Effectiveness of a Community Health Worker-Led Diabetes Intervention among Older and Younger Latino Participants: Results from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Interventions
2.3. Study Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
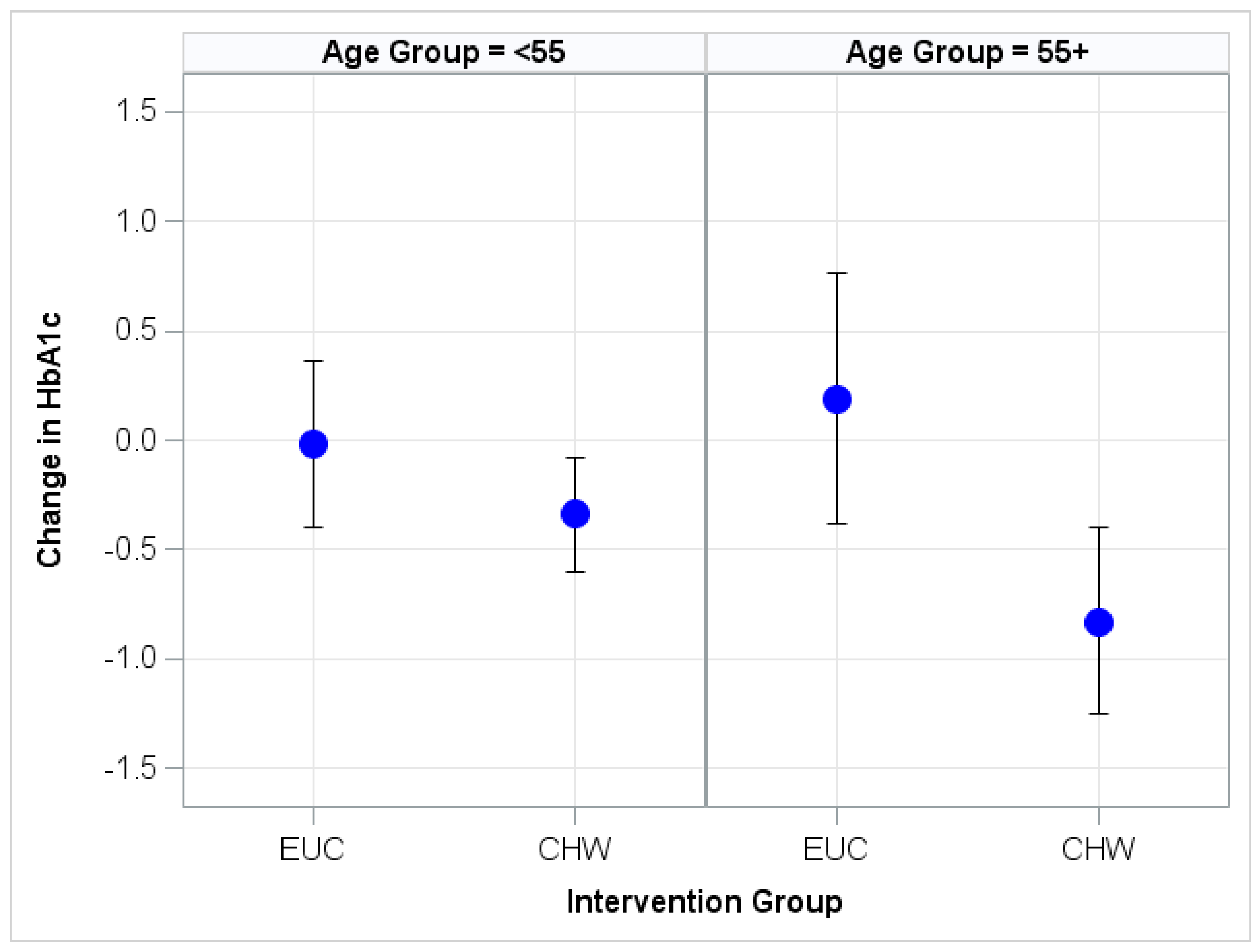
Appendix A. Illustration of HbA1c Change
Appendix B. Linear Regression Model and SAS Code
- Let BL_HbA1c = Hemoglobin HbA1c at baseline.
- Let M6_HbA1c = Hemoglobin HbA1c at 6 months.
- Let M6BL_HbA1c = M6_HbA1c–BL_HbA1c.
- Let GenderInd = 1 if male, 0 if female (reference).
