Illness Representation and Self-Care Ability in Older Adults with Chronic Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Sample Characteristics
3.2. Latent Profile Analysis
3.3. Group Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ward, B.W.; Schiller, J.S.; Goodman, R.A. Multiple chronic conditions among us adults: A 2012 update. Prev. Chronic Dis. 2014, 11, E62. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Chronic Care: Making the Case for Ongoing Care; Robert Wood Johnson Foundation: Princeton, NJ, USA, 2010. [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion. Chronic Disease Overview. Available online: https://www.cdc.gov/chronicdisease/overview/index.htm (accessed on 26 November 2017).
- Billings, J.; Raven, M.C. Dispelling an urban legend: Frequent emergency department users have substantial burden of disease. Health Aff. (Millwood) 2013, 32, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Rogers, A.; Bower, P. Support for self care for patients with chronic disease. BMJ 2007, 335, 968–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, H.G.; Dickson, V.V.; Fida, R.; Riegel, B.; D’Agostino, F.; Alvaro, R.; Vellone, E. Predictors of hospitalization and quality of life in heart failure: A model of comorbidity, self-efficacy and self-care. Int. J. Nurs. Stud. 2015, 52, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Lee, M.C.; Liang, S.Y.; Lu, Y.Y.; Wang, T.J.; Tung, H.H. Effectiveness of a self-efficacy program for persons with diabetes: A randomized controlled trial. Nurs. Health Sci. 2011, 13, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Dickson, V.V.; Buck, H.; Riegel, B. Multiple comorbid conditions challenge heart failure self-care by decreasing self-efficacy. Nurs. Res. 2013, 62, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Franek, J. Self-management support interventions for persons with chronic disease: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2013, 13, 1–60. [Google Scholar] [PubMed]
- Jonkman, N.H.; Schuurmans, M.J.; Groenwold, R.H.H.; Hoes, A.W.; Trappenburg, J.C.A. Identifying components of self-management interventions that improve health-related quality of life in chronically ill patients: Systematic review and meta-regression analysis. Patient Educ. Couns. 2016, 99, 1087–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Shorey, S.; Seah, B.; Chan, W.X.; Tam, W.W.S.; Wang, W. The effectiveness of psychological interventions on self-care, psychological and health outcomes in patients with chronic heart failure—A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018, 78, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Jolly, K.; Majothi, S.; Sitch, A.J.; Heneghan, N.R.; Riley, R.D.; Moore, D.J.; Bates, E.J.; Turner, A.M.; Bayliss, S.E.; Price, M.J.; et al. Self-management of health care behaviors for copd: A systematic review and meta-analysis. Int. J. Chronic Obstruct. Pulm. Dis. 2016, 11, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Wu, S.V.; Hsieh, N.C.; Tsai, J.M. Self-management programs on egfr, depression, and quality of life among patients with chronic kidney disease: A meta-analysis. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2016, 10, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, H.; Nerenz, D.R.; Steele, D.J. Illness representation and coping with health threats. In Social Psychological Aspects of Health; Baum, A., Taylor, S.E., Singer, J.E., Eds.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1984; Volume 4, pp. 219–252. [Google Scholar]
- Brandes, K.; Mullan, B. Can the common-sense model predict adherence in chronically ill patients? A meta-analysis. Health Psychol. Rev. 2014, 8, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Dempster, M.; Howell, D.; McCorry, N.K. Illness perceptions and coping in physical health conditions: A meta-analysis. J. Psychosom. Res. 2015, 79, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, S.; Allen, W.; Franklin, M.; Peters, R.M. Illness beliefs in african americans with hypertension. West J. Nurs. Res. 2014, 36, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Scharloo, M.; Abbink, J.; van’t Hul, A.; van Ranst, D.; Rudolphus, A.; Weinman, J.; Rabe, K.; Kaptein, A.A. The dynamics of illness perceptions: Testing assumptions of leventhal’s common-sense model in a pulmonary rehabilitation setting. Br. J. Health Psychol. 