Factors Influencing the Development of Mild Cognitive Impairment in Community-Dwelling People Aged 75 Years and Older
Abstract
:1. Introduction
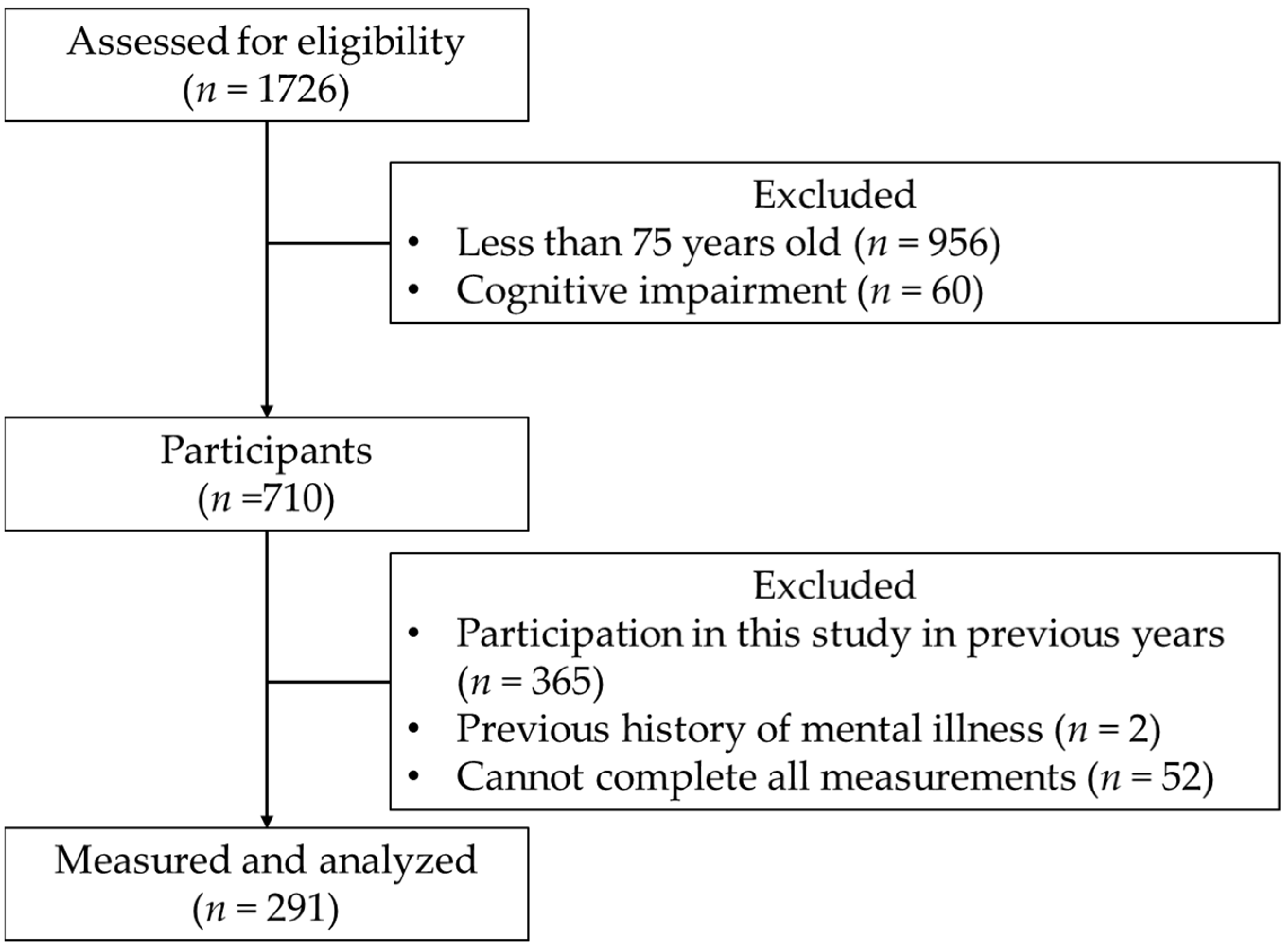
2. Materials and Methods
- (1)
- Older adults aged ≥ 75 years;
- (2)
- Individuals not suspected to have cognitive decline, measured via an MMSE score ≥ 24 [14].
