Relationship of Prevalent Fragility Fracture in Dementia Patients: Three Years Follow up Study
Abstract
1. Introduction
2. Methodology
2.1. Design
2.2. Setting
2.3. Data and Statistical Analysis
3. Results
3.1. Demographic Profile
3.2. Clinical Characteristics
3.3. Clinical Outcomes
3.4. Discharge Destination
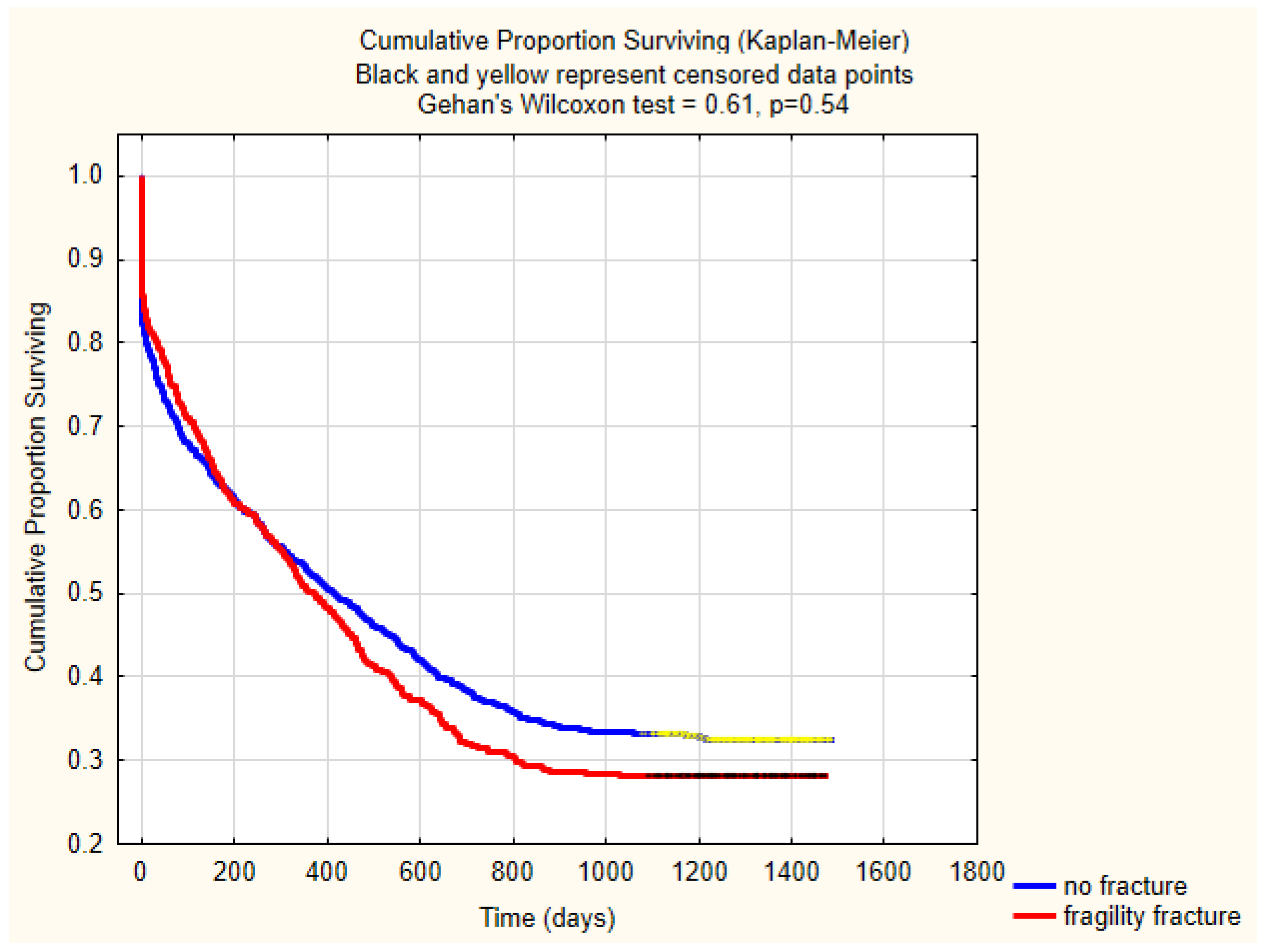
3.5. Mortality
3.6. Poor Outcome Predictors
3.7. Follow-Up Hip Fracture
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patterson, C. The State of the Art of Dementia Research: New Frontiers; World Alzheimer Report 2018; Azheirmer’s Disease International: London, UK, 2018. [Google Scholar]
- Prince, M.; Knapp, M.; Guerchet, M.; McCrone, P.; Prina, M.; Comas-Herrera, A.; Wittenberg, R.; Adelaja, B.; Hu, B.; King, D.; et al. Dementia UK: Update; Alzheimer’s Society: London, UK, 2014. [Google Scholar]
- Royal College of Psychiatrists. Who Cares Wins. Improving the Outcome for Older People Admitted to the General Hospital: Guidelines for the Development of Liaison Mental Health Services for Older People; Report of a Working Group for the Faculty of Old Age Psychiatry; Royal College of Psychiatrists: London, UK, 2005; Available online: https://www.bgs.org.uk/sites/default/files/content/resources/files/2018-05-18/WhoCaresWins.pdf (accessed on 12 February 2020).
- Briggs, R.; Dyer, A.; Nabeel, S.; Collins, R.; Doherty, J.; Coughlan, T.; O’Neill, D.; Kennelly, S.P. Dementia in the acute hospital: The prevalence and clinical outcomes of acutely unwell patients with dementia. QJM Int. J. Med. 2017, 110, 33–37. [Google Scholar] [CrossRef]
- Fogg, C.; Griffiths, P.; Meredith, P.; Bridges, J. Hospital outcomes of older people with cognitive impairment: An integrative review. Int. J. Geriatr. Psychiatry 2018, 33, 1177–1197. [Google Scholar] [CrossRef]
- Fogg, C.; Meredith, P.; Bridges, J.; Gould, G.P.; Griffiths, P. The relationship between cognitive impairment, mortality and discharge characteristics in a large cohort of older adults with unscheduled admissions to an acute hospital: A retrospective observational study. Age Ageing 2017, 46, 794–801. [Google Scholar] [CrossRef]