- Let HSGrad =1 if high school graduate, 0 if less than high school education (reference).
- Let EmpBin =1 if employed full or part-time; 0 if unemployed (reference).
- Let M6_MoreDiabetesMeds = 1 if medication intensification, 0 if not (reference).
- Let RandomizationBinN = −1 if intervention group, 0 if control group (reference); (RandomizationBinN = −1) is 1 if RandomizationBinN = −1 & 0 if RandomizationBinN = 0.
- Let AgeGE55 = −1 if age ≥55, 0 if age <55 (reference); (AgeGE55N = −1) is 1 if AgeGE55N = −1 & 0 if AgeGE55N = 0.
- β0–β7 are regression coefficients.
- ε = random error.
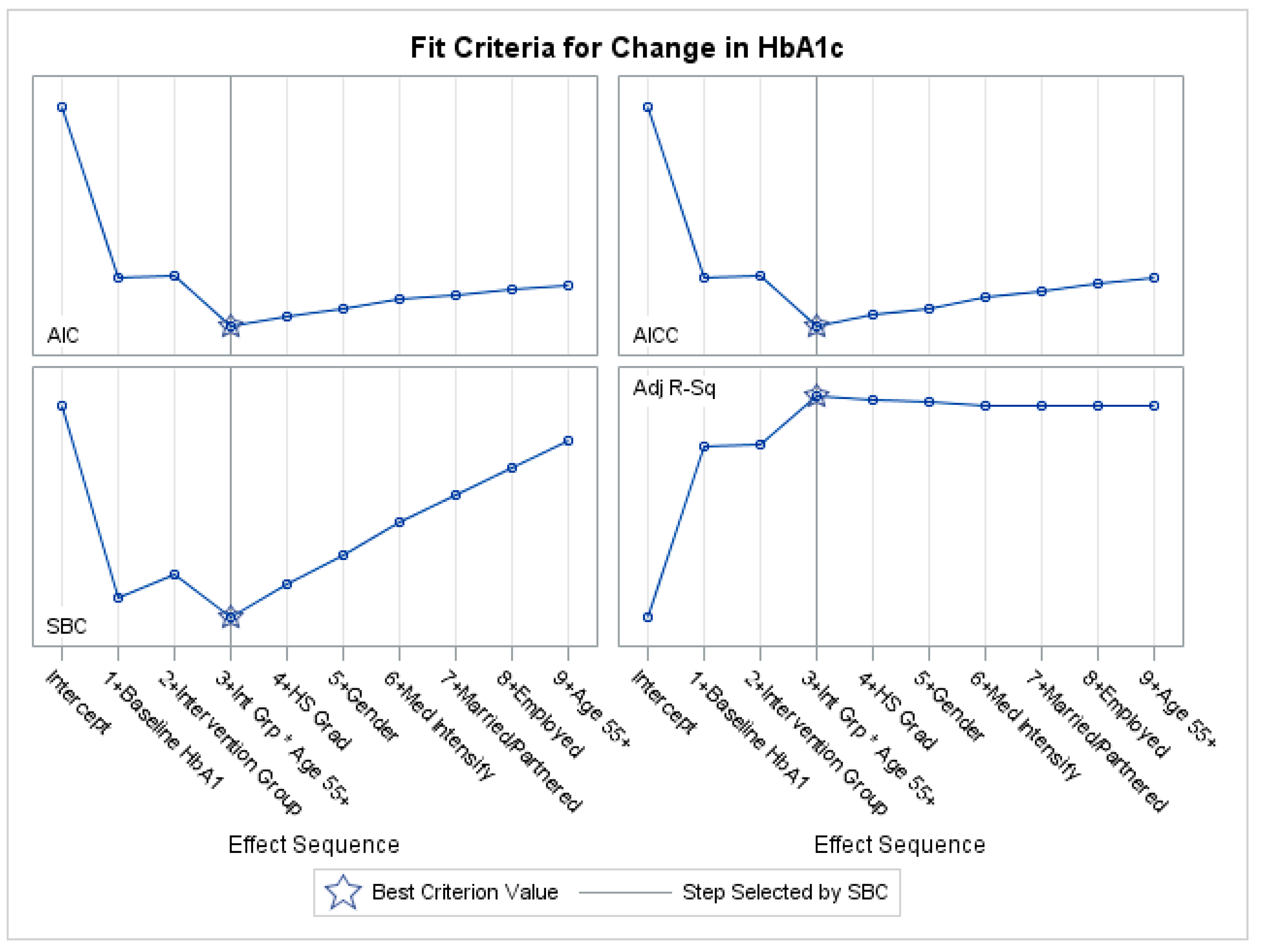
Appendix C. Variable Selection with Penalized Regression
References
- Zimmet, P.Z. Diabetes and its drivers: The largest epidemic in human history? Clin. Diabetes Endocrinol. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 22 September 2017).
