Risk Factors for Mortality in Nursing Home Residents: An Observational Study
Abstract
1. Introduction
2. Methods
2.1. Design and Participants
2.2. Outcome Measures
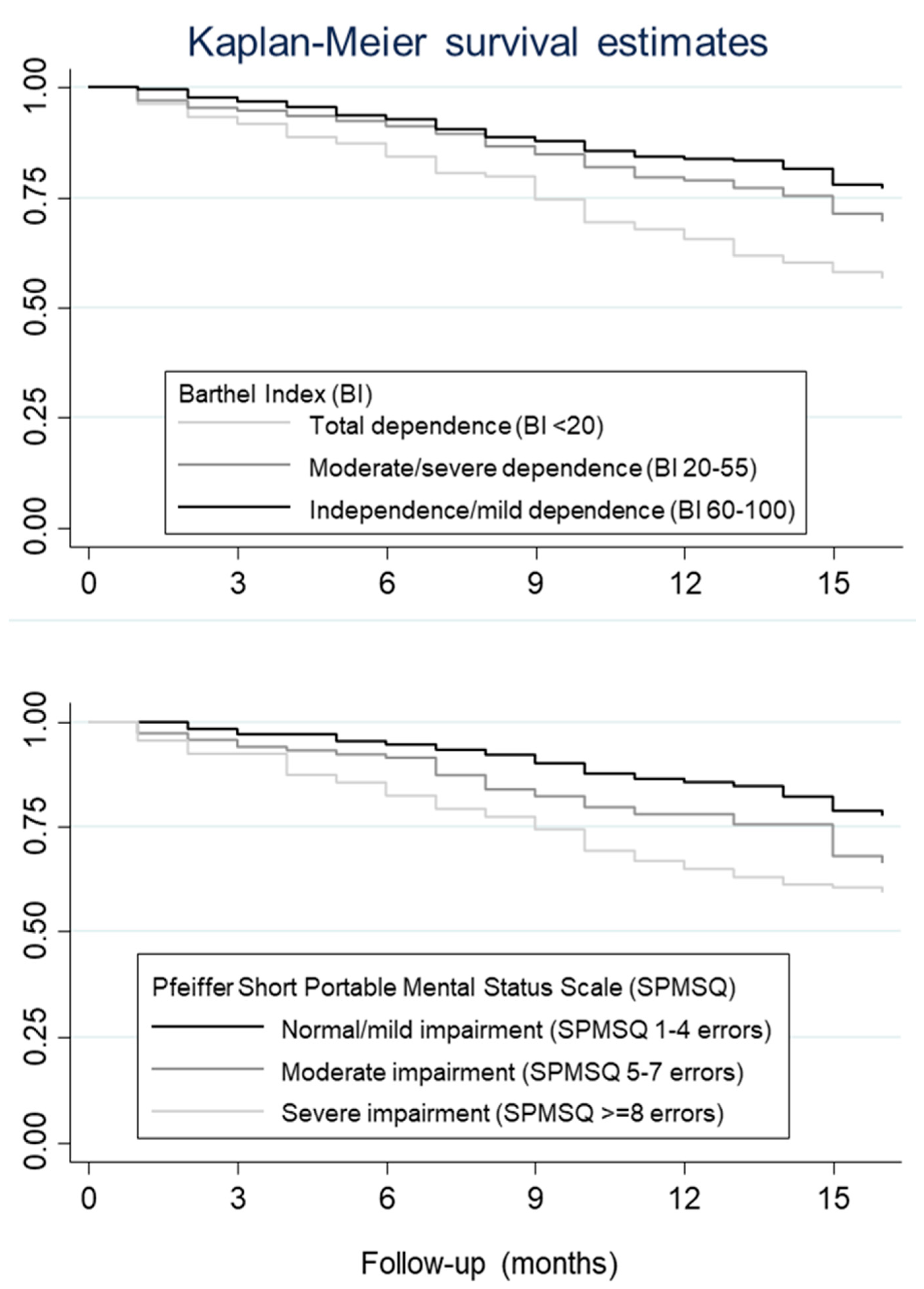
2.2.1. Functional Status Indicators
2.2.2. Cognitive Status Indicators
2.2.3. Nutritional Assessment
2.2.4. Comorbidities
2.2.5. Other Health Variables
2.3. Pharmacological Treatment
2.4. Data Analysis
2.5. Ethical Considerations
3. Results
Mortality Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowlegements
Conflicts of Interest
References
- Li:, Q.; Zheng, N.T.; Temkin-Greener, H. End of Life Quality of Care Among Long-Term Nursing Home Decedent Residents With and Without Dementia. J. Am. Geriatr. Soc. 2013, 61, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- AEsteban-Burgos, A.; El Mansouri-Yachou, J.; Ramírez, R.M. Prognostic Models Associated with 6-Month Survival of Patients Admitted to Nursing Homes. Gerontology 2018, 65, 44. [Google Scholar] [CrossRef] [PubMed]
- Amblàs-Novellas, J.; Santaeugènia, S.; Vela, E.; Clèries, M.; Contel, J. What lies beneath: A retrospective, population-based cohort study investigating clinical and resource-use characteristics of institutionalized older people in Catalonia. BMC Geriatr. 2020, 20, 187. [Google Scholar] [CrossRef]
- Smets, T.; Pivodic, L.; Piers, R.; Pasman, H.; Engels, Y.; Szczerbinska, K. The palliative care knowledge of nursing home staff: The EU FP7 PACE cross-sectional survey in 322 nursing homes in six European countries. Palliat. Med. 2018, 32, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Hjaltadóttir, I.; Hallberg, I.R.; Ekwall, A.K.; Nyberg, P. Predicting mortality of residents at admission to nursing home: A longitudinal cohort study. BMC Health Serv. Res. 2011, 11, 86. [Google Scholar] [CrossRef]
- Etkind, S.; Koffman, J. Approaches to managing uncertainty in people with life-limiting conditions: Role of communication and palliative care. Postgrad. Med. J. 2016, 92, 412–417. [Google Scholar] [CrossRef]
- Kennedy, C.; Brooks-Young, P.; Gray, C.B.; Larkin, P.; Conolly, M.; Wilde-Larson, B. Diagnosing dying: An integrative literature review. BMJ Support. Palliat. Care 2014, 4, 263–270. [Google Scholar] [CrossRef]
- Porock, D.; Parker-Oliver, D.; Petroski, G.F.; Rantz, M. The MDS mortality risk index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res. Notes 2010, 3, 200. [Google Scholar] [CrossRef]
