A.; Lavorini, F. Cough Motor Mechanisms. Respir. Physiol. Neurobiol. 2006, 152, 266–281. [Google Scholar] [CrossRef]
- Dimyan, M.A.; Cohen, L.G. Neuroplasticity in the Context of Motor Rehabilitation after Stroke. Nat. Rev. Neurol. 2011, 7, 76–85. [Google Scholar] [CrossRef]
- Toukan, N.; Kulnik, S.T.; Lewko, A.; ElShaer, A. Therapeutic applications of capsaicin in humans to target conditions of the respiratory system: A scoping review. Respir. Med. 2022, 106772. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Yang, L.; Shen, F.; Ma, C.; Shen, M. Effect of Capsaicin Atomization-Induced Cough on Sputum Excretion in Tracheotomized Patients After Hemorrhagic Stroke: A Randomized Controlled Trial. J. Speech Lang. Hear. Res. 2021, 64, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Jinnouchi, O.; Agawa, S.; Kondo, E.; Kawata, I.; Okamoto, H.; Azuma, T.; Sato, G.; Kitamura, Y.; Abe, K.; et al. Daily Auricular Stimulation with Capsaicin Ointment Improved Cough Reflex Sensitivity in Elderly Patients with Dysphagia: A Pilot Study. Acta Otolaryngol. 2020, 140, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Sekizawa, K.; Nakazawa, H.; Sasaki, H. Capsaicin and Swallowing Reflex. Lancet 1993, 341, 432. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Martin, A.; Clavé, P. Natural Capsaicinoids Improve Swallow Response in Older Patients with Oropharyngeal Dysphagia. Gut 2013, 62, 1280–1287. [Google Scholar] [CrossRef]
- Ebihara, T.; Takahashi, H.; Ebihara, S.; Okazaki, T.; Sasaki, T.; Watando, A.; Nemoto, M.; Sasaki, H. Capsaicin Troche for Swallowing Dysfunction in Older People. J. Am. Geriatr. Soc. 2005, 53, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Yin, Q.; Wu, C.; Shen, M.; Zhang, Y.; Ma, C.; Zhang, H.; Shen, F. Capsaicin Combined with Ice Stimulation Improves Swallowing Function in Patients with Dysphagia after Stroke: A Randomised Controlled Trial. J. Oral. Rehabil. 2020, 47, 1297–1303. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Fang, Q.; Shen, M.; Zhang, L.; Liu, X. Effects of Capsaicin on Swallowing Function in Stroke Patients with Dysphagia: A Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1744–1751. [Google Scholar] [CrossRef]
