Waist–Calf Circumference Ratio Is Associated with Body Composition, Physical Performance, and Muscle Strength in Older Women
Abstract
1. Introduction
2. Materials and Methods
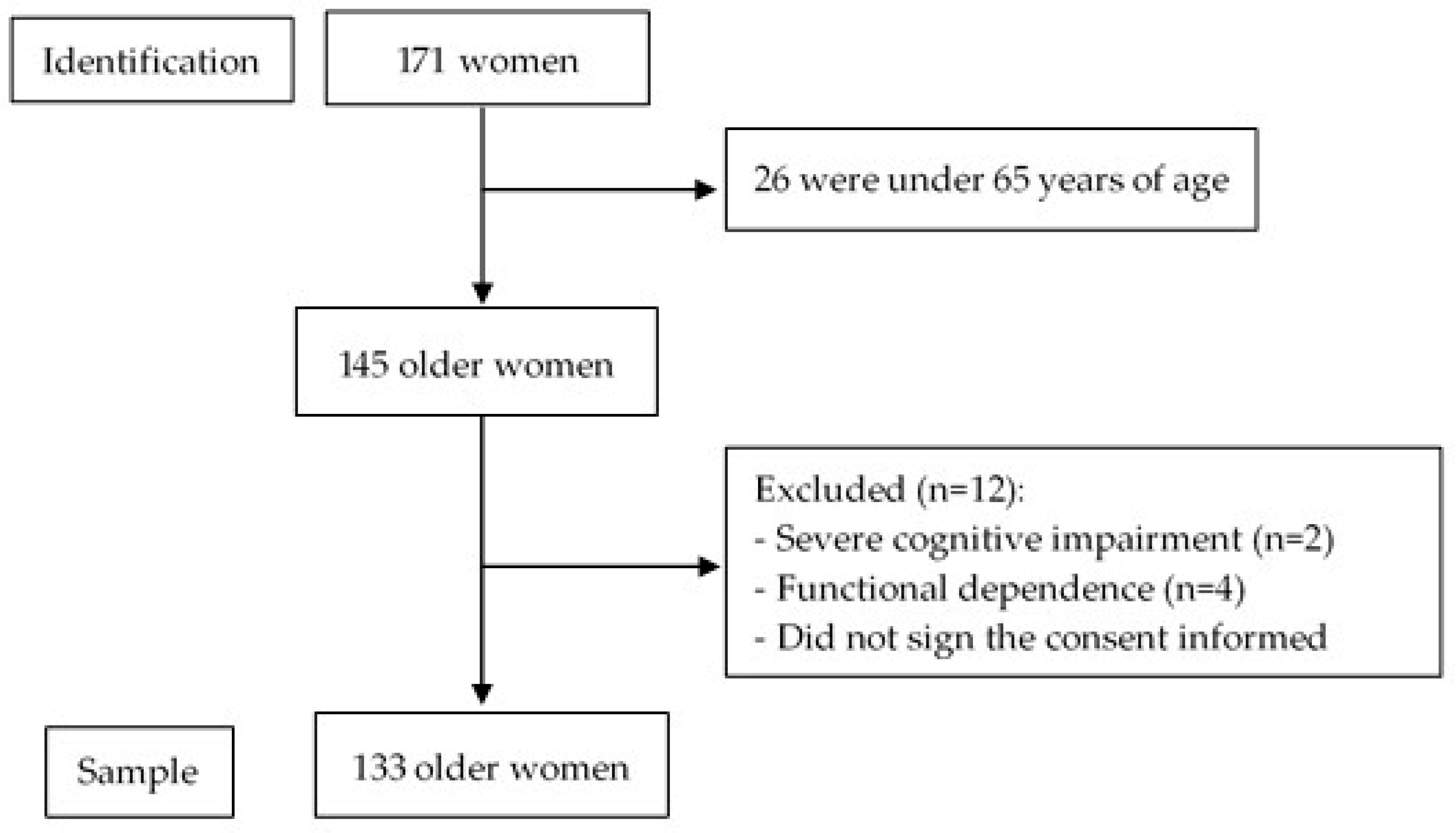
2.1. Participants
2.2. Anthropometric Measures
2.2.1. Waist Circumference Assessment
2.2.2. Calf Circumference
2.3. Body Composition Analysis
2.4. Muscle Function Assessment
2.5. Muscle Strength
2.6. Other Variables
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