- Gallo, L.C.; Fortmann, A.L.; Mccurley, J.L.; Isasi, C.R.; Penedo, F.J.; Daviglus, M.L.; Schneiderman, N. Associations of structural and functional social support with diabetes prevalence in U.S. Hispanics/Latinos: Results from the HCHS/SOL sociocultural ancillary study. J. Behav. Med. 2015, 38, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, N.; Llabre, M.; Cowie, C.C.; Barnhart, J.; Carnethon, M.; Gallo, L.C.; Giachello, A.L.; Heiss, G.; Kaplan, R.C.; Lavange, L.M.; et al. Prevalence of diabetes among hispanics/latinos from diverse backgrounds: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care 2014, 37, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Munshi, M.; Grande, L.; Hayes, M.; Ayres, D.; Suhl, E.; Capelson, R.; Weinger, K. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care 2006, 29, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Meneilly, G.S.; Tessier, D. Diabetes in elderly adults. J. Gerontol. 2001, 56, M5–M13. [Google Scholar] [CrossRef]
- Lin, E.H.; Katon, W.; Von Korff, M.; Rutter, C.; Simon, G.E.; Oliver, M.; Young, B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care 2004, 27, 2154–2216. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. Special considerations with insulin therapy in older adults with diabetes mellitus. Drugs Aging 2011, 28, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Golden, S.H.; Cefalu, W.T. Diabetes and aging: Unique considerations and goals of care. Diabetes Care 2017, 40, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in older adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef] [PubMed]
- Nicklett, E.J.; Omidpanah, A.; Whitener, R.; Howard, B.V.; Manson, S.M. Access to care and diabetes management among older American Indians with Type 2 Diabetes. J. Aging Health 2016, 29, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.; Maryniuk, M.; Beck, J.; Cox, C.E.; Duker, P.; Edwards, L.; McLaughlin, S. National standards for diabetes self-management education and support. Diabetes Care 2012, 35, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Blaum, C.S. Management of diabetes mellitus in older adults: Are national guidelines appropriate? J. Am. Geriatr. Soc. 2002, 50, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Beverly, E.A.; Fitzgerald, S.; Sitnikov, L.; Ganda, O.P.; Caballero, A.E.; Weinger, K. Do older adults aged 60–75 years benefit from diabetes behavioral interventions? Diabetes Care 2013, 36, 1501–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Two Feathers, J.; Kieffer, E.C.; Palmisano, G.; Anderson, M.; Sinco, B.; Janz, N.; Wisdom, K. Racial and ethnic approaches to community health (REACH) detroit partnership: Improving diabetes-related outcomes among African American and Latino adults. Am. J. Public Health 2005, 95, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Palmas, W.; March, D.; Darakjy, S.; Findley, S.E.; Teresi, J.; Carrasquillo, O.; Luchsinger, J.A. Community health worker interventions to improve glycemic control in people with diabetes: A systematic review and meta-analysis. J. Gen. Int. Med. 2015, 30, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Cho, J.S.; Choi, E.; Nieman, C.L.; Joo, J.H.; Lin, F.R.; Han, H.R. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: A systematic review. Am. J. Public Health 2016, 106, e3–e28. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Kaselitz, E.; Heisler, M. The role of community health workers in diabetes: Update on current literature. Curr. Diabetes Rep. 2013, 13, 163–171. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Community Health Workers. American Public Health Association Web Site. Available online: https://www.apha.org/apha-communities/member-sections/community-health-workers (accessed on 19 February 2018).