- (1)
- Participation in this study in previous years;
- (2)
- Past medical history of mental illness;
- (3)
- Inability to complete the measurement of all items.
3. Results
4. Discussion
- (1)
- (2)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization and Alzheimer’s Disease International. Dementia: A Public Health Priority; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Campos, L. A Perspective on the OECD Report “Health at a Glance 2017”. Acta Med. Port. 2018, 31, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Ohara, T.; Hata, J.; Shibata, M.; Hirakawa, Y.; Honda, T.; Furuta, Y.; Oishi, E.; Sakata, S.; Kanba, S.; et al. Lifetime cumulative incidence of dementia in a community-dwelling elderly population in Japan. Neurology 2020, 95, e508–e518. [Google Scholar] [CrossRef]
- Sado, M.; Ninomiya, A.; Shikimoto, R.; Ikeda, B.; Baba, T.; Yoshimura, K.; Mimura, M. The estimated cost of dementia in Japan, the most aged society in the world. PLoS ONE 2018, 13, e0206508. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Epidemiology and Impact of Dementia: Current State and Future Trends. Available online: https://www.who.int/mental_health/neurology/dementia/dementia_thematicbrief_epidemiology.pdf (accessed on 26 October 2021).
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C. Mild Cognitive Impairment. N. Eng. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef] [Green Version]
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Sanford, A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef]
- Pandhita, S.G.; Sutrisna, B.; Wibowo, S.; Adisasmita, A.C.; Rahardjo, T.B.W.; Amir, N.; Rustika, R.; Kosen, S.; Syarif, S.; Wreksoatmodjo, B.R. Decision tree clinical algorithm for screening of mild cognitive impairment in the elderly in primary health care: Development, test of accuracy, and time-effectiveness analysis. Neuroepidemiology 2020, 54, 243–250. [Google Scholar] [CrossRef]
- Ogawa, M.; Sone, D.; Maruo, K.; Shimada, H.; Suzuki, K.; Watanabe, H.; Matsuda, H.; Mizusawa, H. Analysis of risk factors for mild cognitive impairment based on word list memory test results and questionnaire responses in healthy Japanese individuals registered in an online database. PLoS ONE 2018, 13, e0197466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H.; Suzuki, T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Doi, T.; Lee, S.; Makizako, H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: A 4-year longitudinal study. Alzheimers Res. Ther. 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugishita, M.; Hemmi, I.; Takeuchi, T. Reexamination of the Validity and Reliability of the Japanese Version of the Mini-Mental State Examination (MMSE-J). Jpn. J. Cogn. Neurosci. 2016, 18, 168–183. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Ideno, Y.; Takayama, M.; Hayashi, K.; Takagi, H.; Sugai, Y. Evaluation of a Japanese version of the Mini-Mental State Examination in elderly persons. Geriatr. Gerontol. Int. 2012, 12, 310–316. [Google Scholar] [CrossRef]