3.1. Characteristics of the Subjects
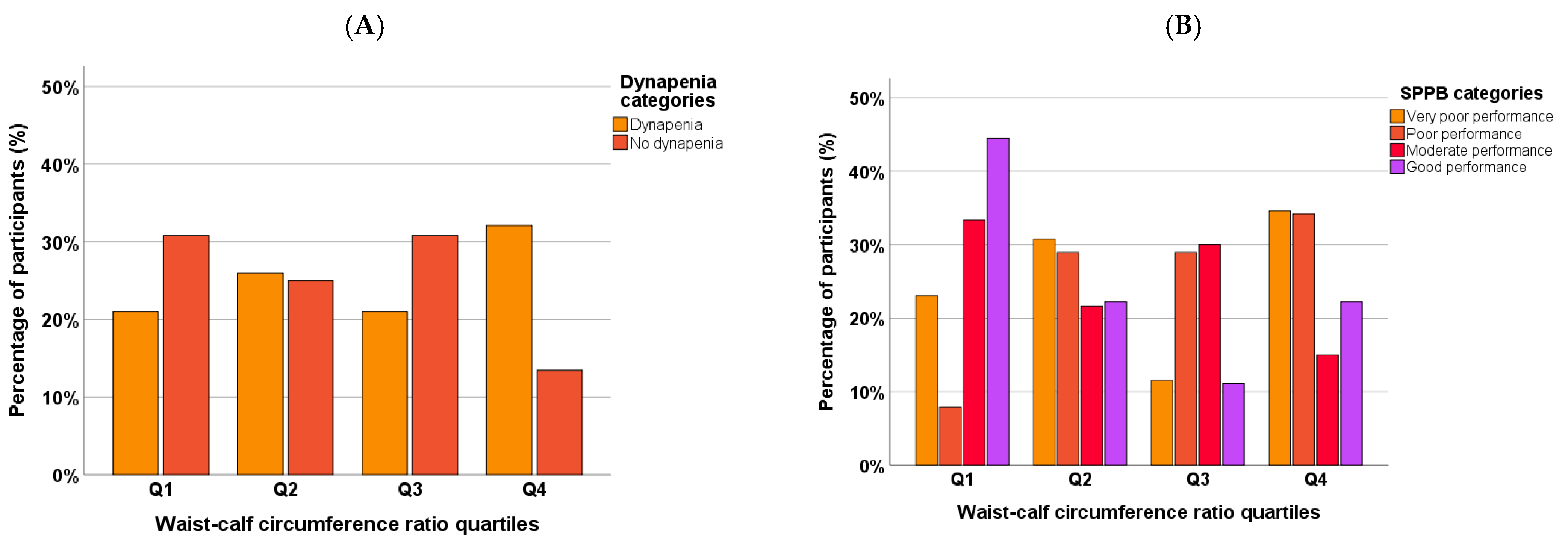
3.2. Waist-to-Calf Ratio (WCR)
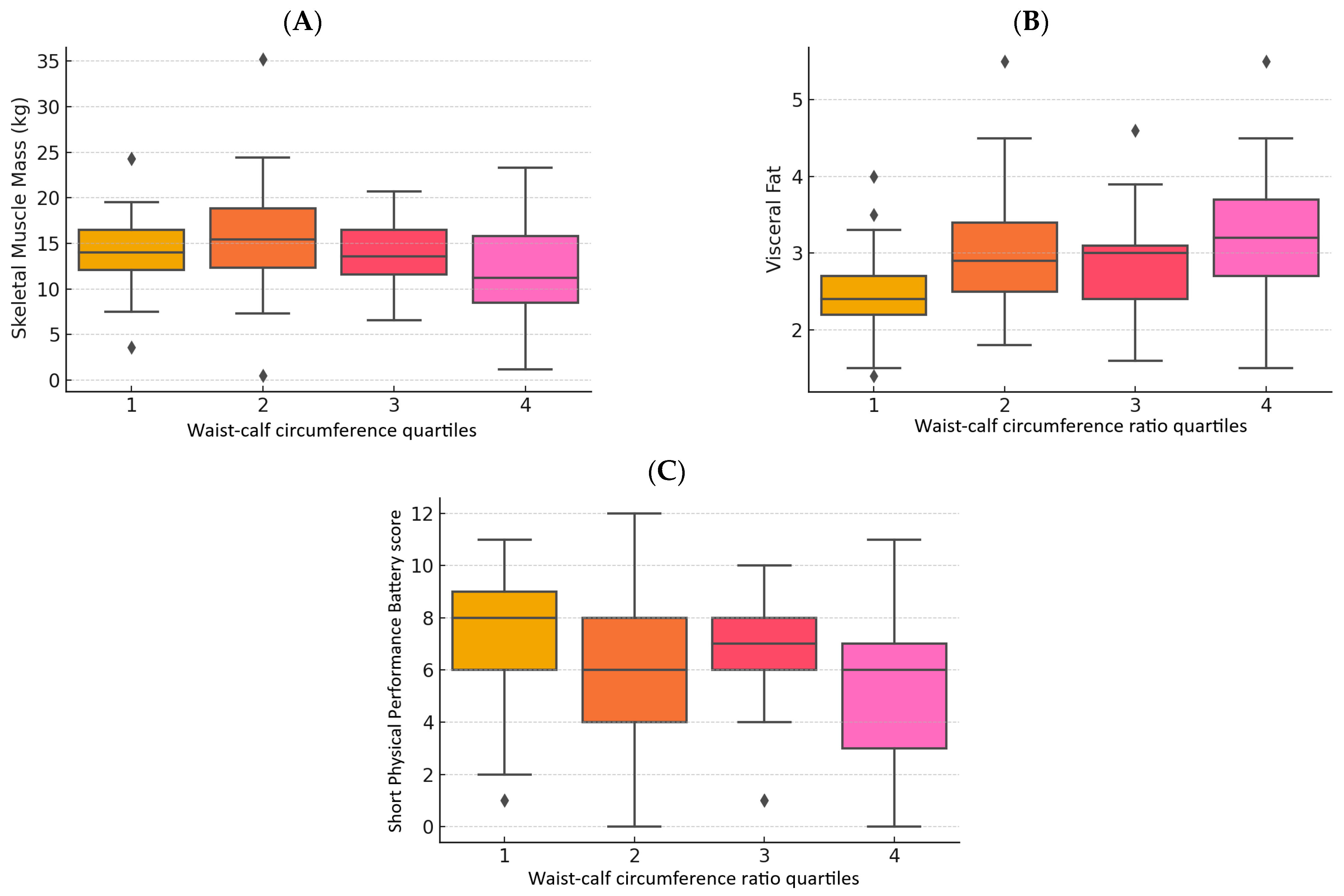
3.3. Body Composition
3.4. Muscle Strength
3.5. Muscle Function
3.6. Association Between WCR and Body Composition, and Muscle Strength and Function
3.7. Regression Model Results
3.8. Post Hoc Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| WCR | Waist–calf circumference ratio |
| FFM | Fat-free mass |
| SMM | Skeletal muscle mass |
| ASM | Appendicular skeletal muscle mass |
| ASMI | Appendicular muscle mass index |
| VF | Visceral fat |
| FM | Fat mass |
| FMI | Fat mass index |
| HGS | Handgrip strength |
| SPPB | Short Physical Performance Battery test |
| WC | Waist circumference |
| CC | Calf circumference |
| BMI | Body mass index |
References
- World Health Organization Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 4 April 2025).
- Global Health Estimates Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 10 March 2025).