- Ballard, C.G.; Shaw, F.; Lowery, K.; Mckeith, I.; Kenny, R. The prevalence, assessment and associations of falls in dementia with Lewy bodies and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1999, 10, 97–103. [Google Scholar] [CrossRef]
- Singh, I.; Edwards, C.; Okeke, J. Impact of cognitive impairment on inpatient falls in single room setting and its adverse outcomes. J. Gerontol. Geriatr. Res. 2015, 2015, 1–4. [Google Scholar]
- Singh, I.; Hooton, K.; Edwards, C.; Lewis, B.; Anwar, A.; Johansen, A. Inpatient hip fractures: Understanding and addressing the risk of this common injury. Age Ageing 2020, 49, 481–486. [Google Scholar] [CrossRef]
- Barceló, M.; Francia, E.; Romero, C.; Ruiz, D.; Casademont, J.; Torres, O.H. Hip fractures in the oldest old. Comparative study of centenarians and nonagenarians and mortality risk factors. Injury 2018, 49, 2198–2202. [Google Scholar] [CrossRef]
- Rapp, K. People with Alzheimer’s disease are at increased risk of hip fracture and of mortality after hip fracture. Evid. Based Nurs. 2011, 14, 78–79. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, P.; Liang, X.; Wu, Z.; Wang, J.; Liang, Y. Association between dementia and mortality in the elderly patients undergoing hip fracture surgery: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 298. [Google Scholar] [CrossRef]
- Berry, S.D.; Rothbaum, R.R.; Kiel, D.P.; Lee, Y.; Mitchell, S.L. Association of clinical outcomes with surgical repair of hip fracture vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern. Med. 2018, 178, 774–780. [Google Scholar] [CrossRef]
- Menzies, I.B.; Mendelson, D.A.; Kates, S.; Friedman, S.M. Prevention and clinical management of hip fractures in patients with dementia. Geriatr. Orthop. Surg. Rehabil. 2010, 1, 63–72. [Google Scholar] [CrossRef]
- Morrison, R.S.; Siu, A.L. Survival in end-stage dementia following acute illness. JAMA 2000, 284, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Waran, E.; William, L. Hip fractures and dementia: Clinical decisions for the future. Oxf. Med. Case Rep. 2016, 2016, 19–21. [Google Scholar] [CrossRef][Green Version]
- Welsh Government. Making Prudent Healthcare Happen—An Update. Welsh Heath Circular, WHC/002/14. Available online: http://www.wales.nhs.uk/sitesplus/documents/866/PHW%20Prudent%20Healthcare%20Booklet%20Final%20English.pdf (accessed on 18 February 2020).
