Validation of a Commercial Indirect ELISA Kit for the Detection of Bovine alphaherpesvirus1 (BoHV-1)-Specific Glycoprotein E Antibodies in Bulk Milk Samples of Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. gE-ELISA (ERADIKIT)
2.2. Criteria for Assay Validation
- Repeatability
- Analytical sensitivity (ASe)
- Diagnostic sensitivity (DSe)
- Diagnostic specificity (DSp)
- Diagnostic accuracy
2.3. Reference Samples
2.4. Repeatability
2.5. Analytical Sensitivity (ASe)
2.6. Diagnostic Sensitivity (DSe) and Diagnostic Specificity (DSp)
2.7. Diagnostic Accuracy
2.8. Statistical Analysis: Tools and Settings
3. Results
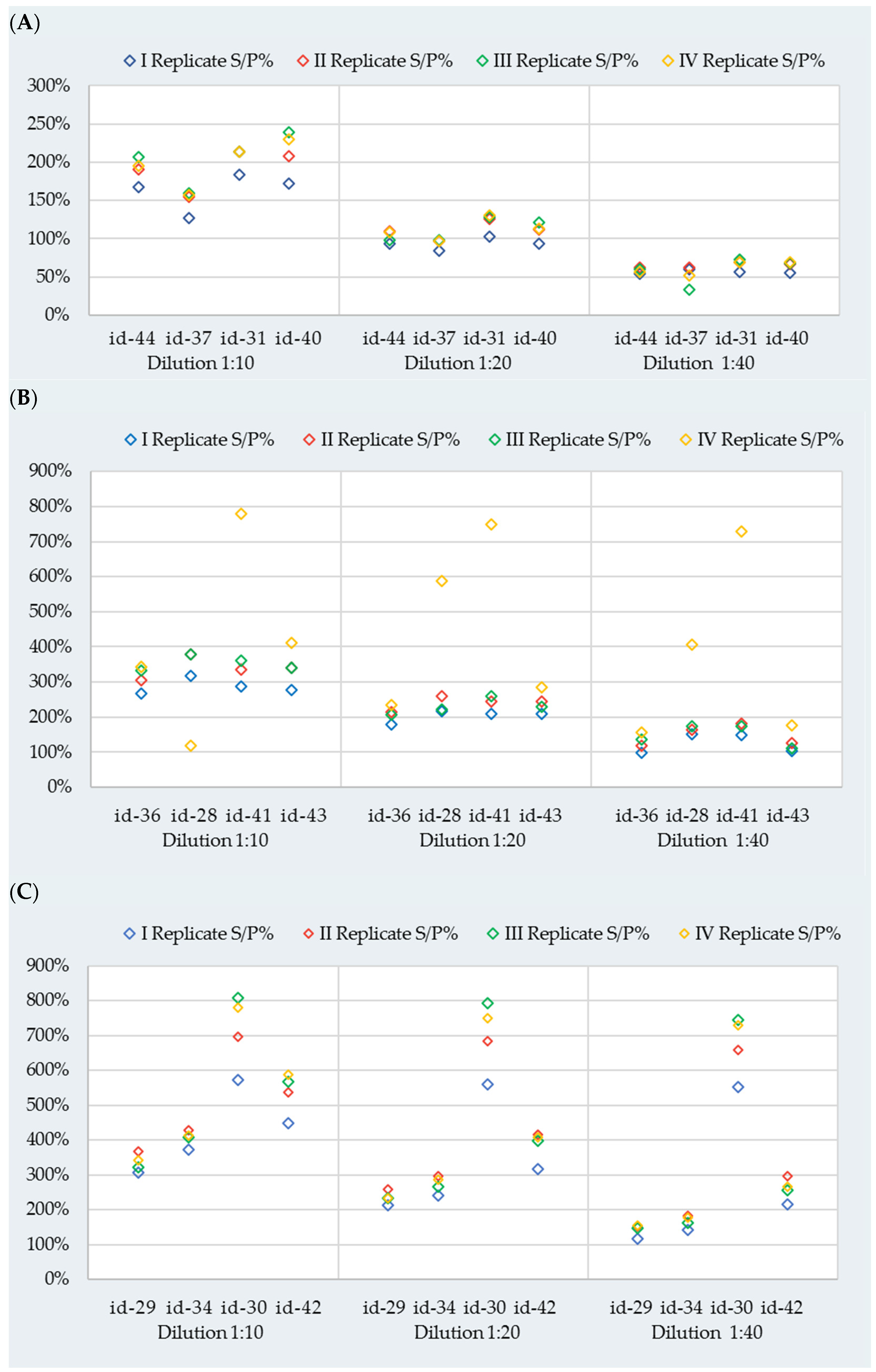
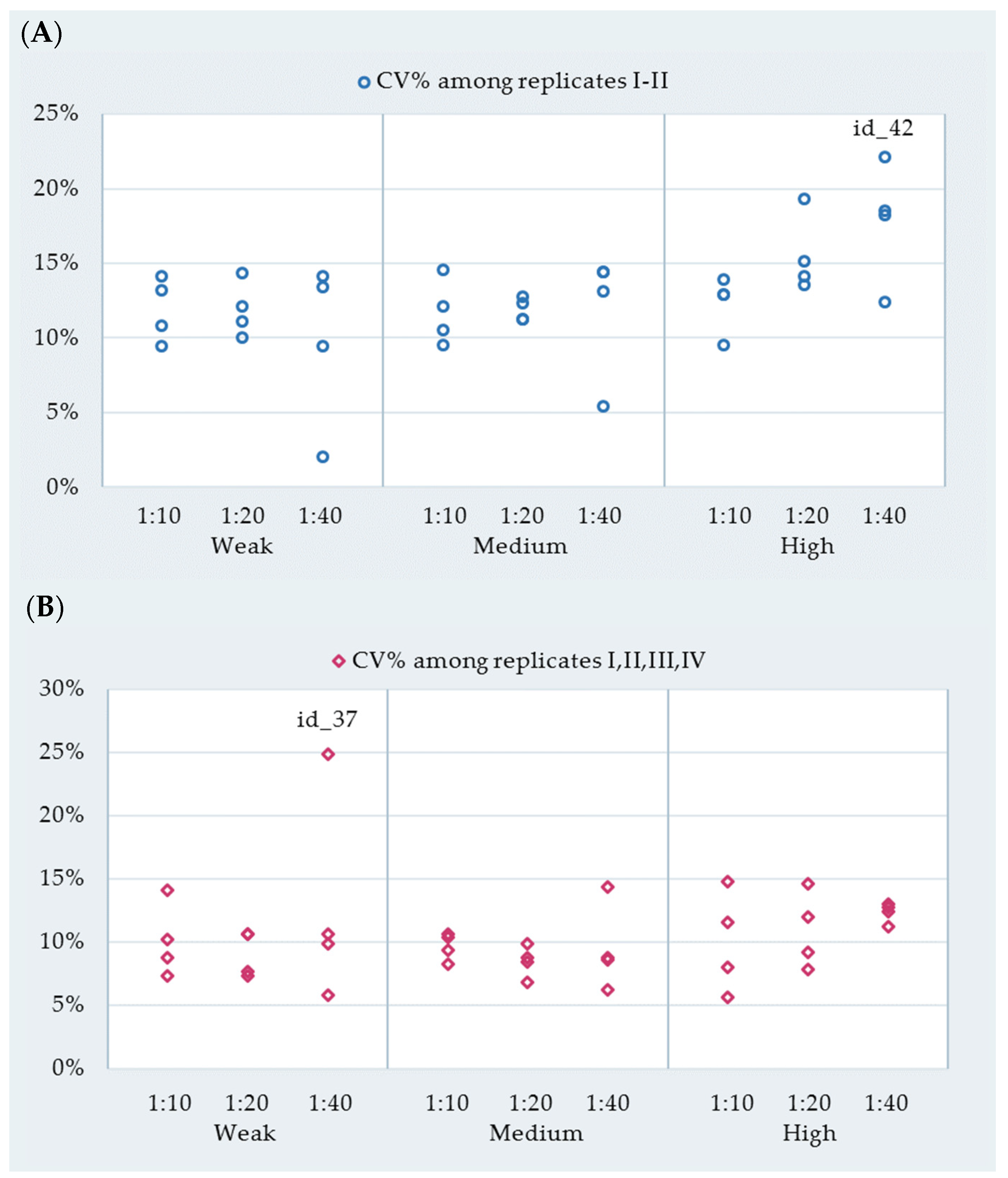
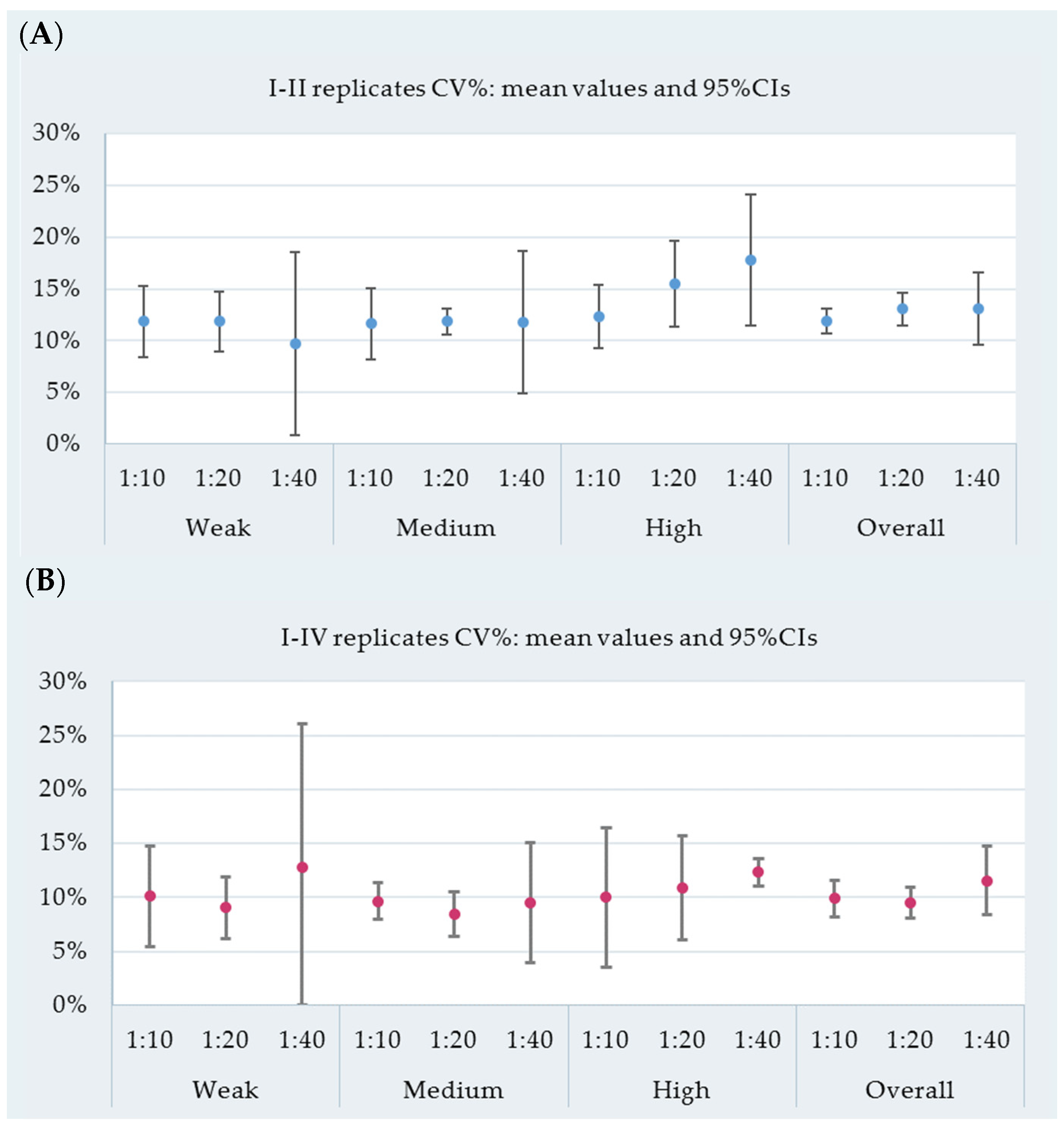
3.1. Repeatability and ASe
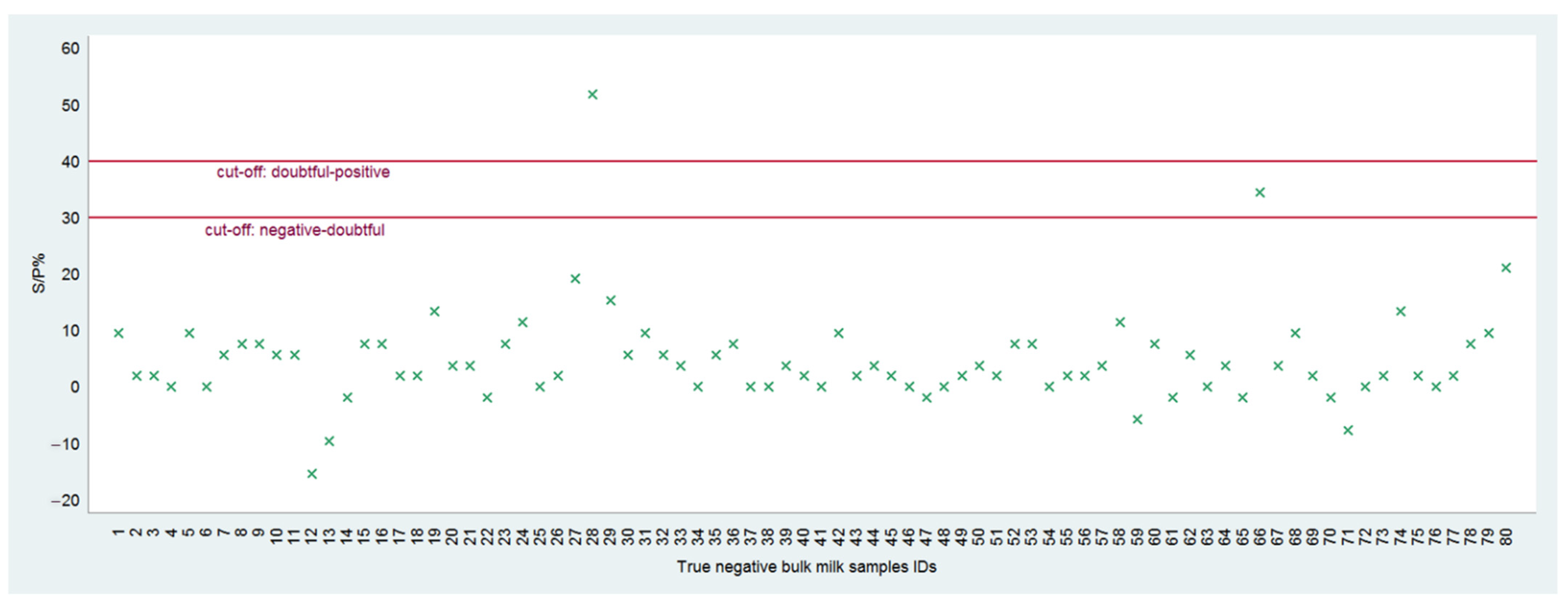
3.2. Diagnostic Performances: DSe, DSp, and Diagnostic Accuracy
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV. ICTV 9th Report (2011): Herpesviridae. Available online: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/dsdna-viruses-2011/w/dsdna viruses/91/ (accessed on 15 October 2021).