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2017. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2019; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2020. [Google Scholar]
- de Magalhães, J.P. Ageing as a Software Design Flaw. Genome Biol. 2023, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Hirono, T.; Takeda, R.; Nishikawa, T.; Ueda, S.; Akamatsu, Y.; Uchida, K.; Mita, Y.; Watanabe, K. Age-Related Decline of Motor Unit Firing Rate in Community-Dwelling Healthy Older Adults and Daily Ingestion of Fish Protein of Alaska Pollack: A Randomised Trial. Int. J. Food Sci. Nutr. 2024, 75, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kamwa, V.; Welch, C.; Hassan-Smith, Z.K. The Endocrinology of Sarcopenia and Frailty. Minerva Endocrinol. 2021, 46, 453–468. [Google Scholar] [CrossRef]
- Frias-Toral, E.; Chapela, S.; de los Angeles Carignano, M.; Moretti, D.; Martinuzzi, A.; Rodríguez-Veintimilla, D.; Guerrero, M.; Pugliese, G.; Muscogiuri, G.; Barrea, L. Mediterranean Diet and Physical Activity for Successful Aging: An Update for Nutritionists and Endocrinologists. Endocrines 2021, 2, 366–383. [Google Scholar] [CrossRef]
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Ransdell, L.B.; Wayment, H.A.; Lopez, N.; Lorts, C.; Schwartz, A.L.; Pugliesi, K.; Pohl, P.S.; Bycura, D.; Camplain, R. The Impact of Resistance Training on Body Composition, Muscle Strength, and Functional Fitness in Older Women (45–80 Years): A Systematic Review (2010–2020). Women 2021, 1, 143–168. [Google Scholar] [CrossRef]
- Vuolo, L.; Barrea, L.; Savanelli, M.C.; Savastano, S.; Rubino, M.; Scarano, E.; Soprano, M.; Illario, M.; Colao, A.; Di Somma, C. Nutrition and Osteoporosis: Preliminary Data of Campania Region of European PERsonalised ICT Supported Service for Independent Living and Active Ageing. Transl. Med. UniSa 2015, 13, 13–18. [Google Scholar]
- Guevara-Ramírez, P.; Tamayo-Trujillo, R.; Cadena-Ullauri, S.; Ruiz-Pozo, V.; Paz-Cruz, E.; Annunziata, G.; Verde, L.; Frias-Toral, E.; Simancas-Racines, D.; Zambrano, A.K. Heavy Metals in the Diet: Unraveling the Molecular Pathways Linked to Neurodegenerative Disease Risk. Food Agric. Immunol. 2024, 35, 2434457. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Ganji, S.S.; Kalyani, R.R. Body Composition Changes in Diabetes and Aging. J. Diabetes Complicat. 2019, 33, 451–459. [Google Scholar] [CrossRef]
- Cassotta, M.; Quiles, J.L.; Giampieri, F.; Battino, M. Aging, Age-Related Diseases, Oxidative Stress and Plant Polyphenols: Is This a True Relationship? Mediterr. J. Nutr. Metab. 2024, 17, 255–259. [Google Scholar] [CrossRef]
- Wiley, E.; Noguchi, K.S.; Moncion, K.; Stratford, P.W.; Tang, A. Sex Differences in Functional Capacity in Older Adults With Stroke: An Analysis of Data From the National Health and Aging Trends Study. Phys. Ther. 2022, 102, pzac077. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.; Raffin, J.; Gonzalez-Bautista, E.; Ritz, P.; Abellan Van Kan, G.; Pillard, F.; Faruch-Bilfeld, M.; Guyonnet, S.; Dray, C.; Vellas, B.; et al. Body Composition and Aging: Cross-Sectional Results from the INSPIRE Study in People 20 to 93 Years Old. Geroscience 2025, 47, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Starkoff, B.E.; Nickerson, B.S. The Evolution of Bioimpedance Analysis: From Traditional Methods to Wearable Technology. Nutrition 2025, 129, 112601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, S.; Yu, J. Evaluating the Association between Anthropometric Parameters and Successful Aging in Older Adults. Geriatr. Nurs. 2023, 50, 240–246. [Google Scholar] [CrossRef]