2010, 15, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Wilski, M.; Tasiemski, T. Illness perception, treatment beliefs, self-esteem, and self-efficacy as correlates of self-management in multiple sclerosis. Acta Neurol. Scand. 2016, 133, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kunschitz, E.; Friedrich, O.; Schoppl, C.; Maitz, J.; Sipotz, J. Illness perception patterns in patients with coronary artery disease. Psychol. Health Med. 2017, 22, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Frostholm, L.; Hornemann, C.; Ornbol, E.; Fink, P.; Mehlsen, M. Using illness perceptions to cluster chronic pain patients: Results from a trial on the chronic pain self-management program. Clin. J. Pain 2018. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Robertson, N.; Graham, C.D.; Williams, J.; Steiner, M.C.; Morgan, M.D.; Singh, S.J. Can we identify patients with different illness schema following an acute exacerbation of copd: A cluster analysis. Respir. Med. 2014, 108, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.; Xavier, R.F.; Ac Pereira, A.C.; Stelmach, R.; Fernandes, F.; Harrison, S.L.; Carvalho, C.R. Identifying copd patients at risk for worse symptoms, hrqol, and self-efficacy: A cluster analysis. Chronic Illn. 2018. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.A.; Posner, S.F.; Huang, E.S.; Parekh, A.K.; Koh, H.K. Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Prev. Chronic Dis. 2013, 10, E66. [Google Scholar] [CrossRef] [PubMed]
- Moss-Morris, R.; Weinman, J.; Petrie, K.J.; Horne, R.; Cameron, L.D.; Buick, D. The revised illness perception questionnaire (IPQ-R). Psychol. Health 2002, 17, 1–16. [Google Scholar] [CrossRef]
- Weinman, J.; Petrie, K.J.; Moss-Morris, R.; Horne, R. The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychol. Health 1996, 11, 431–435. [Google Scholar] [CrossRef]
- Cherrington, C.C.; Lawson, T.N.; Clark, K.B. Illness representation of patients with systolic heart failure. Prog. Cardiovasc. Nurs. 2006, 21, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.; Baas, L.S. Illness representations in patients with chronic kidney disease on maintenance hemodialysis. Nephrol. Nurs. J. 2006, 33, 173–174, 179–186. [Google Scholar] [PubMed]
- Chilcot, J.; Wellsted, D.; Farrington, K. Illness representations are associated with fluid nonadherence among hemodialysis patients. J. Psychosom. Res. 2010, 68, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, J.H.; Stockard, J.; Mahoney, E.R.; Tusler, M. Development of the patient activation measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 2004, 39, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Zimbudzi, E.; Lo, C.; Ranasinha, S.; Fulcher, G.R.; Jan, S.; Kerr, P.G.; Polkinghorne, K.R.; Russell, G.; Walker, R.G.; Zoungas, S. Factors associated with patient activation in an australian population with comorbid diabetes and chronic kidney disease: A cross-sectional study. BMJ Open 2017, 7, e017695. [Google Scholar] [CrossRef] [PubMed]
- Aung, E.; Donald, M.; Williams, G.M.; Coll, J.R.; Doi, S.A. Joint influence of patient-assessed chronic illness care and patient activation on glycaemic control in type 2 diabetes. Int. J. Qual Health Care 2015, 27, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, J.H.; Mahoney, E.R.; Stockard, J.; Tusler, M. Development and testing of a short form of the patient activation measure. Health Serv. Res. 2005, 40, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Bos-Touwen, I.; Schuurmans, M.; Monninkhof, E.M.; Korpershoek, Y.; Spruit-Bentvelzen, L.; Ertugrul-van der Graaf, I.; de Wit, N.; Trappenburg, J. Patient and disease characteristics associated with activation for self-management in patients with diabetes, chronic obstructive pulmonary disease, chronic heart failure and chronic renal disease: A cross-sectional survey study. PLoS ONE 2015, 10, e0126400. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.D.; Gibbs, C.R.; Lip, G.Y. Abc of heart failure. Clinical features and complications. BMJ 2000, 320, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic kidney disease and its complications. Prim. Care 2008, 35, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.; Zieve, D. Chronic Obstructive Pulmonary Disease. Available online: http://www.umm.edu/health/medical/reports/articles/chronic-obstructive-pulmonary-disease (accessed on 26 November 2017).
