Systematic Review of Cholinesterase Inhibitors on Cognition and Behavioral Symptoms in Patients of Chinese Descent with Alzheimer’s Disease, Vascular Dementia, or Mixed Dementia
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection and Quality Assessment
2.4. Discrepancy Resolution
3. Results
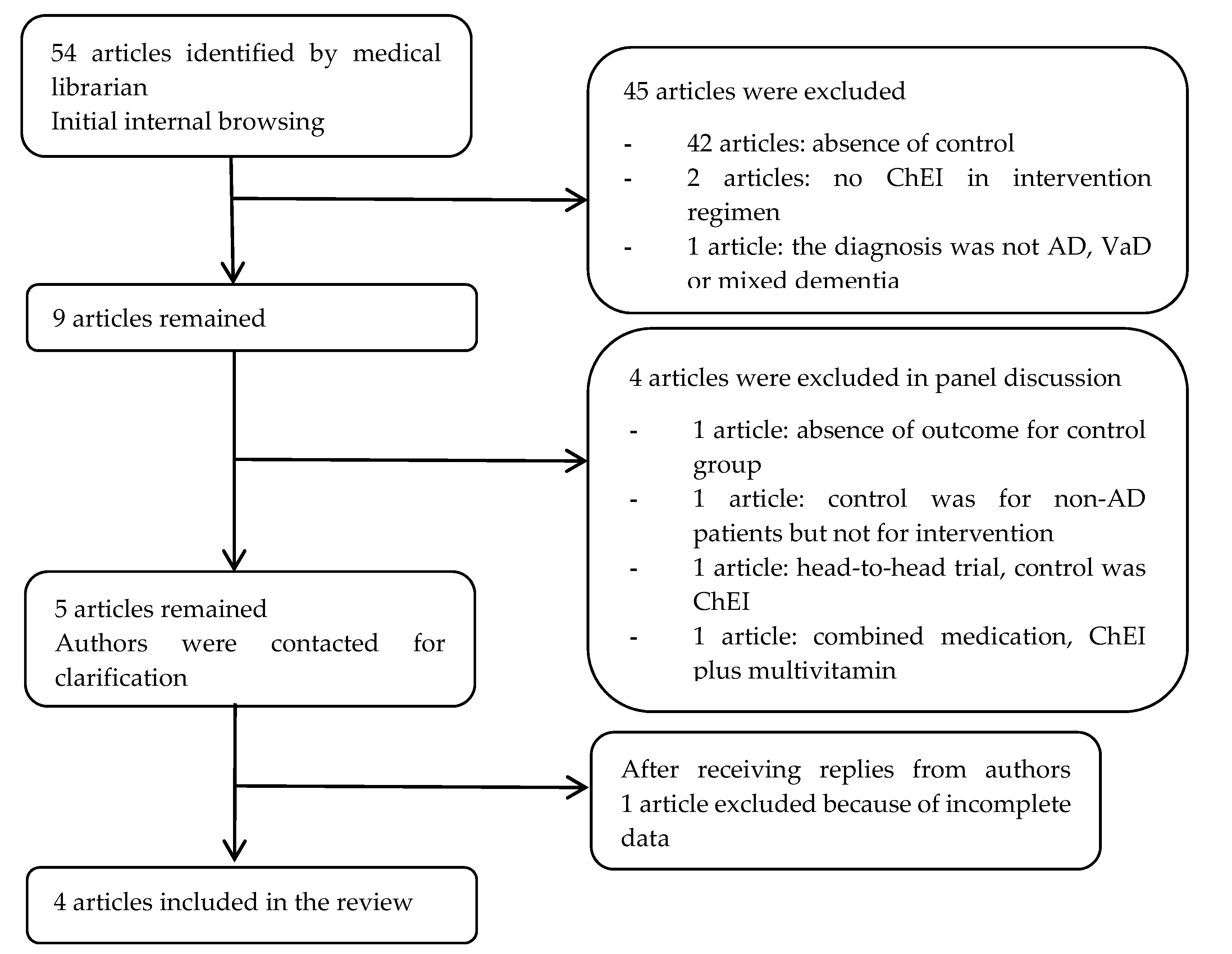
3.1. Literature Search Flow
3.2. Characteristics of Trials and Participants
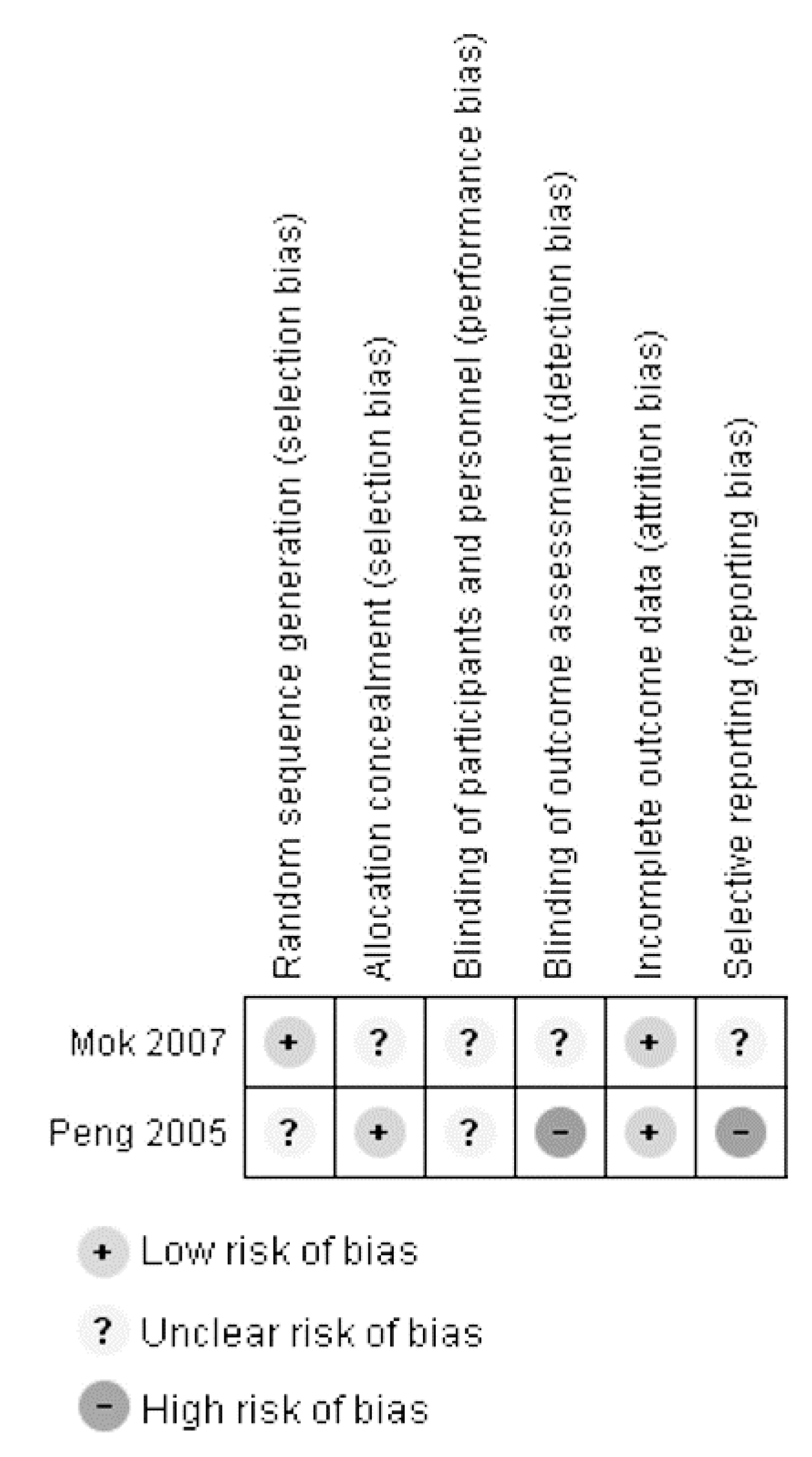
3.3. Assessment of Study Quality
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.; Ferri, C.P.; Acosta, D.; Albanese, E.; Guerra, M.; Huang, Y.; Jacob, K.S.; Jotheeswaran, A.T.; Rodriguez, J.J.; Pichardo, G.R.; et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: A 10/66 Dementia Research Group population-based survey. Lancet 2009, 374, 1821–1830. [Google Scholar] [CrossRef]
- Sousa, R.M.; Ferri, C.P.; Acosta, D.; Guerra, M.; Huang, Y.; Jacob, K.; Jotheeswaran, A.; Hernandez, M.A.; Liu, Z.; Pichardo, G.R.; et al. The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: A 10/66 Dementia Research Group population-based survey. BMC Geriatr. 2010, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; Delon, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Finkel, S.I.; Costa e Silva, J.; Cohen, G.; Miller, S.; Sartorius, N. Behavioral and psychological signs and symptoms of dementia: A consensus statement on current knowledge and implications for research and treatment. Int. Psychogeriatr. 1996, 8 (Suppl. 3), 497–500. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.; Ayub, A.; Boustani, M.A.; Fox, C.; Farlow, M.; Maidment, I.; Howards, R. Impact of cholinesterase inhibitors on behavioral and psychological symptoms of Alzheimer’s disease: A meta-analysis. Clin. Interv. Aging 2008, 3, 719–728. [Google Scholar] [PubMed]