- Bartos, A.; Raisova, M. The Mini-Mental State Examination: Czech Norms and Cutoffs for Mild Dementia and Mild Cognitive Impairment due to Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2016, 42, 50–57. [Google Scholar] [CrossRef]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Hoyl, M.T.; Alessi, C.A.; Harker, J.O.; Josephson, K.R.; Pietruszka, F.M.; Koelfgen, M.; Mervis, J.R.; Fitten, L.J.; Rubenstein, L.Z. Development and testing of a five-item version of the Geriatric Depression Scale. J. Am. Geriatr. Soc. 1999, 47, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Murata, C.; Hirai, H.; Kondo, N.; Kondo, K.; Ueda, K.; Ichida, N. Predictive validity of GDS5 using AGES project data. Kousei no Shihyou 2014, 61, 7–12. (In Japanese) [Google Scholar]
- Abe, T.; Yaginuma, Y.; Fujita, E.; Thiebaud, R.S.; Kawanishi, M.; Akamine, T. Associations of sit-up ability with sarcopenia classification measures in Japanese older women. Interv. Med. Appl. Sci. 2016, 8, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Nadamoto, M.; Mimura, K.-I.; Itoh, M. Validation of a 30-sec chair-stand test for evaluating lower extremity muscle strength in Japanese elderly adults. Taiikugaku kenkyu (Jpn. J. Phys. Educ. Health Sport Sci.) 2002, 47, 451–461. [Google Scholar] [CrossRef]
- Macrae, P.G.; Lacourse, M.; Moldavon, R. Physical performance measures that predict faller status in community-dwelling older adults. J. Orthop. Sports Phys. Ther. 1992, 16, 123–128. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Amano, T.; Tanaka, S.; Ito, H.; Morikawa, S.; Uchida, S. Quantifying walking ability in Japanese patients with knee osteoarthritis: Standard values derived from a multicenter study. J. Orthop. Sci. 2018, 23, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys. Ther. 1986, 66, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Souma, M.; Murata, S.; Iwase, H.; Murata, J.; Kamijou, K.; Kubo, A.; Edo, A. Relationship between Performance in the 30-sec Chair-Stand Test and Physical Function of Community-dwelling Elderly People. J. Phys. Ther. Sci. 2016, 31, 759–763. [Google Scholar]
- Nonaka, K.; Murata, S.; Shiraiwa, K.; Abiko, T.; Nakano, H.; Iwase, H.; Naito, K.; Horie, J. Physical Characteristics Vary According to Body Mass Index in Japanese Community-Dwelling Elderly Women. Geriatrics 2018, 3, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyahara, Y.; Kamijo, K.; Inoue, T.; Tanaka, J.; Nando, M.; Nakamura, T. A study of cognitive function in elderly people living in the community. J. Health Welf. Stat. 2017, 64, 1–4. (In Japanese) [Google Scholar]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Kaneko, Y.; Motohashi, Y. Male Gender and Low Education with Poor Mental Health Literacy: A Population-based Study. J. Epidemiol. 2007, 17, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, Y.; Okubo, T.; Yanai, H.; Doba, N.; Paasche-Orlow, M.K. Development and Validation of a 15-Item Japanese Health Knowledge Test. J. Epidemiol. 2010, 20, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svendsen, M.T.; Bak, C.K.; Sørensen, K.; Pelikan, J.; Riddersholm, S.J.; Skals, R.K.; Mortensen, R.N.; Maindal, H.T.; Bøggild, H.; Nielsen, G.; et al. Associations of health literacy with socioeconomic position, health risk behavior, and health status: A large national population-based survey among Danish adults. BMC Public Health 2020, 20, 565. [Google Scholar] [CrossRef] [PubMed]