- Gupta, S.; Goren, A.; Phillips, A.L.; Dangond, F.; Stewart, M. Self-reported severity among patients with multiple sclerosis in the U.S. And its association with health outcomes. Mult. Scler. Relat. Disord. 2014, 3, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Muthen, B.; Muthen, L.K. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol. Clin. Exp. Res. 2000, 24, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.; Villiers-Tuthill, A.; Barker, M.; McGee, H. The contribution of illness perception to psychological distress in heart failure patients. BMC Psychol. 2014, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, Y.; Chilcot, J.; Dekker, F.W.; Halbesma, N.; van Dijk, S. Health-related quality of life trajectories during predialysis care and associated illness perceptions. Health Psychol. 2017, 36, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Halding, A.G.; Grov, E.K. Self-rated health aspects among persons living with chronic obstructive pulmonary disease. Int. J. Chronic Obstruct. Pulmon. Dis. 2017, 12, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Shively, M.J.; Gardetto, N.J.; Kodiath, M.F.; Kelly, A.; Smith, T.L.; Stepnowsky, C.; Maynard, C.; Larson, C.B. Effect of patient activation on self-management in patients with heart failure. J. Cardiovasc. Nurs. 2013, 28, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Porter, A.; Snooks, H.; Button, L.; Evans, B.A. The association between illness representation profiles and use of unscheduled urgent and emergency health care services. Br. J. Health Psychol. 2011, 16, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.Y.; Chang, C.; Chen, C.D. An investigation on illness perception and adherence among hypertensive patients. Kaohsiung J. Med. Sci. 2012, 28, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.; Corte, C. Person-centered analysis of illness representations: A systematic review of the literature. In Proceedings of the Midwest Nursing Research Society Conference, Minneapolis, MN, USA, 6–9 April 2017. [Google Scholar]
- Korpershoek, Y.; Bos-Touwen, I.D.; de Man-van Ginkel, J.M.; Lammers, J.W.; Schuurmans, M.J.; Trappenburg, J. Determinants of activation for self-management in patients with copd. Int. J. Chronic Obstruct. Pulmon. Dis. 2016, 11, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Shifren, K. Individual differences in the perception of optimism and disease severity: A study among individuals with parkinson’s disease. J. Behav. Med. 1996, 19, 241–271. [Google Scholar] [CrossRef] [PubMed]
- Flora, P.K.; Anderson, T.J.; Brawley, L.R. Illness perceptions and adherence to exercise therapy in cardiac rehabilitation participants. Rehabil. Psychol. 2015, 60, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Skinner, T.C.; Carey, M.E.; Cradock, S.; Dallosso, H.M.; Daly, H.; Davies, M.J.; Doherty, Y.; Heller, S.; Khunti, K.; Oliver, L.; et al. Comparison of illness representations dimensions and illness representation clusters in predicting outcomes in the first year following diagnosis of type 2 diabetes: Results from the desmond trial. Psychol. Health 2011, 26, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Fitzpatrick, L.; Considine, J.; Shaban, R.Z.; Curtis, K. Emergency department utilisation among older people with acute and/or chronic conditions: A multi-centre retrospective study. Int. Emerg. Nurs. 2018, 37, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.S.; Khare, R.K.; Courtney, D.M.; Feinglass, J. Lower mortality in sepsis patients admitted through the ed vs direct admission. Am. J. Emerg. Med. 2012, 30, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Berk, L.; Dodd, S.; Jacka, F.N.; Fitzgerald, P.B.; de Castella, A.R.; Filia, S.; Filia, K.; Kulkarni, J.; Jackson, H.J.; et al. Psychometric properties of a scale to measure investment in the sick role: The illness cognitions scale. J. Eval. Clin. Pract. 2012, 18, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, A.A.; Bijsterbosch, J.; Scharloo, M.; Hampson, S.E.; Kroon, H.M.; Kloppenburg, M. Using the common sense model of illness perceptions to examine osteoarthritis change: A 6-year longitudinal study. Health Psychol. 2010, 29, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. What Is Precision Medicine? Available online: https://ghr.nlm.nih.gov/primer/precisionmedicine/definition (accessed on 20 December 2017).