- Daramola, O.O.; Rhee, J.S. Rating Evidence in Medical Literature. Virtual Mentor. 2011, 13, 46–51. [Google Scholar] [CrossRef]
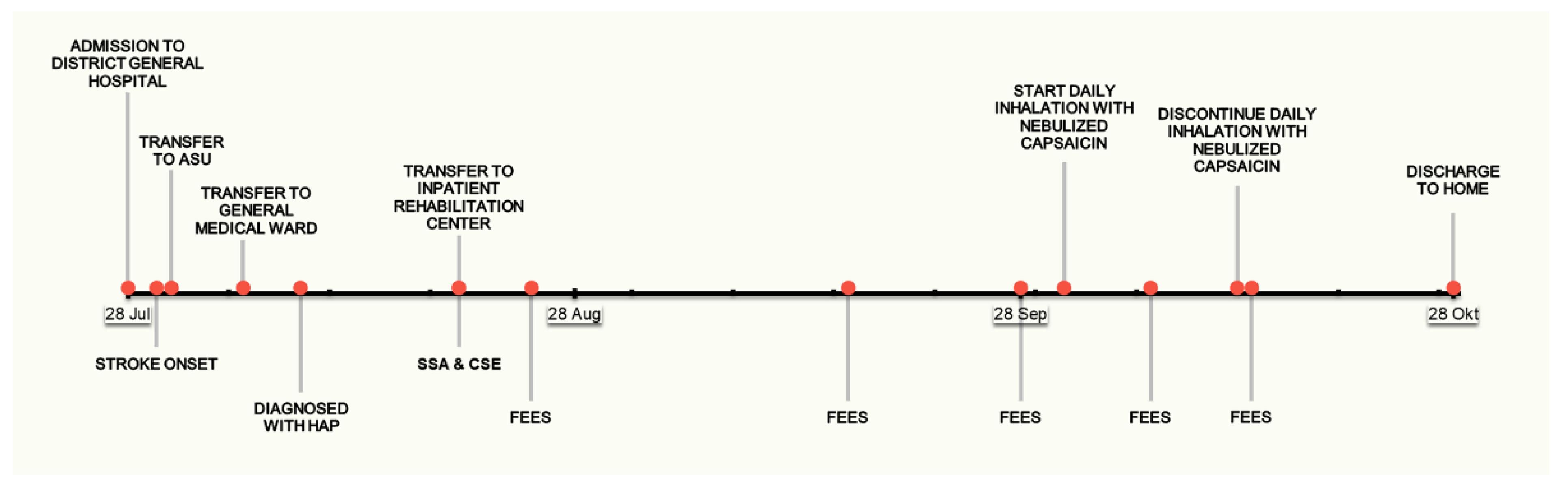
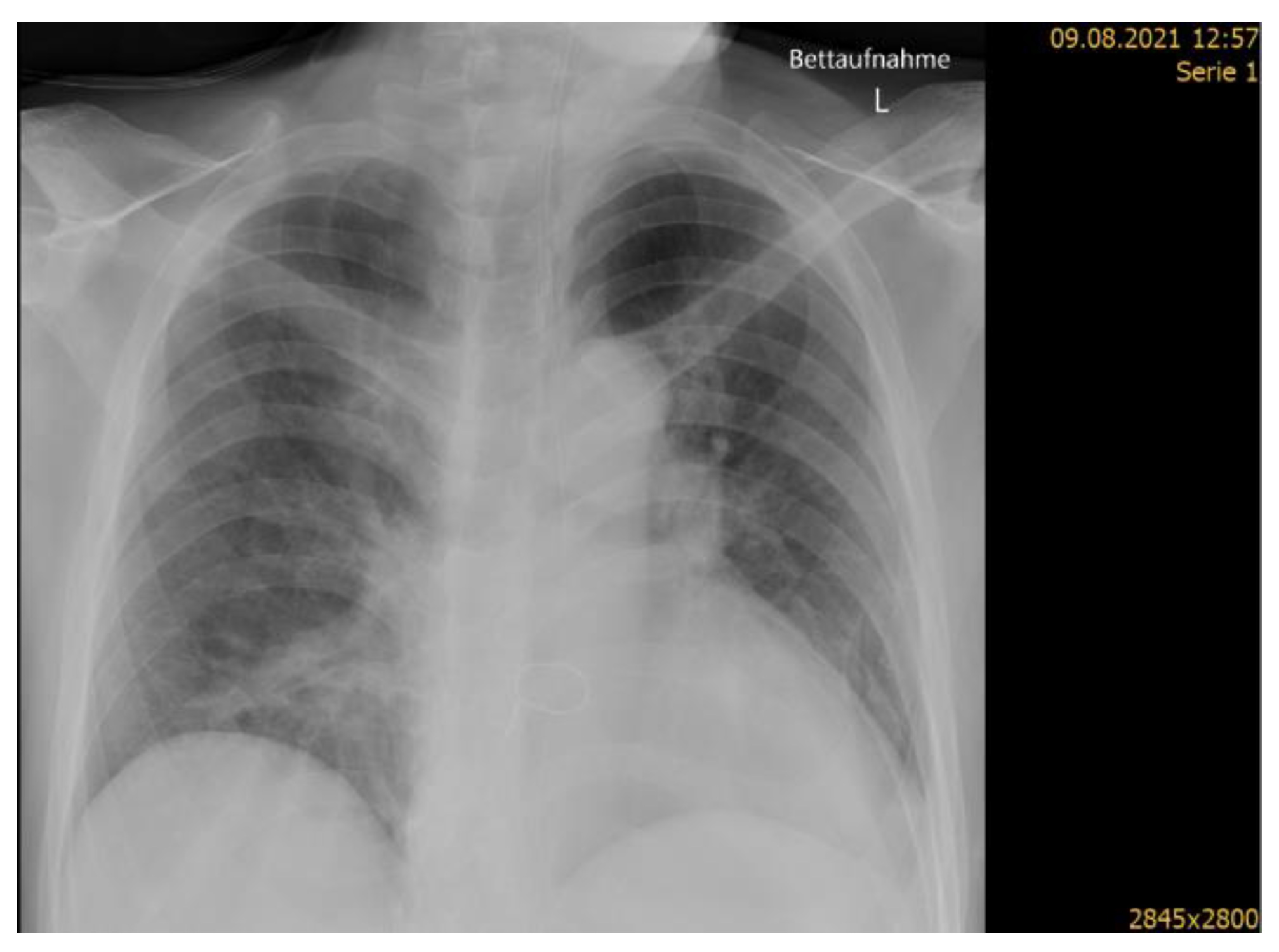
| Measure | Admission to DGH | Stroke Onset | Admission to ASU | Discharge from Acute Hospital |
|---|---|---|---|---|
| NIHSS (points) | n/a | 25/42 | 15/42 | 1/42 |
| mRS (points) | 0 | 5 | 5 | 4 |
| Time Point | Observation | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | Session 7 | Session 8 |
|---|---|---|---|---|---|---|---|---|---|
| Pre-inhalation | Self-rated patient comfort * | 2 | 6 | 5 | 6 | 6 | 5 | 6 | 5 |
| SpO2 (%) | 94 | 92 | 95 | 92 | 92 | 92 | 92 | 93 | |
| Sputum swallowing frequency (swallows per minute) | 1 | 0 | 1 | 13.1 | 1 | 0 | 1 | 0 | |
| Intraoral saliva (i.s.) status | Little i.s. | Little i.s. | No excess i.s | Right cheek accumulating i.s | No excess i.s | Foamy saliva at soft palate | Foamy saliva and coated tongue | Unremarkable | |
| Quality of voice | Wet voice | Normal | Normal | Normal | Normal | Wet voice | Normal | Normal | |
| During inhalation | Coughing | Observed, prompt, throughout | |||||||
| Throat clearing | Observed, prompt, throughout | ||||||||
| Reflex swallow | Observed, prompt, throughout | ||||||||
| Other observations | Coughed and cleared respiratory secretions | None | Bronchial secretion expectorated, runny nose, mild burning sensation | Runny nose, coughed and cleared secretions, mild burning sensation | Runny nose, moderate burning sensation | Runny nose, continuous throat clearing, wet voice improved | Increasing secretions, adequate throat clearing | Adequate throat clearing, strong burning sensation | |
| Post-inhalation | Self-rated patient comfort | 5 | 4 | 5 | 6 | 6 | 6 | 7 | 5 |
| SpO2 (%) | 90 | 91 | 91 | 90 | 90 | 91 | 91 | 91 | |
| Sputum swallowing frequency (swallows per minute) | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | |
| Intraoral saliva status | Unremarkable | Unremarkable | Unremarkable | Foamy saliva | Unremarkable | Unremarkable | Unremarkable | Unremarkable | |
| Quality of voice | Wet voice improved | Wet voice, immediately clearing | Adequate clearing | Normal | Normal | Wet voice, adequately clearing | Normal | Normal | |