- Aaby, A.; Friis, K.; Christensen, B.; Rowlands, G.; Maindal, H.T. Health literacy is associated with health behaviour and self-reported health: A large population-based study in individuals with cardiovascular disease. Eur. J. Prev. Cardiol. 2017, 24, 1880–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokokawa, H.; Fukuda, H.; Yuasa, M.; Sanada, H.; Hisaoka, T.; Naito, T. Association between health literacy and metabolic syndrome or healthy lifestyle characteristics among community-dwelling Japanese people. Diabetol. Metab. Syndr. 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wu, Y.; Zhang, D.; Nie, J. Associations between heart failure and risk of dementia: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2020, 99, e18492. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Hering, D. Hypertension and cognitive dysfunction in elderly: Blood pressure management for this global burden. BMC Cardiovasc. Disord. 2016, 16, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- Bahureksa, L.; Najafi, B.; Saleh, A.; Sabbagh, M.; Coon, D.; Mohler, M.J.; Schwenk, M. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology 2017, 63, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Kuan, Y.C.; Huang, L.K.; Wang, Y.H.; Hu, C.J.; Tseng, I.J.; Chen, H.C.; Lin, L.F. Balance and gait performance in older adults with early-stage cognitive impairment. Eur. J. Phys. Rehabil. Med. 2020, 57, 560–567. [Google Scholar]
- Yoon, B.; Choi, S.H.; Jeong, J.H.; Park, K.W.; Kim, E.J.; Hwang, J.; Jang, J.W.; Kim, H.J.; Hong, J.Y.; Lee, J.M.; et al. Balance and Mobility Performance Along the Alzheimer’s Disease Spectrum. J. Alzheimers Dis. 2020, 73, 633–644. [Google Scholar] [CrossRef]
- Sabia, S.; Dugravot, A.; Dartigues, J.F.; Abell, J.; Elbaz, A.; Kivimäki, M.; Singh-Manoux, A. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ 2017, 357, j2709. [Google Scholar] [CrossRef] [Green Version]
- Goto, E.; Ishikawa, H.; Okuhara, T.; Kiuchi, T. Relationship of health literacy with utilization of health-care services in a general Japanese population. Prev. Med. Rep. 2019, 14, 100811. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Williamson, J. Blood Pressure and Statin Effects on Cognition: A Review. Curr. Hypertens. Rep. 2019, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Penn, I.W.; Sung, W.H.; Lin, C.H.; Chuang, E.; Chuang, T.Y.; Lin, P.H. Effects of individualized Tai-Chi on balance and lower-limb strength in older adults. BMC Geriatr. 2019, 19, 235. [Google Scholar] [CrossRef] [Green Version]
- Hishikawa, N.; Takahashi, Y.; Fukui, Y.; Tokuchi, R.; Furusawa, J.; Takemoto, M.; Sato, K.; Yamashita, T.; Ohta, Y.; Abe, K. Yoga-plus exercise mix promotes cognitive, affective, and physical functions in elderly people. Neurol. Res. 2019, 41, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
| Variable | MCI | Non-MCI | p-Value | |
|---|---|---|---|---|
| (n = 138) | (n = 153) | |||
| Attribute | Age (years) | 79.49 ± 3.67 | 78.79 ± 2.88 | 0.25 |
| Gender: Male/Female (n) * | 42/96 | 47/106 | 1.00 | |
| Height (cm) | 152.5 ± 8.6 | 153.63 ± 8.5 | 0.25 | |
| Weight (kg) ☨ | 52.26 ± 10.18 | 53.77 ± 8.94 | 0.18 | |
| BMI (kg/m2) ☨ | 22.32 ± 3.16 | 22.76 ± 3.21 | 0.24 | |