| Subscale | Definition | High Score Indicates | Number of Items |
|---|---|---|---|
| Timeline | Whether an individual perceives their disease to be acute or chronic in nature | A more chronic disease perception | 6 |
| Consequences | The extent of perceived consequences of the disease | More disease consequences | 6 |
| Personal Control | The amount of perceived control a person has over their disease | More control | 6 |
| Treatment Control | The amount of perceived control the medical treatments have over the disease | More control | 5 |
| Coherence | The perceived level of understanding of their disease | More understanding | 5 |
| Cyclical Timeline | Whether an individual perceives their disease to have a stable or unstable pattern from day today | More unstable disease pattern | 4 |
| Emotion | Amount of negative emotion an individual attribute to their disease | More negative emotion | 6 |
| Variables | Entire Sample n = 187 | Groups Derived from Latent Profile Analysis | Chi-Square or ANOVA | ||
|---|---|---|---|---|---|
| Overwhelmed n = 80 | Stable n = 61 | Confident n = 46 | |||
| Chronic Disease | 5.228 | ||||
| HF | 63 (33.7%) | 29 (36.3%) | 16 (26.2%) | 18 (39.1%) | |
| CKD | 65 (34.8%) | 24 (30.0%) | 28 (45.9%) | 13 (28.3%) | |
| COPD | 59 (31.6%) | 27 (33.8%) | 17 (27.9%) | 15 (32.6%) | |
| Age | 64.9 (SD = 8.9) | 64.5 (8.5) | 66.8 (8.8) | 63.1 (9.6) | 2.468 |
| Range: 50–88 | |||||
| Gender | 1.704 | ||||
| Female | 100 (53.8%) | 46 (57.5%) | 33 (55.0%) | 21 (45.7%) | |
| Male | 86 (46.2%) | 34 (42.5%) | 27 (45.0%) | 25 (54.3%) | |
| Race/ethnicity | 9.201 | ||||
| Black | 137 (73.7%) | 53 (66.3%) | 45 (75.0%) | 39 (84.8%) | |
| Hispanic | 15 (8.1%) | 7 (8.8%) | 5 (8.3%) | 3 (6.5%) | |
| White | 30 (16.1%) | 16 (20.0%) | 10 (16.7%) | 4 (8.7%) | |
| Asian | 4 (2.2%) | 4 (5.0%) | 0 (0.0%) | 0 (0.0%) | |
| Education | 0.798 | ||||
| ≤High school | 96 (51.9%) | 38 (48.1%) | 33 (55.0%) | 25 (54.3%) | |
| >High school | 89 (48.1%) | 41 (51.9%) | 27 (45.0%) | 21 (45.7%) | |
| Severity variables | |||||