- Kieffer, E.; Willis, S.; Odoms-Young, A.; Guzman, R.; Allen, A.; Two Feathers, J.; Loveluck, J. Reducing disparities in diabetes among African American and Latino residents of Detroit: The essential role of community planning focus groups. Ethnic. Dis. 2004, 14, S1–S27. [Google Scholar]
- Spencer, M.; Rosland, A.; Kieffer, E.; Sinco, B.; Palmisano, G.; Anderson, M.; Guzman, J.R.; Heisler, M. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am. J. Public Health 2011, 101, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Kieffer, E.C.; Sinco, B.; Piatt, G.; Palmisano, G.; Hawkins, J.; Lebron, A.; Espitia, N.; Tang, T.; Funnell, M.; et al. 18-Month outcomes from a community health worker and peer leader diabetes self-management program for Latino adults. Diabetes Care 2018. [Google Scholar] [CrossRef] [PubMed]
- Two Feathers, J.; Kieffer, E.C.; Palmisano, G.; Anderson, M.; Janz, N.; Spencer, M.S.; James, S.A. The development, implementation, and process evaluation of the reach detroit partnerships diabetes lifestyle intervention. Diabetes Educ. 2007, 33, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Arsie, M.P.; Marchioro, L.; Lapolla, A.; Giacchetto, G.F.; Bordin, M.R.; Rizzotti, P.; Fedele, D. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol. 2000, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Glasgow, R.E.; McKay, H.G.; Boles, S.M.; Feil, E.G. Do internet-based support interventions change perceptions of social support? An experimental trial of approaches for supporting diabetes self-management. Am. J. Commun. Psychol. 2002, 30, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.T.; Anderson, R.M.; Gruppen, L.D.; Davis, W.K.; Aman, L.C.; Jacober, S.J.; Grunberger, G. The reliability of the diabetes care profile for African Americans. Eval. Health Prof. 1998, 21, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Lorig, K.; Stewart, A.; Ritter, P.; González, V.; Laurent, D.; Lynch, J. Outcome Measures for Health Education and Other Health Care Interventions; Sage Publications: Thousand Oaks, CA, USA, 1996; pp. 24–25, 41–45. [Google Scholar]
- Hibbard, J.H.; Mahoney, E.R.; Stockard, J.; Tusler, M. Development and testing of a short form of the patient activation measure. Health Serv. Res. 2005, 40, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Fisher, L.; Earles, J.; Dudl, R.J.; Lees, J.; Mullan, J.; Jackson, R.A. Assessing psychosocial distress in diabetes: Development of the diabetes distress scale. Diabetes Care 2005, 28, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Toobert, D.J.; Hampson, S.E.; Glasgow, R.D. The summary of diabetes self-care activities measure results from 7 studies and a revised scale. Diabetes Care 2000, 23, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Int. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Cochran, W.G. Some methods for strengthening the common chi-squared tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Armitage, P. Tests for linear trends in proportions and frequencies. Biometrics 1955, 11, 375–386. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 360. [Google Scholar]
- Agresti, A.; Wackerly, D.; Boyett, J.M. Exact conditional tests for cross-classifications: Approximation of attained significance levels. Psychometrika 1979, 44, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; CRC Press: New York, NY, USA, 1994. [Google Scholar]
- Edwards, D.; Berry, J.J. The efficiency of simulation-based multiple comparisons. Biometrics 1987, 913–928. [Google Scholar] [CrossRef]
- Trento, M.; Passera, P.; Borgo, E.; Tomalino, M.; Bajardi, M.; Cavallo, F.; Porta, M. A 5-year randomized controlled study of learning, problem solving ability, and quality of life modifications in people with type 2 diabetes managed by group care. Diabetes Care 2004, 27, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Shim, R.; Ford, K.L.; Baker, T.A. The relation between diabetes self-efficacy and psychological distress among older adults. J. Aging Health 2014, 27, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.A.; Bardsley, J.; Cypress, M.; Duker, P.; Funnell, M.M.; Fischl, A.H.; Vivian, E. Diabetes self-management education and support in type 2 diabetes: A joint position statement of the American diabetes association, the American association of diabetes educators, and the academy of nutrition and dietetics. Diabetes Educ. 2017, 43, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Suhl, E.; Bonsignore, P. Diabetes self-management education for older adults: General principles and practical application. Diabetes Spectr. 2006, 19, 234–240. [Google Scholar] [CrossRef]
- International Diabetes Federation. Managing Older People with Type 2 Diabetes: IDF Global Guideline. Action in Global Advocacy. Available online: https://www.actioninglobaladvocacy.com/managing-older-people-with-type-2-diabetes-idf-global-guideline/ (accessed on 26 June 2018).
- AARP. AARP Foundation Study, Framework for Isolation in Adults. Available online: https://www.aarp.org/content/dam/aarp/aarp_foundation/2012_PDFs/AARP-Foundation-Isolation-Framework-Report.pdf (accessed on 25 June 2018).