- Haibo, X.; Shifu, X.; Pin, N.T.; Chao, C.; Guorong, M.; Xuejue, L.; Shiming, B.; Wenli, F.; Jun, L.; Mingyuan, Z.; et al. Prevalence and severity of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Chinese: Findings from the Shanghai three districts study. Aging Ment. Health 2013, 17, 748–752. [Google Scholar] [CrossRef]
- Robert, P. Understanding and managing behavioural symptoms in Alzheimer’s disease and related dementias: Focus on rivastigmine. Curr. Med. Res. Opin. 2002, 18, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Schilstrom, B.; Ivanov, V.B.; Wiker, C.; Svensson, T.H. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology 2007, 32, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tangwongchai, S.; Thavichachart, N.; Senanarong, V.; Poungvarin, N.; Phanthumchinda, K.; Praditsuwan, R.; Nidhinandana, S.; Chankrachang, S. Galantamine for the treatment of BPSD in Thai patients with possible Alzheimer’s disease with or without cerebrovascular disease. Am. J. Alzheimers Dis. Other Demen. 2008, 23, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Erkinjuntti, T.; Kurz, A.; Gauthier, S.; Bullock, R.; Lilienfeld, S.; Damaraju, C.V. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: A randomised trial. Lancet 2002, 359, 1283–1290. [Google Scholar] [CrossRef]
- Birks, J.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, Cd001190. [Google Scholar] [CrossRef]