- Kanis, J.A.; Oden, A.; Johnell, O.; Jonsson, B.; De Laet, C.; Dawson, A. The burden of osteoporotic fractures: A method for setting intervention thresholds. Osteoporos. Int. 2001, 12, 417–427. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Beard, C.M.; Kokmen, E.; Atkinson, E.J.; O’Fallon, W.M. Fracture risk in patients with Alzheimer’s disease. J. Am. Geriatr. Soc. 1994, 42, 614–619. [Google Scholar] [CrossRef]
- Wang, H.-K.; Hung, C.-M.; Lin, S.-H.; Tai, Y.-C.; Lu, K.; Liliang, P.-C.; Lin, C.-W.; Lee, Y.-C.; Fang, P.-H.; Chang, L.-C.; et al. Increased risk of hip fractures in patients with dementia: A nationwide population-based study. BMC Neurol. 2014, 14, 175. [Google Scholar] [CrossRef]
- Fisher, A.A.; Davis, M.W.; Srikusalanukul, W.; Budge, M.M. Six-fold increased risk of hip fracture in older Australians (≥60 years) with dementia. Arch. Osteoporos. 2006, 1, 13–19. [Google Scholar] [CrossRef][Green Version]
- Jeon, J.-H.; Park, J.H.; Oh, C.; Chung, J.K.; Song, J.Y.; Kim, S.; Lee, S.-H.; Jang, J.-W.; Kim, Y.-J. Dementia is Associated with an Increased Risk of Hip Fractures: A Nationwide Analysis in Korea. J. Clin. Neurol. 2019, 15, 243–249. [Google Scholar] [CrossRef]
- Chang, K.H.; Chung, C.J.; Lin, C.L.; Sung, F.C.; Wu, T.N.; Kao, C.H. Increased risk of dementia in patients with osteoporosis: A population-based retrospective cohort analysis. Age 2014, 36, 967–975. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chuang, C.S.; Hung, C.-H.; Lin, C.-L.; Sung, F.-C.; Tang, C.-H.; Hsu, H.-C.; Chung, C.-J. Fracture as an independent risk factor of dementia: A nationwide population-based cohort study [published correction appears in Medicine (Baltimore). Medicine 2014, 93, e188. [Google Scholar] [CrossRef]
- Stephan, B.C.M.; Birdi, R.; Tang, E.Y.H.; Cosco, T.D.; Donini, L.M.; Licher, S.; Ikram, M.A.; Siervo, M.; Robinson, L. Secular trends in dementia prevalence and incidence worldwide: A systematic review. J. Alzheimers Dis. 2018, 66, 653–680. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health and Care Guidance (NICE) Clinical Guideline [CG146]. Osteoporosis: Assessing the Risk of Fragility Fracture. 2017. Available online: https://www.nice.org.uk/guidance/cg146 (accessed on 12 February 2020).
- Friedman, S.M.; Menzies, I.B.; Bukata, S.V.; Mendelson, D.A.; Kates, S.L. Dementia and hip fractures: Development of a pathogenic framework for understanding and studying risk. Geriatr. Orthop. Surg. Rehabil. 2010, 1, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.D.; Silber, J.H.; Magaziner, J.S.; Passarella, M.A.; Mehta, S.; Werner, R.M. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern. Med. 2014, 174, 1273–1280. [Google Scholar] [CrossRef]
- National Institute of Health and Care Guidance (NICE) Clinical Guideline [CG124]. Hip Fracture: Management. 2011. Available online: www.nice.org.uk/guidance/cg124 (accessed on 12 February 2020).