| Years with disease | 6.9 (SD = 8.6) | 5.3 (5.2) a | 10.3 (12.0) b | 5.3 (6.9) a | 6.550 ** |
| Range: 1–55 | |||||
| Complications | 1.68 (SD = 1.0) | 1.84 (1.0) a | 1.38 (1.0) b | 1.80 (1.1) ab | 3.965 * |
| Range: 0–4 | |||||
| Comorbid conditions | 3.2 (SD = 1.7) | 1.32 | |||
| Range: 0–8 | 3.3 (1.8) | 2.9 (1.4) | 3.3 (1.6) | ||
| Severity | 8.713 | ||||
| Mild | 56 (31.3%) | 19 (24.7%) | 24 (42.1%) | 13 (28.9%) | |
| Moderate | 82 (45.8%) | 34 (44.2%) | 23 (40.4%) | 25 (55.6%) | |
| Severe | 41 (22.9%) | 24 (31.2%) | 10 (17.5%) | 7 (15.6%) | |
| Illness representation variables | |||||
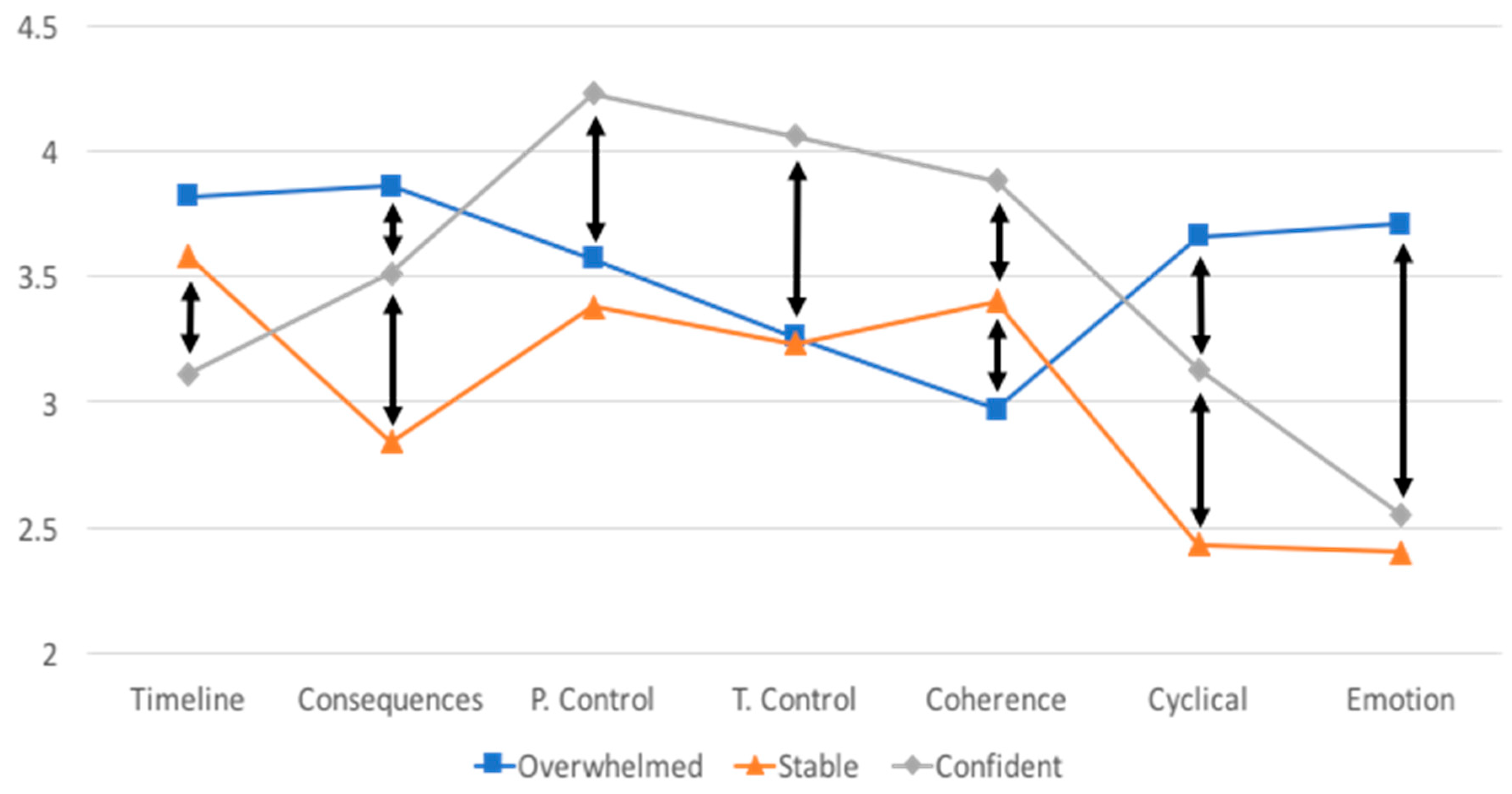
| Timeline | 3.57 (SD = 0.87) | 3.82 (0.76) a | 3.58 (0.81) a | 3.11 (0.98) b | 10.750 *** |
| Range: 1.2–5 | |||||
| Consequences | 3.44 (SD = 0.76) | 3.86 (0.61) a | 2.84 (0.60) b | 3.51 (0.65) c | 48.49 *** |
| Range: 1.2–5 | |||||
| Personal Control | 3.67 (SD = 0.65) | 3.57 (0.57) a | 3.38 (0.62) a | 4.23 (0.47) b | 32.49 *** |
| Range: 2–5 | |||||
| Treatment Control | 3.45 (SD = 0.64) | 3.26 (0.58) a | 3.23 (0.54) a | 4.06 (0.45) b | 39.12 *** |
| Range: 1.8–5 | |||||
| Coherence | 3.33 (SD = 0.85) | 2.97 (0.85) a | 3.40 (0.77) b | 3.88 (0.61) c | 20.80 *** |
| Range: 1–5 | |||||
| Cyclical | 3.13 (SD = 0.87) | 3.66 (0.67) a | 2.43 (0.63) b | 3.13 (0.80) c | 54.37 *** |
| Range: 1–5 | |||||
| Emotion | 3.00 (SD = 0.93) | 3.71 (0.72) a | 2.40 (0.62) b | 2.55 (0.74) b | 73.75 *** |
| Range: 1–5 | |||||
| Outcome variables | |||||
| ED visits | 3.976 | ||||
| 0 | 83 (44.4%) | 29 (36.3%) | 32 (52.5%) | 22 (47.8%) | |
| ≥1 | 104 (55.6%) | 51 (63.8%) | 29 (47.5%) | 24 (52.2%) | |
| Hospitalizations | 7.842 * | ||||
| 0 | 86 (46.0%) | 31 (38.8%) | 37 (60.7%) | 18 (39.1%) | |
| ≥1 | 101 (54.0%) | 49 (61.3%) a | 24 (39.3%) b | 28 (60.9%) a | |
| PAM score | 5.783 | ||||
| Low activation | 84 (44.9%) | 42 (52.5%) | 28 (45.9%) | 14 (30.4%) | |
| High activation | 103 (55.1%) | 38 (47.5%) a | 33 (54.1%) ab | 32 (69.6%) b | |