- National Hispanic Council on Aging. Status of Hispanic Older Adults: Insights from the Field. Available online: http://www.nhcoa.org/wp-content/uploads/2017/09/2017-Status-of-Hispanic-Older-Adults-FV.pdf (accessed on 25 June 2018).
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B 1996, 58, 267–288. [Google Scholar]
- Zou, H. The adaptive lasso and its oracle properties. J. Am. Stat. Assoc. 2006, 101, 1418–1429. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
| Intervention Group | Enhanced Usual Care (n = 73) | Community Health Worker (n = 149) | ||||
|---|---|---|---|---|---|---|
| Age Group | <55 Years (N = 53) | ≥55 Years (N = 20) | p-Value | <55 Years (N = 105) | ≥55 Years (N = 44) | p-Value |
| Demographic Characteristics | ||||||
| Female, n (%) | 34 (64.2%) | 15 (75.0%) | 0.379 a | 66 (62.9%) | 20 (45.5%) | 0.049 a |
| Spanish speaking, n (%) | 44 (83.0%) | 17 (85.0%) | >0.999 b | 87 (82.9%) | 41 (93.2%) | 0.124 b |
| ≥High School Education, n (%) | 26 (49.1%) | 6 (30.0%) | 0.143 a | 27 (25.7%) | 9 (20.5%) | 0.494 a |
| Employed, n (%) | 24 (45.3%) | 8 (40.0%) | 0.685 a | 50 (47.6%) | 13 (29.5%) | 0.042 a |
| Place of Birth | 0.751 b | 0.055 b | ||||
| Mexico | 33 (63.5%) | 12 (60.0%) | 74 (71.2%) | 33 (75.0%) | ||
| Other Latin American Country | 10 (19.2%) | 3 (15.0%) | 18 (17.3%) | 2 (4.5%) | ||
| US | 9 (17.3%) | 5 (25.0%) | 12 (11.5%) | 9 (20.5%) | ||
| US residence for ≥20 years, n (%) | 20 (38.5%) | 11 (55.0%) | 0.204 a | 51 (48.6%) | 20 (45.5%) | 0.728 a |
| Married or partnered, n (%) | 40 (75.5%) | 12 (60.0%) | 0.193 a | 77 (73.3%) | 27 (61.4%) | 0.147 a |
| Health Related Characteristics | ||||||
| Diabetes duration (years), mean (sd) | 4.42 (4.99) | 7.56 (5.54) | 0.055 c | 5.60 (5.54) | 8.00 (6.48) | 0.037 b |
| Antihyperglycemic medication, n (%) | >0.999 d | 0.585 d | ||||
| No medications | 3 (5.7%) | 0 (0.0%) | 6 (5.7%) | 1 (2.3%) | ||
| Only oral diabetes medication | 34 (64.2%) | 15 (75.0%) | 73 (69.5%) | 36 (81.8%) | ||
| Insulin, with or without medication | 16 (30.2%) | 5 (25.0%) | 26 (24.8%) | 7 (15.9%) | ||
| Medication Intensification e, n (%) | 8 (15.1%) | 9 (45.0%) | 0.012 b | 32 (30.5%) | 12 (27.3%) | 0.844 b |
| Outcome Values at Baseline | ||||||
| Diabetes Support, mean (sd) g | 3.97 (1.16) | 4.06 (1.01) | 0.749 f | 4.25 (1.04) | 4.02 (1.13) | 0.233 f |
| Understanding of Diabetes Management h, mean (sd) | 2.79 (0.90) | 2.75 (0.85) | 0.842 f | 2.80 (0.84) | 2.79 (0.72) | 0.952 f |
| Self-Efficacy i, mean (sd) | 7.11 (1.72) | 7.61 (1.48) | 0.249 f | 6.82 (1.71) | 7.38 (1.52) | 0.058 f |
| Patient Activation Measure j (PAM), mean (sd) | 56.03 (10.38) | 56.51 (7.31) | 0.829 f | 56.90 (8.36) | 55.14 (4.19) | 0.090 f |
| Diabetes Distress k, mean (sd) | 2.08 (0.93) | 1.89 (1.01) | 0.462 f | 2.26 (1.11) | 1.69 (0.65) | <0.001 f |