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 36. [Google Scholar] [CrossRef]
- Klotzbuecher, C.M.; Ross, P.D.; Landsman, P.B.; Abbott, T.A.; Berger, M. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J. Bone Min. Res. 2000, 15, 721–739. [Google Scholar] [CrossRef]
- Van Geel, T.A.C.M.; Huntjens, K.M.B.; Van Den Bergh, J.P.W.; Dinant, G.-J.; Geusens, P.P. Timing of subsequent fractures after an initial fracture. Curr. Osteoporos. Rep. 2010, 8, 118–122. [Google Scholar] [CrossRef]
- Van Geel, T.A.C.M.; Van Helden, S.; Geusens, P.P.; Winkens, B.; Dinant, G.-J. Clinical subsequent fractures cluster in time after first fractures. Ann. Rheum. Dis. 2009, 68, 99–102. [Google Scholar] [CrossRef]
- Van Helden, S.; Cals, J.; Kessels, F.; Brink, P.; Dinant, G.J.; Geusens, P. Risk of new clinical fractures within 2 years following a fracture. Osteoporos. Int. 2006, 17, 348–354. [Google Scholar] [CrossRef]
- Center, J.R.; Bliuc, D.; Nguyen, T.V.; Eisman, J.A. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007, 297, 387–394. [Google Scholar] [CrossRef]
- Vun, J.S.H.; Ahmadi, M.; Panteli, M.; Pountos, I.; Giannoudis, P.V. Dementia and fragility fractures: Issues and solutions. Injury 2017, 48, S10–S16. [Google Scholar] [CrossRef]
- Downey, C.L.; Young, A.; Burton, E.F.; Graham, S.M.; Macfarlane, R.J.; Tsapakis, E.-M.; Tsiridis, E. Dementia and osteoporosis in a geriatric population: Is there a common link? World J. Orthop. 2017, 8, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.L.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Nordin, B.E.; Need, A.G.; Steurer, T.; Morris, H.A.; Chatterton, B.E.; Horowitz, M. Nutrition, osteoporosis, and aging. Ann. N. Y. Acad. Sci. 1998, 854, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.; Wells, G.; Cranney, A.; Zytaruk, N.; Robinson, V.; Griffith, L.E.; Ortiz, Z.; Peterson, J.; Adachi, J.; Tugwell, P.; et al. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr. Rev. 2002, 23, 552–559. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, X.; Wang, J.; Liu, L. Association between calcium or vitamin d supplementation and fracture incidence in community–dwelling older adults: A systematic review and meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J. Alzheimers Dis. 2010, 20, S487–S498. [Google Scholar] [CrossRef]
- Small, D.H. Dysregulation of calcium homeostasis in Alzheimer’s disease. Neurochem. Res. 2009, 34, 1824–1829. [Google Scholar] [CrossRef]
- Tong, B.C.; Wu, A.J.; Li, M.; Cheung, K.H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1745–1760. [Google Scholar]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. Calmodulin Binding Proteins and Alzheimer’s Disease: Biomarkers, Regulatory Enzymes and Receptors That Are Regulated by Calmodulin. Int. J. Mol. Sci. 2020, 21, 7344. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Bruyere, O.; Coxam, V.; Cruz-Jentoft, A.; Czerwinski, E.; et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos. Int. 2017, 28, 447–462. [Google Scholar]
- Singh, I. Training and professional development for nurses and healthcare support workers: Supporting foundation for quality and good practice for care of the acutely ill older person. Int. Arch. Nurs. Health Care 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Singh, I.; Varanasi, A.; Williamson, K. Assessment and management of dementia in the general hospital setting. Rev. Clin. Gerontol. 2014, 24, 205–218. [Google Scholar] [CrossRef]