- Van der Steen, T.; Heymans, M.W.; Steyerberg, E.W.; Kruse, R.L.; Mehr, D.R. The difficulty of predicting mortality in nursing home residents. Eur. Geriatr. Med. 2011, 2, 79–81. [Google Scholar] [CrossRef]
- Sung, K. Predictive factors associated with death of elderly in nursing homes. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2014, 8, 143–149. [Google Scholar] [CrossRef]
- Vossius, C.; Selbæk, G.; Benth, J.; Bergh, S. Mortality in nursing home residents: A longitudinal study over three years. PLoS ONE 2018, 13, e203480. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.S.; Ogarek, J.A.; Teno, J.M.; Gozalo, P.L.; Mor, V. Development and Validation of the Nursing Home Minimum Data Set 3.0 Mortality Risk Score (MRS3) Authors. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Scherer, Y. Advantages and disadvantages of using MDS data in nursing research. J. Gerontol. Nurs. 2009, 35, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Yourman, L.C.; Lee, S.J.; Schonberg, M.A.; Widera, E.W.; Smith, A.K. Prognostic indices for older adults: A systematic review. JAMA 2012, 307, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Heppenstall, C.; Broad, J.; Boyd, M.; Gott, M.; Connolly, M. Progress towards predicting 1-year mortality in older people living in residential long-term care. Age Ageing 2015, 44, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Cooney, L.; Fried, T. Systematic review: Health-related characteristics of elderly hospitalized adults and nursing home residents associated with short-term mortality. J. Am. Geriatr. Soc. 2013, 61, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.; Parker, D.; Oliver, D.; Mehr, D.; Petroski, G.; Swenson, L. Using mortality risk scores for long-term prognosis of nursing home residents: Caution is recommended. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1235–1241. [Google Scholar] [CrossRef]
- Granger, C.; Albrecht, G.; Hamilton, B. Outcome of comprehensive medical rehabilitation: Measurement by PULSES Profile and the Barthel Index. Arch. Phys. Med. Rehabil. 1979, 60, 145–154. [Google Scholar]