- Mok, V.; Wong, A.; Ho, S.; Leung, T.; Lam, W.W.; Wong, K.S. Rivastigmine in Chinese patients with subcortical vascular dementia. Neuropsychiatr. Dis. Treat. 2007, 3, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Yik, P.Y.; Mok, W.; Chung, C.P. A 2-year open-label study of galantamine therapy in Chinese Alzheimer’s disease patients in Hong Kong. Int. J. Clin. Pract. 2007, 61, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Chong, L.Y.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 9, Cd001191. [Google Scholar] [CrossRef] [PubMed]
- Black, S.E.; Doody, R.; Li, H.; McRae, T.; Jambor, K.M.; Xu, Y.; Sun, Y.; Perdomo, C.A.; Richardson, S. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 2007, 69, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M.; Alzheimer’s Disease International. World Alzheimer Report 2015: The Global Impact of Dementia; Alzheimer’s Disease International: London, UK, 2015; pp. 58–67. [Google Scholar]
- Wu, Y.T.; Lee, H.Y.; Norton, S.; Chen, C.; Chen, H.; He, C.; Fleming, J.; Matthews, F.E.; Brayne, C. Prevalence studies of dementia in mainland China, Hong Kong and Taiwan: A systematic review and meta-analysis. PLoS ONE 2013, 8, e66252. [Google Scholar] [CrossRef]
- Cacabelos, R.; Llovo, R.; Fraile, C.; Fernandez-Novoa, L. Pharmacogenetic aspects of therapy with cholinesterase inhibitors: The role of CYP2D6 in Alzheimer’s disease pharmacogenetics. Curr. Alzheimer Res. 2007, 4, 479–500. [Google Scholar] [CrossRef]
- Bradford, L.D. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002, 3, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available online: www.handbook.cochrane.org (accessed on 30 October 2016).