- Singh, I.; Anwar, A.; Rasuly, A.; Edwards, C.; Farooq, A.H.; Majeed, S.; Singh, N.; Waheed, A. Prevalence of fragility fractures according to quality and outcomes framework (QOF) of the general medical services (GMC) contract and quality initiatives to improve osteoporosis care in the general practice within Caerphilly County Borough, Wales, UK: A feasibility study. MOJ Gerontol. Ger. 2018, 3, 98–104. [Google Scholar]

| Dementia % (n) | Group 1: No Fracture | Group 2: All Fracture | Group 3: Fragility Fracture | p-Value Group 1 vs. Group 2 | p-Value Group 1 vs. Group 3 | |
|---|---|---|---|---|---|---|
| 65.9% (1363/2067) | 34.1% (704/2067) | 28.7% (595/2067) | ||||
| Mean age (SD) | 83.8 (8.0) | 85.9 (6.9) | 85.8 (6.9) | <0.0001 | <0.0001 | |
| Female % (n) | 55.2% (752/1363) | 76.1% (536/704) | 76.7% (457/595) | <0.0001 | <0.0001 | |
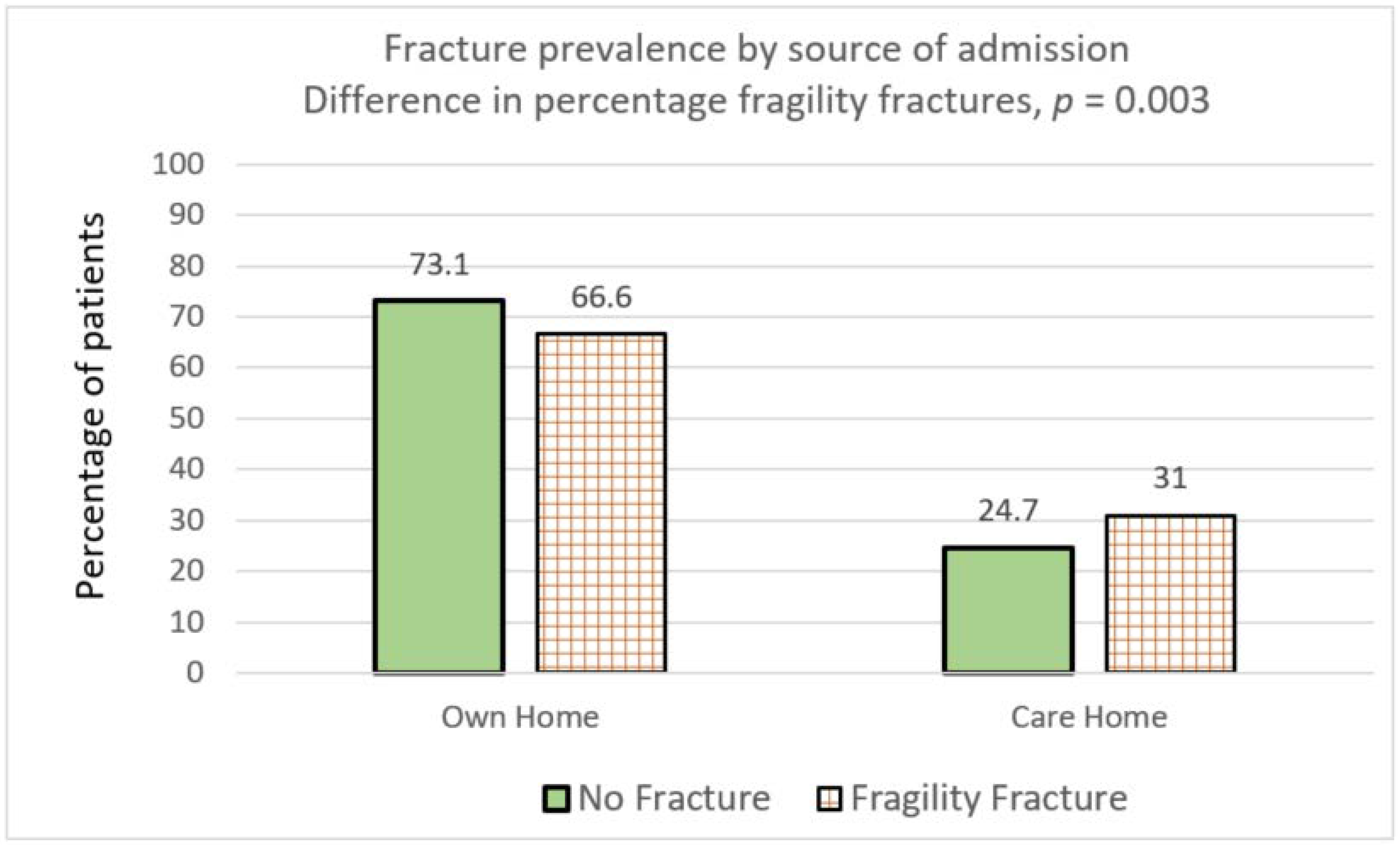
| Living in % (n) | Own Home | 73.1% (997/1363) | 66.8% (470/704) | 66.6% (396/595) | 0.0028 | 0.0035 |
| Care Home | 24.7% (337/1363) | 30.9% (218/704) | 31.0% (185/595) | 0.0026 | 0.0037 | |
| Mean CCI (SD) | 5.92 (1.47) | 6.9 (1.42) | 6.02 (1.42) | 0.17 | 0.16 | |
| Mean Drugs (SD) | 7.76 (3.8) | 8.24 (3.5) | 8.32 (3.7) | 0.005 | 0.002 | |
| Mean Anti-psychotics % (n) | 16.4% (220/1341) | 17.1% (120/700) | 16.2% (96/592) | 0.69 | 0.091 | |
| Dementia Patients % (n) | Group 1: No Fracture % (n) | Group 2: All Fracture | Group 3: Fragility Fracture | p-Value Group 1 vs. Group 2 | p-Value Group 1 vs. Group 3 | |
|---|---|---|---|---|---|---|
| 65.9% (1363/2067) | 34.1% (704/2067) | 28.7% (595/2067) | ||||
| Mean LoS | 17.37 (23.4) | 18.8 (23.2) | 18.6 (2.37) | 0.20 | 0.35 | |
| Discharge destination | Own home | 46.3% (632/1363) | 32.5% (229/704) | 30.7% (183/595) | <0.0001 | <0.0001 |