| Observation | 25 August 2021 | 16 September 2021 | 28 September 2021 | 7 October 2021 | 14 October 2021 |
|---|---|---|---|---|---|
| ROS | 3 | 3 | 2 | 1 | 0 |
| PAS at IDDSI level 6 | n/a | n/a | 5 | 3 | 1 |
| PAS at IDDSI level 4 | 3 | 3 | 3 | 3 | 1 |
| PAS at IDDSI level 2 | 5 | 5 | 5 | 5 | 1 |
| PAS at IDDSI level 1 | n/a | n/a | 8 | 8 | 1 |
| PAS at IDDSI level 0 | n/a | n/a | n/a | n/a | 3 |
| Sensory test | None | None | Minimal response (pharyngeal constriction), redness | Moderate response (pharyngeal constriction) | Normal response (prompt coughing) |
| Diet Recommendation | IDDSI 4 | IDDSI 4 | IDDSI 4 | IDDSI 5 | IDDSI 6 |
| Fluids Recommendation | IDDSI 2 | IDDSI 2 | IDDSI 2 | IDDSI 2 | IDDSI 0 |
| Mealtime supervision (staff to patient ratio) | 1:1 | 1:2 | 1:2 | None | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekacka-Egli, A.M.; Herrmann, J.; Spielmanns, M.; Goerg, A.; Schulz, K.; Zenker, E.; Windisch, W.; Kulnik, S.T. Inhalation Therapy with Nebulized Capsaicin in a Patient with Oropharyngeal Dysphagia Post Stroke: A Clinical Case Report. Geriatrics 2022, 7, 27. https://doi.org/10.3390/geriatrics7020027
Pekacka-Egli AM, Herrmann J, Spielmanns M, Goerg A, Schulz K, Zenker E, Windisch W, Kulnik ST. Inhalation Therapy with Nebulized Capsaicin in a Patient with Oropharyngeal Dysphagia Post Stroke: A Clinical Case Report. Geriatrics. 2022; 7(2):27. https://doi.org/10.3390/geriatrics7020027
Chicago/Turabian StylePekacka-Egli, Anna Maria, Jana Herrmann, Marc Spielmanns, Arthur Goerg, Katharina Schulz, Eveline Zenker, Wolfram Windisch, and Stefan Tino Kulnik. 2022. "Inhalation Therapy with Nebulized Capsaicin in a Patient with Oropharyngeal Dysphagia Post Stroke: A Clinical Case Report" Geriatrics 7, no. 2: 27. https://doi.org/10.3390/geriatrics7020027
APA StylePekacka-Egli, A. M., Herrmann, J., Spielmanns, M., Goerg, A., Schulz, K., Zenker, E., Windisch, W., & Kulnik, S. T. (2022). Inhalation Therapy with Nebulized Capsaicin in a Patient with Oropharyngeal Dysphagia Post Stroke: A Clinical Case Report. Geriatrics, 7(2), 27. https://doi.org/10.3390/geriatrics7020027