- Norton, D.; McLaren, R.; Exton-Smith, A.N. Investigation of Geriatric Nursing Problems in Hospitals; Churchill Livingstone: London, UK, 1962; pp. 193–224. [Google Scholar]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Lobo, A.; Ezquerra, J.; Burgada, F.G.; Sala, J.M.; Díaz, A.S. El miniexamen, cognoscitivo (un test sencillo, práctico, para detectar alteraciones intelectuales en pacientes médicos). Actas Luso Esp. Neurol. Psiquiatr. Cien. Afines 1979, 7, 189–202. [Google Scholar]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.; Fukushima, R.; Higashiguchi, T.; Cederholm, T.; Jensen, G. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Guigoz, Y.; Garry, P.; F, N.; Bennahum, D.; Lahuque, S. The Mini Nutrtional Assessement and its use in grading nutritional state of elderly patients. Nutrtion 1999, 15, 116–122. [Google Scholar]
- Clave, P.; Terré, R.; de Kraa, M.; Serra, M. Approaching orofaringeal disphagia. Rev. Esp. Enferm. Dig. 2004, 96, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Wells, M.T.; Ullman, R.; King, F.; Schmukler, C. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS ONE 2014, 9, e112479. [Google Scholar]
- American, D.A. Standars of medical care in diabetes 2012. Diabetes Care 2012, 35, 11–63. [Google Scholar]
- Aronow, W.; Fleg, J.; Pepine, C.; Artinian, N.; Bakris, G.; Brown, A.; Ferdinand, K. Expert consensus document on hypertension in the elderly: A report of the American College of cardiology foundation task Force on clinical expert consensus documents. J. Am. Soc. Hypertens. 2011, 5, 259–352. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [PubMed]
- Inouye, S.; van Dyck, C.; Alessi, C.; Balkin, S.; Siegal, A. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.; Manuguerra, M.; Chow, R. How to analize the Visual Analogue Scale (VAS); miths, truths and clinical relevance. Scand. J. Pain 2016, 13, 67–75. [Google Scholar] [CrossRef]
- Warden, V.; Hurley, A.; Volicer, L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J. Am. Dir. Assoc. 2003, 4, 9–15. [Google Scholar] [CrossRef]
- Perna, S.; Francis, M.; Bologna, C.; Moncaglieri, F.; Riva, A.; Morazzoni, P. Performance of Edmonton Frail Scale on frailty assessment: Its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gnjidic, D.; Hilmer, S.; Blyth, F.; Naganatan, V.; Waite, L. Polypharmacy cutoff and outcomes: Five or more medicines were ussed to identify communit- dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 2012, 65, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Español, E. Ley Orgánica 3/2018, de 5 de diciembre, de Protección de Datos Personales y garantía de los derechos digitales. Boletín Oficial del Estado 2018, 119, 778–857. [Google Scholar]
- WMA Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 25 May 2018).