| Variables | ED Visits (≥1) | Hospitalizations (≥1) | Self-Care Activation (High) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | Z | p | OR | Z | p | OR | Z | p | |
| Model 1: Groups only (n = 187) | |||||||||
| Stable = 0 Overwhelmed = 1 | 1.942 | −1.92 | 0.055 | 2.439 | −2.56 | 0.011 | 0.768 | 0.78 | 0.438 |
| Confident = 0 Overwhelmed = 1 | 1.613 | −1.27 | 0.204 | 1.016 | −0.04 | 0.966 | 0.387 | 2.37 | 0.018 |
| Stable = 0 Confident = 1 | 1.204 | 0.47 | 0.635 | 2.437 | 2.19 | 0.029 | 1.939 | 1.61 | 0.107 |
| Model 2: Groups plus disease years and complications (n = 171) | |||||||||
| Stable = 0 Overwhelmed = 1 | 1.848 | −1.56 | 0.118 | 1.701 | −1.37 | 0.171 | 0.938 | 0.17 | 0.866 |
| Confident = 0 Overwhelmed = 1 | 1.730 | −1.38 | 0.169 | 0.975 | −0.06 | 0.949 | 0.352 | 2.52 | 0.012 |
| Stable = 0 Confident = 1 | 1.068 | 0.15 | 0.879 | 1.751 | 1.28 | 0.200 | 2.662 | 2.15 | 0.031 |
| Years since diagnosis | 1.016 | 0.78 | 0.437 | 0.995 | −0.27 | 0.786 | 1.030 | 1.41 | 0.159 |
| Complications | 1.460 | 2.32 | 0.020 | 1.524 | 2.57 | 0.010 | 0.915 | −0.56 | 0.573 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, E.; Corte, C.; Steffen, A.; DeVon, H.A.; Collins, E.G.; McCabe, P.J. Illness Representation and Self-Care Ability in Older Adults with Chronic Disease. Geriatrics 2018, 3, 45. https://doi.org/10.3390/geriatrics3030045
Rivera E, Corte C, Steffen A, DeVon HA, Collins EG, McCabe PJ. Illness Representation and Self-Care Ability in Older Adults with Chronic Disease. Geriatrics. 2018; 3(3):45. https://doi.org/10.3390/geriatrics3030045
Chicago/Turabian StyleRivera, Eleanor, Colleen Corte, Alana Steffen, Holli A. DeVon, Eileen G. Collins, and Pamela J. McCabe. 2018. "Illness Representation and Self-Care Ability in Older Adults with Chronic Disease" Geriatrics 3, no. 3: 45. https://doi.org/10.3390/geriatrics3030045
APA StyleRivera, E., Corte, C., Steffen, A., DeVon, H. A., Collins, E. G., & McCabe, P. J. (2018). Illness Representation and Self-Care Ability in Older Adults with Chronic Disease. Geriatrics, 3(3), 45. https://doi.org/10.3390/geriatrics3030045