- Fuh, J.L.; Pwu, R.F.; Wang, S.J.; Chen, Y.H. Measuring Alzheimer’s disease progression with transition probabilities in the Taiwanese population. Int. J. Geriatr. Psychiatry 2004, 19, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Xu, X.; Wang, L. Efficiency and safety assessment of donepezil for treating mild and moderate Alzheimer disease. Chin. J. Clin. Rehabil. 2005, 9, 170–172. [Google Scholar]
- Review Manager (RevMan), Version 5.3.; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014.
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst. Rev. 2006, Cd001747. [Google Scholar] [CrossRef]
- Rodda, J.; Morgan, S.; Walker, Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int. Psychogeriatr. 2009, 21, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Tariot, P.N.; Cummings, J.L.; Katz, I.R.; Mintzer, J.; Perdomo, C.A.; Schwam, E.M.; Whalen, E. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J. Am. Geriatr. Soc. 2001, 49, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Black, S.; Bedard, M.A.; Tran, T.; Lussier, I. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: A multi-centre, primary care, open-label study. Int. J. Geriatr. Psychiatry 2007, 22, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Frisardi, V.; Seripa, D.; D’Onofrio, G.; Santamato, A.; Masullo, C.; Logroscino, G.; Solfrizzi, V.; Pilotto, A. Apolipoprotein E genotypes and neuropsychiatric symptoms and syndromes in late-onset Alzheimer’s disease. Ageing Res. Rev. 2012, 11, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.; Quaranta, D.; Profice, P.; Pilato, F.; Capone, F.; Iodice, F.; Di Lazzaro, V.; Gainotti, G. Central cholinergic dysfunction measured “in vivo” correlates with different behavioral disorders in Alzheimer’s disease and dementia with Lewy body. Brain Stimul. 2012, 5, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, R.B.; Zuidema, S.U.; de Jonghe, J.F.; Verhey, F.R.; Koopmans, R.T. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am. J. Geriatr. Psychiatry 2010, 18, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Selbaek, G.; Engedal, K.; Benth, J.S.; Bergh, S. The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int. Psychogeriatr. 2014, 26, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H. Neuropsychiatric aspects of dementia. Maturitas 2014, 79, 209–215. [Google Scholar] [CrossRef]
- Wynn, Z.J.; Cummings, J.L. Cholinesterase inhibitor therapies and neuropsychiatric manifestations of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2004, 17, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Trzepacz, P.T.; Cummings, J.; Konechnik, T.; Forrester, T.D.; Chang, C.; Dennehy, E.B.; Willis, B.A.; Shuler, C.; Tabas, L.B.; Lyketsos, C. Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer’s disease. Int. Psychogeriatr. 