- Güner, M.; Öztürk, Y.; Ceylan, S.; Okyar Baş, A.; Koca, M.; Balci, C.; Doğu, B.B.; Cankurtaran, M.; Halil, M.G. Evaluation of Waist-to-Calf Ratio as a Diagnostic Tool for Sarcopenic Obesity: A Cross-Sectional Study from a Geriatric Outpatient Clinic. Eur. Geriatr. Med. 2024, 15, 1469–1475. [Google Scholar] [CrossRef]
- Liu, M.; He, P.; Zhou, C.; Zhang, Z.; Zhang, Y.; Li, H.; Ye, Z.; Wu, Q.; Yang, S.; Zhang, Y.; et al. Association of Waist-Calf Circumference Ratio with Incident Cognitive Impairment in Older Adults. Am. J. Clin. Nutr. 2022, 115, 1005–1012. [Google Scholar] [CrossRef]
- Dai, M.; Xia, B.; Xu, J.; Zhao, W.; Chen, D.; Wang, X. Association of Waist-Calf Circumference Ratio, Waist Circumference, Calf Circumference, and Body Mass Index with All-Cause and Cause-Specific Mortality in Older Adults: A Cohort Study. BMC Public Health 2023, 23, 1777. [Google Scholar] [CrossRef]
- Álvarez Córdova, L.R.; Artacho Martín-Lagos, R.; Arteaga Pazmiño, C.; Fonseca Pérez, D.M.; Sierra Nieto, V.H.; Ruiz-López, M.D. Comprehensive geriatric assessment in a marginal community of Ecuador. Nutr. Hosp. 2020, 37, 926–932. [Google Scholar] [CrossRef]
- Lean, M.E.; Han, T.S.; Morrison, C.E. Waist Circumference as a Measure for Indicating Need for Weight Management. BMJ 1995, 311, 158–161. [Google Scholar] [CrossRef]
- Dai, M.; Song, Q.; Yue, J.; Lin, W.; Wang, X.; Ge, N. Is Waist-Calf Circumference Ratio Associated with Frailty in Older Adults? Findings from a Cohort Study. BMC Geriatr 2023, 23, 492. [Google Scholar] [CrossRef]
- Cacciatore, S.; Martone, A.M.; Ciciarello, F.; Galluzzo, V.; Gava, G.; Massaro, C.; Calvani, R.; Tosato, M.; Marzetti, E.; Landi, F. Waist-to-Calf Circumference Ratio as a Potential Indicator of Diabetes Risk: Results from the Longevity Check-Up (Lookup) 8+. Sci. Rep. 2024, 14, 28882. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing Appendicular Skeletal Muscle Mass with Bioelectrical Impedance Analysis in Free-Living Caucasian Older Adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Cildan Uysal, S.; Tonak, H.A.; Kitis, A. Validity, Reliability and Test-Retest Study of Grip Strength Measurement in Two Positions with Two Dynamometers: Jamar® Plus and K-Force® Grip. Hand Surg. Rehabil. 2022, 41, 305–310. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cereda, E. Mini Nutritional Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 29–41. [Google Scholar] [CrossRef]
- Thompson, M.Q.; Theou, O.; Tucker, G.R.; Adams, R.J.; Visvanathan, R. FRAIL Scale: Predictive Validity and Diagnostic Test Accuracy. Australas. J. Ageing 2020, 39, e529–e536. [Google Scholar] [CrossRef]
- Bahat, G.; Erdoğan, T.; İlhan, B. SARC-F and Other Screening Tests for Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 37–42. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research Involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Correa-Rodríguez, M.; Izquierdo, M.; Schmidt-RioValle, J.; González-Jiménez, E. Muscle Fitness to Visceral Fat Ratio, Metabolic Syndrome and Ideal Cardiovascular Health Metrics. Nutrients 2018, 11, 24. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Brasacchio, C.; Laudisio, D.; Lubrano, C.; Pivari, F.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) group Sarcopenic Obesity: What about in the Cancer Setting? Nutrition 2022, 98, 111624. [Google Scholar] [CrossRef]