- Sarabia-Cobo, C.; Pérez, V.; de Lorena, P.; Dominguez, E.; Hermosilla, C. The incidence and prognostic implications of dysphagia in elderly patients institutionalized: A multicenter study in Spain. Appl. Nurs. Res. 2016, 30, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Damián, J.; Pastor-Barriuso, R.; López, F.G.; de Pedro-Cuesta, J. Urinary incontinence and mortality among older adults residing in care homes. J. Adv. Nurs. 2017, 73, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Genao, L.; Buhr, G. Urinary tract infections in older adults residing in long-term care facilities. Ann. Long-Term Care 2012, 20, 33–38. [Google Scholar]
- Torisson, G.; Stavenow, L.; Minthon, L.; Londos, E. Importance and added value of functional impairment to predict mortality: A cohort study in Swedish medical inpatients. BMJ Open 2017, 7, e014464. [Google Scholar] [CrossRef]
- Hendriks, S.; Smalbrugge, M.; van Gageldonk-Lafeber, A. Pneumonia, Intake Problems, and Survival among Nursing Home Residents with Variable Stages of Dementia in the Netherlands: Results from a Prospective Observational Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 200–208. [Google Scholar] [CrossRef]
- Flacker, J.M.; Kiely, D.K. Mortality-related factors and 1-year survival in nursing home residents. J. Am. Geriatr. Soc. 2003, 51, 213–321. [Google Scholar] [CrossRef]
- Onder, G.; Liperoti, R.; Foebel, A.; Fialova, D.; Topinkova, E. Polypharmacy and mortality among nursing home residents with advanced cognitive impairment: Results from the SHELTER study. J. Am. Med. Dir. Assoc. 2013, 14, 450.e7–450.e12. [Google Scholar] [CrossRef]
- Taxis, K.; O´Sullivan, D.; Cullinan, S. Drug utilization in older people. In Drug Utilization Research: Methods and Applications; Wiley-Blackwell: London, UK, 2016; pp. 259–269. [Google Scholar]
- Fox, C.; Smith, T.; Maidement, I.; Wei-Yee, C.; Nelson, B. Effect of medications with anti-colinergic properties on cognitive function, deliium, physical function and mortality: A sistematic review. Age Ageing 2014, 43, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Satoh, H.; Miki, A.; Urushihara, H.; Sawada, Y. Medications associated with falls in older people: Sistematic review of publications from a recent 5-year period. Eur. J. Clin. Pharmacol. 2015, 71, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, M.; Kalish, L.; Shakib, S.; Caughey, G. Predictors of mortality in the older population: The role of polipharmacy and other medication and chronic diseases-related factors. Drugs Aging 2020, 37, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Porock, D.; Oliver, D.P.; Zweig, S.; Rantz, M.; Mehr, D.; Madsen, R.; Petroski, G. Predicting death in the nursing home: Development and validation of the 6-month Minimum Data Set mortality risk index. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 491–498. [Google Scholar] [CrossRef]