| Educational history (years) | 10.9 ± 2.83 | 11.86 ± 2.3 | < 0.01 | |
| Medical visits due to disease | Hypertension: yes/no (n) * | 48/90 | 78/75 | 0.01 |
| Hyperlipidemia: yes/no (n) * | 14/124 | 22/131 | 0.29 | |
| Diabetes: yes/no (n) * | 35/103 | 32/121 | 0.40 | |
| Cerebrovascular disease: yes/no (n) * | 19/119 | 21/132 | 1.00 | |
| Cardiovascular disease: yes/no (n) * | 10/128 | 19/134 | 0.17 | |
| Cancer: yes/no (n) * | 4/134 | 1/152 | 0.19 | |
| Mental and physical indicator | GDS-5 (score) | 0.71 ± 0.96 | 0.63 ± 0.92 | 0.41 |
| Sit-up (repetitions) | 5.95 ± 5.65 | 6.95 ± 6.08 | 0.20 | |
| CS-30 (cycles) | 17.2 ± 5.51 | 18.24 ± 5.02 | 0.05 | |
| OLST (s) | 21.95 ± 26.65 | 32.69 ± 33.62 | < 0.01 | |
| TUG (s) | 6.8 ± 1.81 | 6.35 ± 1.3 | 0.04 | |
| 5-m walking time (s) | 2.98 ± 0.95 | 2.83 ± 0.56 | 0.40 | |
| Grip strength (kg) | 25.41 ± 7.43 | 26.24 ± 6.83 | 0.19 | |
| Knee-extension strength (kg) | 18.14 ± 6.49 | 19.32 ± 6.39 | 0.15 | |
| Toe-grip strength (kg) | 8.42 ± 3.98 | 8.19 ± 3.64 | 0.74 | |
| Body composition | Body fat (kg) | 14.64 ± 5.25 | 15.52 ± 5.83 | 0.31 |
| Skeletal muscle mass (kg) | 19.96 ± 4.3 | 20.25 ± 3.96 | 0.31 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI for OR | 95% CI for OR | |||||||
| OR | Lower | Upper | p | OR | Lower | Upper | p | |
| Age (y) | 1.07 | 0.99 | 1.15 | 0.07 | 1.01 | 0.93 | 1.09 | 0.90 |
| Gender: Male | 0.99 | 0.60 | 1.63 | 0.96 | 1.44 | 0.81 | 2.57 | 0.21 |
| Female | 1.00 | 1.00 | ||||||
| BMI (kg/m2) | 0.96 | 0.89 | 1.03 | 0.24 | 0.96 | 0.88 | 1.04 | 0.30 |
| Educational history (y) | 0.86 | 0.78 | 0.95 | <0.01 | 0.87 | 0.77 | 0.97 | 0.01 |
| Medical visits due to hypertension: Yes | 0.51 | 0.32 | 0.82 | 0.01 | 0.50 | 0.30 | 0.84 | 0.01 |
| No | 1.00 | 1.00 | ||||||
| OLST (s) | 0.99 | 0.98 | 0.99 | <0.01 | 0.99 | 0.98 | 0.99 | 0.04 |
| TUG (s) | 1.21 | 1.03 | 1.41 | 0.02 | 1.10 | 0.90 | 1.35 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, A.; Murata, S.; Shiraiwa, K.; Abiko, T.; Nakano, H.; Nonaka, K.; Iwase, H.; Anami, K.; Kikuchi, Y.; Horie, J. Factors Influencing the Development of Mild Cognitive Impairment in Community-Dwelling People Aged 75 Years and Older. Geriatrics 2021, 6, 104. https://doi.org/10.3390/geriatrics6040104
Goda A, Murata S, Shiraiwa K, Abiko T, Nakano H, Nonaka K, Iwase H, Anami K, Kikuchi Y, Horie J. Factors Influencing the Development of Mild Cognitive Impairment in Community-Dwelling People Aged 75 Years and Older. Geriatrics. 2021; 6(4):104. https://doi.org/10.3390/geriatrics6040104
Chicago/Turabian StyleGoda, Akio, Shin Murata, Kayoko Shiraiwa, Teppei Abiko, Hideki Nakano, Koji Nonaka, Hiroaki Iwase, Kunihiko Anami, Yuki Kikuchi, and Jun Horie. 2021. "Factors Influencing the Development of Mild Cognitive Impairment in Community-Dwelling People Aged 75 Years and Older" Geriatrics 6, no. 4: 104. https://doi.org/10.3390/geriatrics6040104
APA StyleGoda, A., Murata, S., Shiraiwa, K., Abiko, T., Nakano, H., Nonaka, K., Iwase, H., Anami, K., Kikuchi, Y., & Horie, J. (2021). Factors Influencing the Development of Mild Cognitive Impairment in Community-Dwelling People Aged 75 Years and Older. Geriatrics, 6(4), 104. https://doi.org/10.3390/geriatrics6040104