- Nandi, S.; Kumar, M.; Manohar, M.; Chauhan, S. Bovine herpesvirus infections in cattle. Anim. Health Res. Rev. 2009, 19, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turin, L.; Russo, S. BHV-1 infection in cattle: An update. Vet. Bull. 2003, 73, 15–21. [Google Scholar]
- Maresca, C.; Scoccia, E.; Dettori, A.; Felici, A.; Guarcini, R.; Petrini, S.; Quaglia, A.; Filippini, G. National surveillance plan for infectious bovine rhinotracheitis (IBR) in autochthonous Italian cattle breeds: Results of first year of activity. Vet. Microbiol. 2018, 219, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet. J. 2014, 201, 249–256. [Google Scholar] [CrossRef]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [Green Version]
- European Union. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016, on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (Animal Health Law); European Union: Brussels, Belgium, 2016; Volume 84, pp. 1–208. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0429&from=IT (accessed on 18 October 2021).
- European Union. Commission Delegated Regulation (EU) 2018/1629 of 25 July 2018, Amending the List of Diseases Set out in Annex II to Regulation (EU) 2016/429 of the European Parliament and of the Council on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (Animal Health Law); European Union: Brussels, Belgium, 2018; Volume 272, pp. 1–5. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1629&from=IT (accessed on 18 October 2021).
- European Union. Commission Implementing Regulation (EU) 2018/1882 of 3 December 2018, on the Application of Certain Disease Prevention and Control Rules to Categories of Listed Diseases and Establishing a List of Species and Groups of Species Posing a Considerable Risk for the Spread of These Diseases; European Union: Brussels, Belgium, 2018; Volume 308, pp. 1–9. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1882&from=EN (accessed on 18 October 2021).
- European Union. Commission Delegated Regulation (EU) 2020/688 of 17 December 2019 Supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council, as Regards Animal Health Requirements for Movements within the Union of Terrestrial Animals and Hatching Eggs; European Union: Brussels, Belgium, 2020; Volume 174, pp. 140–210. Available online: http://data.europa.eu/eli/reg_del/2020/688/oj (accessed on 18 October 2021).
- European Union. Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 Supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards Rules for Surveillance, Eradication Programmes, and Disease-Free Status for Certain Listed and Emerging Diseases; European Union: Brussels, Belgium, 2020; Volume 174, pp. 211–340. Available online: http://data.europa.eu/eli/reg_del/2020/689/2021-04-21 (accessed on 18 October 2021).
- European Union. Commission Delegated Regulation (EU) 2020/692 of 30 January 2020 Supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards Rules for Entry into the Union, and the Movement and Handling after Entry of Consignments of Certain Animals, Germinal Products and Products of Animal Origin; European Union: Brussels, Belgium, 2020; Volume 174, pp. 379–520. Available online: http://data.europa.eu/eli/reg_del/2020/692/oj (accessed on 18 October 2021).
- European Union. Commission Delegated Regulation (EU) 2020/686 of 17 December 2019 Supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards the Approval of Germinal Product Establishments and the Traceability and Animal Health Requirements for Movements within the Union of Germinal Products of Certain Kept Terrestrial Animals; European Union: Brussels, Belgium, 2020; Volume 174, pp. 1–63. Available online: http://data.europa.eu/eli/reg_del/2020/686/oj (accessed on 18 October 2021).
- European Union. Commission Implementing Regulation (EU) 2021/620 of 15 April 2021 Laying down Rules for the Application of Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards the Approval of the Disease-Free and Non-Vaccination Status of Certain Member States or Zones or Compartments Thereof as Regards Certain Listed Diseases and the Approval of Eradication Programmes for Those Listed Diseases; European Union: Brussels, Belgium, 2021; Volume 131, pp. 78–119. Available online: http://data.europa.eu/eli/reg_impl/2021/620/oj (accessed on 31 January 2022).