- Ogarek, J.A.; McCreedy, E.M.; Thomas, K.S.; Teno, J.M.; Gozalo, P.L. Minimum Data Set Changes in Health, End-Stage Disease and Symptoms and Signs Scale: A Revised Measure to Predict Mortality in Nursing Home Residents. J. Am. Geriatr. Soc. 2018, 66, 976–981. [Google Scholar] [CrossRef]
- Tiainen, K.; Luukkaala, T.; Hervonen, A.; Jylhä, M. Predictors of mortality in men and women aged 90 and older: A nine-year follow-up study in the Vitality 90+ study. Age Ageing 2013, 42, 468–475. [Google Scholar] [CrossRef]
| Sociodemographic and Functional Variables | n(%) | ||||||||||||||
| Gender | Female | 400 (75.3%) | |||||||||||||
| Male | 131 (24.7%) | ||||||||||||||
| Age | <80 years | 86 (16.2%) | |||||||||||||
| ≥80 years | 445 (83.8%) | ||||||||||||||
| Origin of residents | Home | 313 (59%) | |||||||||||||
| Hospital discharge | 160 (30%) | ||||||||||||||
| Other nursing home | 58 (11%) | ||||||||||||||
| Reason for admission | Increased intensity of care | 414(78%) | |||||||||||||
| Post-surgical care | 61 (12%) | ||||||||||||||
| Other reasons | 56 (10%) | ||||||||||||||
| Length of stay (months) | Mean (SD) | 35.46 (61.83) | |||||||||||||
| Dependency level (Barthel index) | Total (<20) | Severe (20–35) | Moderate (40–55) | Mild (60–95) | Independent (100) | ||||||||||
| 135 (25.6%) | 87 (16.5%) | 84 (15.9%) | 203 (38.4%) | 19 (3.6%) | |||||||||||
| Risk of pressure ulcers (Norton) | Very high (5–9) | High (10–12) | Medium (13–14) | Minimum/no risk (>14) | |||||||||||
| 17 (3.2%) | 69 (13.1%) | 110 (20.8%) | 332 (62.9%) | ||||||||||||
| Cognitive Status Indicators | |||||||||||||||
| Pfeiffer Short Portable Mental Status Scale | Normal (<3 errors) | Mild impairment (3–4 errors) | Moderate impairment (5–7 errors) | Severe impairment (≥8 errors) | |||||||||||
| 169 (32.3%) | 75 (14.3%) | 119 (22.7%) | 161 (30.7%) | ||||||||||||
| Assessment of Nutritional Status | |||||||||||||||
| BMI | Mean (SD) | 26.64 (5.45) | |||||||||||||
| MNA | Malnourished (<17) | At risk (17–23) | Well nourished (≥24) | ||||||||||||
| 177 (33.6%) | 268 (50.9%) | 82 (15.6%) | |||||||||||||
| Weight loss (>5 kg in 12 months) | 88 (18.5%) | ||||||||||||||
| Incidence of dysphagia | 115 (21.8%) | ||||||||||||||
| Comorbidities | |||||||||||||||
| Charlson | Median (IQR) | 7 (6–9) | |||||||||||||
| Charlson ≥ 5 | 289 (94.1%) | ||||||||||||||
| AFib/Arrhythmia | 141 (26.7%) | ||||||||||||||
| Chronic heart failure | 217(40.9%) | ||||||||||||||
| High blood pressure | 314 (59.5%) | ||||||||||||||
| Kidney failure(CKD Stage 3 or higher) | 72 (13.7%) | ||||||||||||||
| Pneumonia | 55 (10.4%) | ||||||||||||||
| Anemia | 182 (34.5%) | ||||||||||||||
| Urinary tract infection | 180 (34.2%) | ||||||||||||||
| Risk Factors and Health Variables | |||||||||||||||
| Risk of suffering a fall (TUG > 20 s) | Normal | 223 (68.4%) | |||||||||||||
| High | 103 (31.6%) | ||||||||||||||
| Hip fracture | 93 (17.6%) | ||||||||||||||