2013, 25, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Lyketsos, C.G.; Peskind, E.R.; Porsteinsson, A.P.; Mintzer, J.E.; Scharre, D.W.; De La Gandara, J.E.; Agronin, M.; Davis, C.S.; Nguyen, U.; et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: A randomized clinical trial. JAMA 2015, 314, 1242–1254. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Drye, L.T.; Pollock, B.G.; Devanand, D.P.; Frangakis, C.; Ismail, Z.; Marano, C.; Meinert, C.L.; Mintzer, J.E.; Munro, C.A.; et al. Effect of citalopram on agitation in Alzheimer disease: The CitAD randomized clinical trial. JAMA 2014, 311, 682–691. [Google Scholar] [CrossRef]
| First Author (Year) | Country | Study Design | Intervention | Treatment Duration, Length of Follow-up | Study Population | Mean Age | Baseline Cognition (MMSE) | Baseline BPSD (NPI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | |||||
| Chu (2007) [14] | Hong Kong | Cohort study | Galantamine Week 1–4: 4 mg twice daily Week 5–8: 8 mg twice daily Week 5–24 month: 12 mg twice daily or 8 mg twice daily depending on tolerability | 2 years, 2 years | 42 | 19 | 78.5 | 78.9 | 14.9 a | 16.1 a | 11.0 a | 7.4 a |
| Mok (2007) [13] | Hong Kong | RCT | Rivastigmine Week 1–4: 1.5 mg twice daily Week 5–26: 3.0 mg twice daily | 26 weeks, 26 weeks | 20 | 20 | 75.7 | 74.1 | 13.0 a | 13.4 a | 15.0 a | 10.6 a |
| Peng (2005) [23] | China | RCT | Donepezil 5 mg once daily | 12 weeks, 12 weeks | 46 | 43 | 72.6 | 71.8 | 17.8 b | 18.2 b | Not provided | |
| Fuh (2004) [22] | Taiwan | Longitudinal observational study | Any ChEIs between follow-up No exposure to ChEIs | Mean = 1.6 years (range: 0.1–3.1 years), mean = 29 months (range: 3–109 months) | 194 | 171 | 73.1 | 73.6 | 16.9 | 12.0 | Not provided | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, K.-C.; Li, V.; Ng, Y.-Z.; Chan, T.-T.; Chang, R.S.; Wong, R.Y. Systematic Review of Cholinesterase Inhibitors on Cognition and Behavioral Symptoms in Patients of Chinese Descent with Alzheimer’s Disease, Vascular Dementia, or Mixed Dementia. Geriatrics 2017, 2, 29. https://doi.org/10.3390/geriatrics2030029
Leung K-C, Li V, Ng Y-Z, Chan T-T, Chang RS, Wong RY. Systematic Review of Cholinesterase Inhibitors on Cognition and Behavioral Symptoms in Patients of Chinese Descent with Alzheimer’s Disease, Vascular Dementia, or Mixed Dementia. Geriatrics. 2017; 2(3):29. https://doi.org/10.3390/geriatrics2030029
Chicago/Turabian StyleLeung, Ka-Chun, Victor Li, Yuey-Zhun Ng, Tsz-Tai Chan, Richard S. Chang, and Roger Y. Wong. 2017. "Systematic Review of Cholinesterase Inhibitors on Cognition and Behavioral Symptoms in Patients of Chinese Descent with Alzheimer’s Disease, Vascular Dementia, or Mixed Dementia" Geriatrics 2, no. 3: 29. https://doi.org/10.3390/geriatrics2030029
APA StyleLeung, K.-C., Li, V., Ng, Y.-Z., Chan, T.-T., Chang, R. S., & Wong, R. Y. (2017). Systematic Review of Cholinesterase Inhibitors on Cognition and Behavioral Symptoms in Patients of Chinese Descent with Alzheimer’s Disease, Vascular Dementia, or Mixed Dementia. Geriatrics, 2(3), 29. https://doi.org/10.3390/geriatrics2030029