- Champaiboon, J.; Petchlorlian, A.; Manasvanich, B.-A.; Ubonsutvanich, N.; Jitpugdee, W.; Kittiskulnam, P.; Wongwatthananart, S.; Menorngwa, Y.; Pornsalnuwat, S.; Praditpornsilpa, K. Calf Circumference as a Screening Tool for Low Skeletal Muscle Mass: Cut-off Values in Independent Thai Older Adults. BMC Geriatr. 2023, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.M.; Bertschi, D.; Beerli, N.; Berres, M.; Kressig, R.W.; Fischer, A.M. Calf Circumference as a Surrogate Indicator for Detecting Low Muscle Mass in Hospitalized Geriatric Patients. Aging Clin. Exp. Res. 2024, 36, 25. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Neeland, I.J.; Turer, A.T.; Vega, G.L. Waist Circumference as Measure of Abdominal Fat Compartments. J. Obes. 2013, 2013, 454285. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Lee, S.; Heymsfield, S.B.; Ross, R. Waist Circumference and Abdominal Adipose Tissue Distribution: Influence of Age and Sex2. Am. J. Clin. Nutr. 2005, 81, 1330–1334. [Google Scholar] [CrossRef]
- Arteaga-Pazmiño, C.; Sierra-Nieto, V.; Fonseca-Perez, D.; Álvarez-Córdova, L.; Chedraui, P. Arm Muscle Area and Handgrip Strength in Postmenopausal Women. Maturitas 2023, 173, 117–118. [Google Scholar] [CrossRef]
- Mendes, J.; Afonso, C.; Moreira, P.; Padrão, P.; Santos, A.; Borges, N.; Negrão, R.; Amaral, T.F. Association of Anthropometric and Nutrition Status Indicators with Hand Grip Strength and Gait Speed in Older Adults. JPEN J. Parenter. Enter. Nutr. 2019, 43, 347–356. [Google Scholar] [CrossRef]
- Kemala Sari, N.; Stepvia, S.; Ilyas, M.F. The Association between Anthropometric Measurements and Body Composition with Hand Grip Strength among the Elderly Population in Indonesia. J. Clin. Med. 2024, 13, 4697. [Google Scholar] [CrossRef]
- Álvarez-Córdova, L.R.; Artacho, R.; Chedraui, P.; Arteaga-Pazmiño, C.; Sánchez-Briones, M.; Ruiz-López, M.D. Arm Muscle Area Is Correlated to Handgrip Strength in Postmenopausal Women. Gynecol. Endocrinol. 2024, 40, 2420937. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean Diet and Hand Grip Strength in Older Adult Women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef]
- Paranhos Amorim, D.N.; Nascimento, D.d.C.; Stone, W.; Alves, V.P.; Coelho Vilaça e Silva, K.H. Body Composition and Functional Performance of Older Adults. Osteoporos. Sarcopenia 2022, 8, 86–91. [Google Scholar] [CrossRef] [PubMed]
- de Santana, D.A.; Scolfaro, P.G.; Marzetti, E.; Cavaglieri, C.R. Associations Between Fat and Lean Mass Indexes and Physical Performance in Prefrail and Frail Older Women. J. Frailty Sarcopenia Falls 2024, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Mabe-Castro, D.; Castillo-Aguilar, M.; Mabe-Castro, M.; Méndez Muñoz, R.; Basualto-Alarcón, C.; Nuñez-Espinosa, C.A. Associations between Physical Fitness, Body Composition, and Heart Rate Variability during Exercise in Older People: Exploring Mediating Factors. PeerJ 2024, 12, e18061. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Bettini, S.; Verde, L.; Busetto, L.; Barrea, L.; Muscogiuri, G. Diet and Physical Exercise in Elderly People with Obesity: The State of the Art. Eur. J. Intern. Med. 2024, 130, 9–18. [Google Scholar] [CrossRef]

| Variable | n | % |
|---|---|---|
| Age, years | 75 [IQR: 69.00–85.00] | |
| Ethnicity | ||
| Mestizo | 92 | 69.20 |
| Afro-Ecuadorian | 15 | 11.30 |
| Caucasic | 15 | 11.30 |
| Indigenous | 11 | 8.30 |
| Marital status | ||
| Single | 40 | 30.10 |