| Falls/last year | 1 | 94 (17.8%) | |||||||||||||
| ≥2 | 74 (14%) | ||||||||||||||
| Emergency service/last year | 1 | 82 (15.7%) | |||||||||||||
| ≥2 | 29 (5.6%) | ||||||||||||||
| Hospital./last year | 1 | 89 (17.1%) | |||||||||||||
| ≥2 | 36 (6.9%) | ||||||||||||||
| Ep. delirium/last year | 1 | 34 (8.9%) | |||||||||||||
| ≥2 | 30 (7.9%) | ||||||||||||||
| Incontinence | Urinary incontinence | Fecal incontinence | Mixed incontinence | ||||||||||||
| 147 (28.1%) | 19 (3.6%) | 196 (37.5%) | |||||||||||||
| Pain (VAS or PAINAD ≥4) | 146 (27.5%) | ||||||||||||||
| Edmonton Frailty Scale | Median (IQR) | 9 (6–13) | |||||||||||||
| Pharmacological treatment | |||||||||||||||
| Polymedication (≥5 treatment) | Anticholinergics | Anxiolytics | Anti-depressant | Neuroleptics | |||||||||||
| 422 (79.9%) | 98 (19.2%) | 191 (37.3%) | 162 (31.6%) | 115 (22.4%) | |||||||||||
| Alive n (%) | Expired n (%) | |||
|---|---|---|---|---|
| n = 365 | n = 166 | |||
| Sociodemographic and functional variables | Gender | Female | 286 (78.3%) | 114 (68.6%) |
| Male | 79 (21.6%) | 52 (31.3%) | ||
| Age | <80 years | 67 (18.3%) | 19 (11.4%) | |
| ≥80 years | 298 (81.6%) | 147 (88.5%) | ||
| Dependency level (Barthel index) | Total (<20) | 75 (20.5%) | 60 (36.8%) | |
| Severe (20–35) | 57 (15.6%) | 30 (18.0%) | ||
| Moderate (40–55) | 62 (16.9%) | 22 (13.5%) | ||
| Mild (60–95) | 153 (41.9%) | 50 (30.6%) | ||
| Independence (100) | 18 (4.9%) | 1 (0.6%) | ||
| Risk of pressure ulcers (Norton) | Very high (5–9) | 7 (1.9%) | 10 (6.1%) | |
| High (10–12) | 34 (9.3%) | 35 (21.4%) | ||
| Medium (13–14) | 70 (19.1%) | 40 (24.5%) | ||
| Minimum/no risk (>14) | 254 (69.5%) | 78 (47.8%) | ||
| Cognitive status indicators | Pfeiffer Short Portable Mental Status Scale | Normal (<3 errors) | 132 (36.3%) | 37 (22.9%) |
| Mild impairment (3–4 errors) | 57 (15.7%) | 18 (11.2%) | ||
| Moderate impairment (5–7 errors) | 79 (21.7%) | 40 (24.8%) | ||
| Severe impairment (≥8 errors) | 95 (26.1%) | 66 (40.9%) | ||
| Assessment of nutritional status | BMI | Mean (SD) | 27.19 (29.78) | 25.41 (27.97) |
| MNA | Malnourished (< 17) | 133 (36.5%) | 44 (26.9%) | |
| At risk (17–23) | 174 (47.8%) | 94 (57.6%) | ||
| Well nourished (≥24) | 57 (15.6%) | 25 (15.3%) | ||
| Weight loss (>5 kg in 12 months) | 53 (16.3%) | 35 (23.1%) | ||
| Incidence of dysphagia | 66 (18.0%) | 49 (30.0%) | ||
| Comorbidities | Charlson | Median (IQR) | 7 (6–8) | 8 (6–9) |
| AFib/Arrhythmia | 71 (19.5%) | 46 (28.2%) | ||
| Chronic heart failure | 141 (38.8%) | 76 (45.2%) | ||
| High blood pressure | 209 (57.2%) | 105 (64.4%) | ||
| Kidney failure (CKD stage 3 or higher) | 45 (12.3%) | 27 (16.6%) | ||
| Diabetes mellitus | 83 (22.7%) | 50 (30.7%) | ||
| Parkinson’s | 26 (7.2%) | 15 (9.4%) | ||
| Cancer | 44 (12.1%) | 27 (16.6%) | ||
| Pneumonia | 31 (8.4%) | 24 (14.7%) | ||
| Anemia | 117 (32.0%) | 65 (39.8%) | ||