| Self-Management Behavior l, mean (sd) | 3.23 (1.18) | 4.02 (0.94) | 0.009 f | 3.32 (1.29) | 3.64 (1.06) | 0.144 f |
| Depressive Symptoms m, mean (sd) | 5.04 (3.78) | 4.30 (5.45) | 0.583 f | 6.88 (5.98) | 4.16 (3.70) | 0.001 f |
| HbA1c, mean (sd) | 7.83 (1.92) | 7.40 (1.37) | 0.365 f | 8.01 (2.07) | 7.60 (1.47) | 0.171 f |
| Outcome | Age Group | EUC a,b Change | CHW a,b Change | Intervention Effect c |
|---|---|---|---|---|
| Diabetes Support d | <55 | 0.10 (−0.15, 0.35) | 0.83 (0.67, 1.00) *** | 0.74 (0.34, 1.13) *** |
| ≥55 | 0.32 (−0.05, 0.69) | 0.87 (0.60,1.15) *** | 0.56 (−0.05, 1.17) | |
| Understanding of Diabetes Management e | <55 | 0.19 (0.0002, 0.38) * | 0.58 (0.45, 0.71) *** | 0.39 (0.08, 0.70) ** |
| ≥55 | 0.36 (0.08, 0.65) * | 0.38 (0.17, 0.59) *** | 0.02 (−0.46, 0.49) | |
| Self-Efficacy f | <55 | 0.34 (−0.08, 0.76) | 0.62 (0.33, 0.91) *** | 0.28 (−0.40, 0.96) |
| ≥55 | −0.33 (−0.96, 0.31) | 0.95 (0.48, 1.41) *** | 1.27 (0.23, 2.32) * | |
| Patient Activation Measure g (PAM) | <55 | 2.42 (−0.13, 4.98) | 4.45 (2.72, 6.19) *** | 2.03 (−2.06, 6.11) |
| ≥55 | 1.11 (−2.71, 4.93) | 0.62 (−2.20, 3.44) | −0.49 (−6.79, 5.81) | |
| Diabetes Distress h | <55 | −0.06 (−0.30, 0.17) | −0.36 (-0.52, −0.20) *** | −0.29 (−0.67, 0.08) |
| ≥55 | −0.16 (−0.51, 0.19) | −0.31 (-0.57, −0.05) * | −0.15 (−0.72, 0.42) | |
| Self-Management Behavior i | <55 | −0.13 (−0.46, 0.21) | 0.19 (−0.04, 0.42) | 0.32 (−0.22, 0.86) |
| ≥55 | −0.31 (−0.82, 0.19) | 0.40 (0.03, 0.77) * | 0.72 (−0.11, 1.54) | |
| Depressive Symptoms j | <55 | −0.37 (−1.55, 0.80) | −0.87 (−1.67, −0.06) * | −0.49 (−2.40, 1.41) |
| ≥55 | −1.62 (−3.37, 0.12) | −1.47 (−2.77, −0.18) * | 0.15 (-2.73, 3.03) | |
| HbA1c | <55 | −0.02 (−0.40, 0.36) | −0.34 (−0.60, −0.08) ** | −0.32 (−0.93, 0.28) |
| ≥55 | 0.19 (−0.38, 0.76) | −0.83 (−1.25, −0.40) *** | −1.02 (−1.96, −0.07) * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez Campos, B.; Kieffer, E.C.; Sinco, B.; Palmisano, G.; Spencer, M.S.; Piatt, G.A. Effectiveness of a Community Health Worker-Led Diabetes Intervention among Older and Younger Latino Participants: Results from a Randomized Controlled Trial. Geriatrics 2018, 3, 47. https://doi.org/10.3390/geriatrics3030047
Mendez Campos B, Kieffer EC, Sinco B, Palmisano G, Spencer MS, Piatt GA. Effectiveness of a Community Health Worker-Led Diabetes Intervention among Older and Younger Latino Participants: Results from a Randomized Controlled Trial. Geriatrics. 2018; 3(3):47. https://doi.org/10.3390/geriatrics3030047
Chicago/Turabian StyleMendez Campos, Barbara, Edith C. Kieffer, Brandy Sinco, Gloria Palmisano, Michael S. Spencer, and Gretchen A. Piatt. 2018. "Effectiveness of a Community Health Worker-Led Diabetes Intervention among Older and Younger Latino Participants: Results from a Randomized Controlled Trial" Geriatrics 3, no. 3: 47. https://doi.org/10.3390/geriatrics3030047