| Care home | 32.2% (439/1363) | 50.4% (355/704) | 52.6% (313/595) | <0.0001 | <0.0001 | |
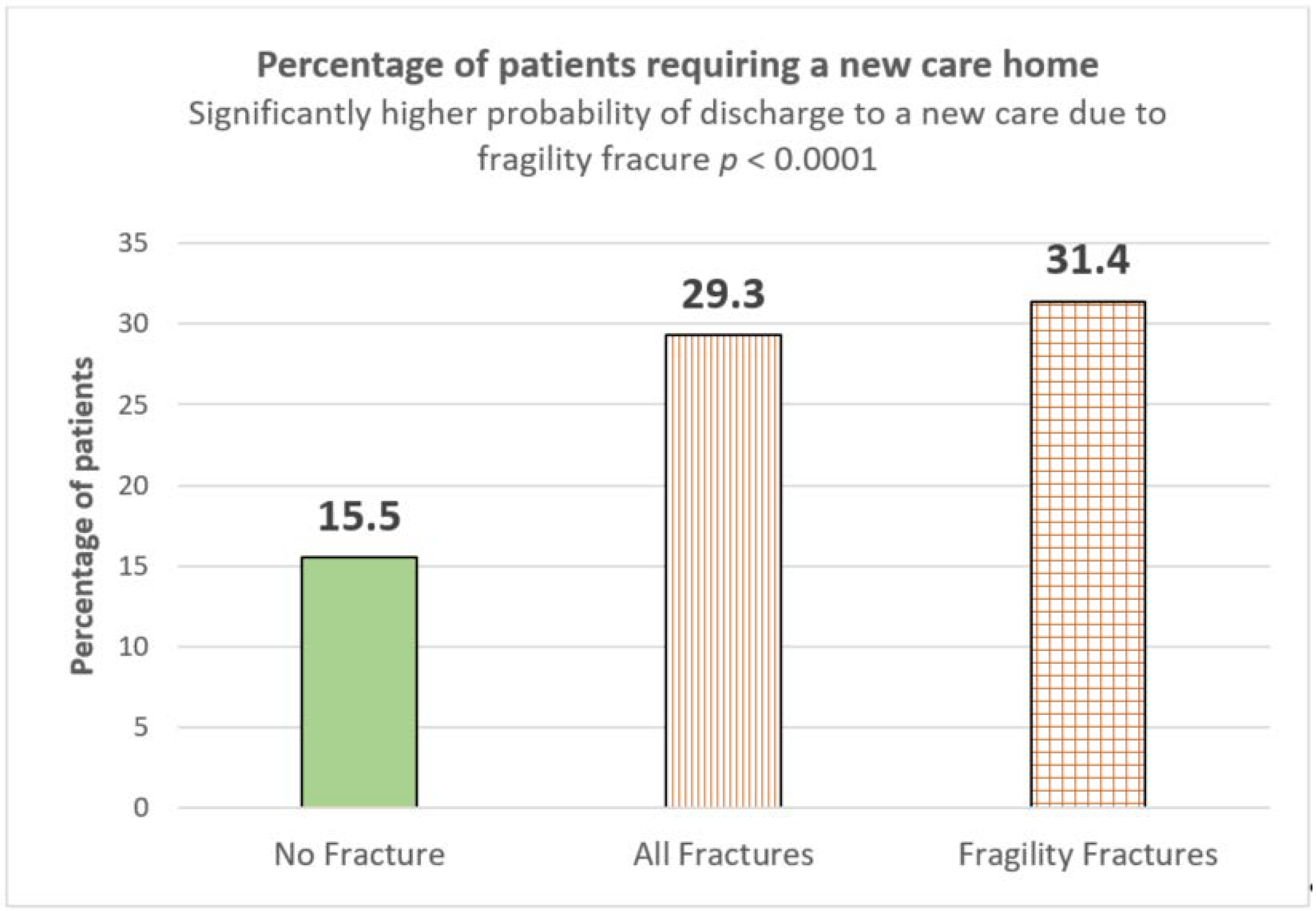
| % Requiring new care home | 15.5% (207/1333) | 29.3% (201/678) | 31.4% (182/580) | <0.0001 | <0.0001 | |
| Mortality | Inpatient | 16.7% (228/1363) | 13.6% (96/704) | 13.3% (79/595) | 0.066 | 0.057 |
| 30-days | 23.5% (320/1363) | 19.2% (135/704) | 19.4% (115/595) | 0.025 | 0.045 | |
| One-year | 47.7% (650/1363) | 50.4% (355/704) | 49.9% (296/595) | 0.2454 | 0.37 | |
| Three-year | 73.5% (352/479) | 65.1% (125/192) | 0.03 | |||
| Inpatient hip fracture | 2.1% (18/1363) | 3.1% (42/354) | 3.7% (42/595) | 0.26 | 0.040 | |
| Post-discharge hip fracture % (n) | 2.9% (34/1135) | 19.9% (121/608) | 21.5 (111/516) | <0.0001 | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, I.; Duric, D.; Motoc, A.; Edwards, C.; Anwar, A. Relationship of Prevalent Fragility Fracture in Dementia Patients: Three Years Follow up Study. Geriatrics 2020, 5, 99. https://doi.org/10.3390/geriatrics5040099
Singh I, Duric D, Motoc A, Edwards C, Anwar A. Relationship of Prevalent Fragility Fracture in Dementia Patients: Three Years Follow up Study. Geriatrics. 2020; 5(4):99. https://doi.org/10.3390/geriatrics5040099
Chicago/Turabian StyleSingh, Inderpal, Daniel Duric, Alfe Motoc, Chris Edwards, and Anser Anwar. 2020. "Relationship of Prevalent Fragility Fracture in Dementia Patients: Three Years Follow up Study" Geriatrics 5, no. 4: 99. https://doi.org/10.3390/geriatrics5040099
APA StyleSingh, I., Duric, D., Motoc, A., Edwards, C., & Anwar, A. (2020). Relationship of Prevalent Fragility Fracture in Dementia Patients: Three Years Follow up Study. Geriatrics, 5(4), 99. https://doi.org/10.3390/geriatrics5040099