| Married | 38 | 28.60 |
| Widowed | 44 | 33.10 |
| Divorced | 11 | 8.30 |
| Instruction | ||
| None | 39 | 29.30 |
| Elementary | 73 | 54.90 |
| High school | 18 | 13.50 |
| University | 3 | 2.30 |
| Height, cm | 147.30 [IQR: 142.85–152.15] | |
| Weight, kg | 60 [IQR: 51.80–66.50] | |
| BMI, kg/m2 | 27.42 ± 4.83 | |
| Waist circumference, cm | 92.37 [IQR: 90.50–94.24] | |
| Abdominal obesity | ||
| Yes | 90 | 67.70 |
| No | 43 | 32.30 |
| Calf circumference, cm | 33.20 [IQR: 29.85–35.90] | |
| Nutritional status | ||
| Malnutrition | 19 | 14.30 |
| Nutritional risk | 61 | 45.90 |
| Normal | 53 | 39.80 |
| Frailty | ||
| Yes | 25 | 18.85 |
| No | 108 | 81.15 |
| Sarcopenia risk | ||
| Yes | 63 | 47.40 |
| No | 70 | 52.60 |
| Variable | Total (n = 133) | Q1 (n = 33) | Q2 (n = 34) | Q3 (n = 33) | Q4 (n = 33) | p Value |
|---|---|---|---|---|---|---|
| FFM, kg | 25.80 [9.45] | 33.40 [6.85] | 37.15 [11.92] | 33.40 [9.90] | 32.60 [10.45] | 0.043 * |
| SMM, kg | 14.10 [6.25] | 14 [4.20] | 15.45 [7.28] | 13.60 [5.20] | 11.20 [7.75] | 0.039 * |
| ASM, kg | 14.25 [3.62] | 14.05 [2.70] | 15.44 [4.89] | 14.46 [3.48] | 13.45 [3.72] | 0.016 * |
| ASMI, kg/m2 | 6.52 [1.36] | 6.50 [1.35] | 7.09 [1.52] | 6.38 [1.41] | 6.28 [1.34] | 0.051 |
| Body fat, kg | 25.80 [9.45] | 25.40 [9.05] | 25.90 [14.12] | 26.60 [8.65] | 24.90 [9.6] | 0.535 |
| BFI, kg/m2 | 11.60 [4.1] | 11.40 [5.15] | 11.60 [6.63] | 12.20 [3.75] | 10.90 [3.65] | 0.568 |
| Visceral fat, l | 2.80 [1.00] | 2.40 [0.60] | 2.90 [0.95] | 3.00 [0.85] | 3.20 [1.10] | 0.000 * |
| HGS, kg | 13 [8.00] | 14 [8.00] | 14 [10.00] | 14 [7.50] | 10 [6.00] | 0.025 * |
| SPPB value | 7 [4.00] | 8.00 [3.00] | 7.00 [3.00] | 6.00 [4.00] | 6.00 [1.5] | 0.029 * |
| Variable | ρ (Spearman) | p Value |
|---|---|---|
| Skeletal muscle mass (kg) | −0.150 | 0.085 |
| ASM (kg) | −0.198 | 0.022 * |
| ASMI (kg/m2) | −0.157 | 0.071 |
| Handgrip strength (kg) | −0.235 | 0.006 * |
| SPPB | −0.156 | 0.074 |
| Visceral fat | 0.387 | <0.001 * |
| Fat mass (kg) | −0.031 | 0.720 |
| Body fat index (kg) | −0.147 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arteaga-Pazmiño, C.; Guzmán-Gurrola, A.L.; Fonseca-Pérez, D.; Galvez-Celi, J.; Aycart, D.F.; Álvarez-Córdova, L.; Frias-Toral, E. Waist–Calf Circumference Ratio Is Associated with Body Composition, Physical Performance, and Muscle Strength in Older Women. Geriatrics 2025, 10, 103. https://doi.org/10.3390/geriatrics10040103
Arteaga-Pazmiño C, Guzmán-Gurrola AL, Fonseca-Pérez D, Galvez-Celi J, Aycart DF, Álvarez-Córdova L, Frias-Toral E. Waist–Calf Circumference Ratio Is Associated with Body Composition, Physical Performance, and Muscle Strength in Older Women. Geriatrics. 2025; 10(4):103. https://doi.org/10.3390/geriatrics10040103
Chicago/Turabian StyleArteaga-Pazmiño, Cecilia, Alma L. Guzmán-Gurrola, Diana Fonseca-Pérez, Javier Galvez-Celi, Danielle Francesca Aycart, Ludwig Álvarez-Córdova, and Evelyn Frias-Toral. 2025. "Waist–Calf Circumference Ratio Is Associated with Body Composition, Physical Performance, and Muscle Strength in Older Women" Geriatrics 10, no. 4: 103. https://doi.org/10.3390/geriatrics10040103
APA StyleArteaga-Pazmiño, C., Guzmán-Gurrola, A. L., Fonseca-Pérez, D., Galvez-Celi, J., Aycart, D. F., Álvarez-Córdova, L., & Frias-Toral, E. (2025). Waist–Calf Circumference Ratio Is Associated with Body Composition, Physical Performance, and Muscle Strength in Older Women. Geriatrics, 10(4), 103. https://doi.org/10.3390/geriatrics10040103