| Urinary tract infection | 113 (31.0%) | 67 (41.1%) | ||
| Risk factors and health variables | Risk of suffering a fall (TUG > 20 s) (n = 326) | High | 78 (31.84%) | 25 (30.86%) |
| Hip fracture | 67 (18.3%) | 26 (15.9%) | ||
| Falls/last year | 1 | 68 (18.6%) | 26 (16.0%) | |
| ≥2 | 48 (13.1%) | 26 (16.0%) | ||
| Emergency service/last year | 1 | 55 (15.3%) | 27 (16.5%) | |
| ≥2 | 16 (4.4%) | 13 (7.9%) | ||
| Hospital./last year | 1 | 58 (16.2%) | 31 (19.0%) | |
| ≥2 | 22 (6.1%) | 14 (8.5%) | ||
| Ep. delirium/last year | 1 | 27 (10.4%) | 7 (5.7%) | |
| ≥2 | 19 (7.3%) | 11 (9.0%) | ||
| Incontinence | Urinary incontinence | 103 (28.4%) | 44 (27.3%) | |
| Fecal incontinence | 12 (3.3%) | 7 (4.3%) | ||
| Mixed incontinence | 121 (33.4%) | 75 (46.5%) | ||
| Pain (VAS or PAINAD ≥ 4) | 102 (28.1%) | 44 (26.2%) | ||
| Edmonton Frailty Scale (n = 413) | Median (IQR) | 9 (6–12) | 10 (7–14) | |
| Not frail (0–5) | 70 (24.73%) | 17 (14.78%) | ||
| At risk (6–7) | 31 (10.95%) | 23 (20%) | ||
| Pre-frail (8–9) | 48 (16.96%) | 19 (16.52%) | ||
| Moderately frail (10–11) | 53 (18.73%) | 16 (13.91%) | ||
| Frail (12–17) | 81 (28.62%) | 40 (34.78%) | ||
| Pharmacological treatment | Polymedication (≥5 treatment) | 287 (78.6%) | 135 (82.8%) | |
| Anticholinergics | 70 (19.8%) | 28 (17.8%) | ||
| Anxiolytics | 129 (36.5%) | 62 (38.9%) | ||
| Anti-depressant | 122 (34.4%) | 40 (25.1%) | ||
| Neuroleptics | 79 (22.3%) | 36 (22.6%) |
| Risk Factor | HR | CI95% HR | p-Value | ||
|---|---|---|---|---|---|
| Sociodemographic and functional variables | Male | 1.45 | 1.04 | 2.01 | 0.027 * |
| ≥80 years | 1.53 | 0.95 | 2.48 | 0.079 | |
| Total dependence (Barthel <20) | 1.93 | 1.41 | 2.62 | <0.001 * | |
| High/very high risk of pressure ulcers (Norton ≤ 12) | 2.51 | 1.78 | 3.54 | <0.001 * | |
| Cognitive status indicators | Moderate cognitive impairment (SPMSQ 5-7 errors) | 1.58 | 1.05 | 2.37 | 0.028 * |
| Severe cognitive impairment (SPMSQ ≥8 errors) | 2.14 | 1.50 | 3.07 | <0.001 * | |
| Assessment of nutritional status | BMI | 0.95 | 0.91 | 0.99 | 0.023 * |
| Malnourished (MNA < 17) | 0.76 | 0.47 | 1.25 | 0.282 | |
| At risk of malnutrition (MNA 17-23) | 1.15 | 0.74 | 1.78 | 0.547 | |
| Weight loss (> 5 kg in 12 months) | 1.40 | 0.96 | 2.04 | 0.084 | |
| Dysphagia | 1.74 | 1.24 | 2.43 | 0.001 * | |
| Comorbidities | Charlson ≥7 | 1.55 | 0.98 | 2.44 | 0.060 |
| AFib/Arrhythmia | 1.56 | 1.11 | 2.20 | 0.010 * | |
| Chronic heart failure | 1.28 | 0.94 | 1.73 | 0.114 | |
| High blood pressure | 1.35 | 0.98 | 1.86 | 0.065 | |
| Kidney failure (CKD stage 3 or higher) | 1.37 | 0.91 | 2.07 | 0.136 | |
| Diabetes mellitus | 1.39 | 0.99 | 1.94 | 0.054 | |
| Parkinson’s | 1.32 | 0.78 | 2.25 | 0.303 | |
| Cancer | 1.35 | 0.89 | 2.04 | 0.153 | |
| Pneumonia | 1.62 | 1.05 | 2.50 | 0.029 * | |
| Anemia | 1.29 | 0.94 | 1.76 | 0.112 | |
| Urinary tract infection | 1.44 | 1.05 | 1.97 | 0.022 * | |
| Risk factors and health variables | High risk of falling (TUG > 20 s) | 0.95 | 0.6 | 1.53 | 0.846 |
| Hip fracture | 0.86 | 0.56 | 1.3 | 0.472 | |
| ≥2 Falls/last year | 1.16 | 0.76 | 1.78 | 0.491 | |
| ≥2 Hospital./last year | 1.36 | 0.78 | 2.37 | 0.277 | |
| ≥2 Emergency service/last year | 1.67 | 0.94 | 2.95 | 0.080 | |
| ≥2 Ep. delirium/last year | 1.26 | 0.68 | 2.35 | 0.461 | |
| Urinary or fecal incontinence | 1.72 | 1.18 | 2.50 | 0.004 * | |
| Pain (VAS or PAINAD ≥4) | 0.91 | 0.65 | 1.29 | 0.607 | |
| Edmonton Frailty Scale | 1.05 | 1.01 | 1.09 | 0.018 * | |
| At-risk/pre-frail (Edmonton Frailty Scale 6-9) | 1.92 | 1.09 | 3.37 | 0.023 * | |
| Moderately frail/frail (Edmonton Frailty Scale 10-17) | 1.61 | 0.94 | 2.77 | 0.085 | |
| Pharmacological treatment | Polymedication (≥5 treatment) | 1.26 | 0.84 | 1.9 | 0.263 |
| Anticholinergics | 0.88 | 0.58 | 1.32 | 0.535 | |
| Anxiolytics | 1.08 | 0.79 | 1.49 | 0.621 | |
| Neuroleptics | 0.99 | 0.69 | 1.44 | 0.975 | |
| Anti-depressants | 0.69 | 0.49 | 0.99 | 0.046 * | |
| Predictor | HR | CI95% HR | p-Value | |
|---|---|---|---|---|
| Male | 1.88 | 1.32 | 2.67 | <0.001 * |
| ≥80 years | 1.73 | 1.04 | 2.86 | 0.034 * |
| Moderate cognitive impairment (SPMSQ 5-7 errors) | 1.59 | 1.04 | 2.43 | 0.031 * |
| Severe cognitive impairment (SPMSQ ≥8 errors) | 1.93 | 1.28 | 2.92 | 0.002 * |
| Total dependence (Barthel <20) | 1.52 | 1.07 | 2.17 | 0.02 * |
| High blood pressure | 1.53 | 1.10 | 2.14 | 0.012 * |
| AFib/Arrhythmia | 1.43 | 1.00 | 2.04 | 0.048 * |
| Pneumonia | 1.65 | 1.05 | 2.58 | 0.029 * |
| ≥2 Emergency service/last year | 1.80 | 0.96 | 3.36 | 0.067 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gollarte, J.F.; García-Andrade, M.M.; Santaeugenia-González, S.J.; Solá Hermida, J.C.; Baixauli-Alacreu, S.; Santabalbina, F.J.T. Risk Factors for Mortality in Nursing Home Residents: An Observational Study. Geriatrics 2020, 5, 71. https://doi.org/10.3390/geriatrics5040071
García-Gollarte JF, García-Andrade MM, Santaeugenia-González SJ, Solá Hermida JC, Baixauli-Alacreu S, Santabalbina FJT. Risk Factors for Mortality in Nursing Home Residents: An Observational Study. Geriatrics. 2020; 5(4):71. https://doi.org/10.3390/geriatrics5040071
Chicago/Turabian StyleGarcía-Gollarte, José Fermín, María Montero García-Andrade, Sebastiá J. Santaeugenia-González, José Carlos Solá Hermida, Susana Baixauli-Alacreu, and Francisco José Tarazona Santabalbina. 2020. "Risk Factors for Mortality in Nursing Home Residents: An Observational Study" Geriatrics 5, no. 4: 71. https://doi.org/10.3390/geriatrics5040071
APA StyleGarcía-Gollarte, J. F., García-Andrade, M. M., Santaeugenia-González, S. J., Solá Hermida, J. C., Baixauli-Alacreu, S., & Santabalbina, F. J. T. (2020). Risk Factors for Mortality in Nursing Home Residents: An Observational Study. Geriatrics, 5(4), 71. https://doi.org/10.3390/geriatrics5040071