- Kramps, J.A.; Perrin, B.; Edwards, S.; Van Oirschot, J.T. A European inter-laboratory trial to evaluate the reliability of serological diagnosis of bovine herpesvirus 1 infections. Vet. Microbiol. 1996, 53, 153–161. [Google Scholar] [CrossRef]
- More, S.; Bøtner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429: Infectious bovine rhinotracheitis (IBR). EFSA J. 2017, 15, e04947. [Google Scholar] [CrossRef]
- Mayers, J.; Sawyer, J. Development and evaluation of a multiplex enzyme-linked immunosorbent assay for the detection of antibodies to bovine respiratory diseases on the Meso Scale Discovery platform. J. Vet. Diagn. Investig. 2012, 24, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Manet, G.; Guilbert, M.; Menard, M.; Perrin, B. Etude serologique de la rhinotracheite infectieuse bovine: Comparaison de quelques reactions. Rev. Med. Vet. 1993, 144, 591–598. [Google Scholar]
- Casarin, E.; Lucchese, L.; Grazioli, S.; Facchin, S.; Realdon, N.; Brocchi, E.; Morpurgo, M.; Nardelli, S. A new ELISA using the ANANAS technology showing high sensitivity to diagnose the bovine rhinotracheitis from individual sera to pooled milk. PLoS ONE 2016, 11, e0145912. [Google Scholar]
- Tignon, M.; De Baere, M.; Hanon, J.B.; Goolaerts, A.; Houtain, J.Y.; Delooz, L.; Cay, A.B. Characterization of three commercial ELISA kits for detection of BOHV-1 gE specific antibodies in serum and milk samples and applicability of bulk milk for determination of herd status. J. Virol. Methods 2017, 245, 66–72. [Google Scholar] [CrossRef]
- Kramps, J.A.; Banks, M.; Beer, M.; Kerkhofs, P.; Perrin, M.; Wellenberg, G.J.; Van Oirschot, J.T. Evaluation of tests for antibodies against bovine herpesvirus 1 performed in national reference laboratories in Europe. Vet. Microbiol. 2004, 102, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Colitti, B.; Muratore, E.; Careddu, M.E.; Bertolotti, L.; Iotti, B.; Giacobini, M.; Profiti, M.; Nogarol, C.; Böttcher, J.; Ponzo, A.; et al. Field application of an indirect gE-ELISA on pooled milk samples for the control of IBR in free and marker vaccinated dairy herds. BMC Vet. Res. 2018, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Horner, S.; Bürger, N.; Engemann, C.; Bange, U.; Knoop, E.V.; Gabert, J. Improving the sensitivity of the IBR gE- ELISA for testing IBR marker vaccinated cows from bulk milk. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 290–296. [Google Scholar] [PubMed]
- Bertolotti, L.; Muratore, E.; Nogarol, C.; Caruso, C.; Lucchese, L.; Profiti, M.; Anfossi, L.; Masoero, L.; Nardelli, S.; Rosati, S. Development and validation of an indirect ELISA as a confirmatory test for surveillance of infectious bovine rhinotracheitis in vaccinated herds. BMC Vet. Res. 2015, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Frankena, K.; Franken, P.; Vandehoek, J.; Koskamp, G.; Kramps, J.A. Probability of detecting antibodies to bovine herpesvirus 1 in bulk milk after the introduction of a positive animal on to a negative farm. Vet. Rec. 1997, 140, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Petrini, S.; König, P.; Righi, C.; Iscaro, C.; Pierini, I.; Casciari, C.; Pellegrini, C.; Gobbi, P.; Giammarioli, M.; De Mia, G.M. Serological cross-reactivity between Bovine alphaherpesvirus 2 and Bovine alphaherpesvirus 1 in a gB-ELISA: A case report in Italy. Front. Vet. 2020, 7, 587–885. [Google Scholar] [CrossRef]
- Valas, S.; Bremaud, I.; Stourm, S.; Croise, B.; Memeteau, S.; Ngwa-Mbot, D.; Tabouret, M. Improvement of eradication program for infectious bovine rhinotracheitis in France inferred by serological monitoring of singleton reactors in certified BoHV-1-free herds. Prev. Vet. Med. 2019, 171, 104743. [Google Scholar] [CrossRef]
- Bottcher, J.; Boje, L.; Janowetz, B.; Alex, M.; Konig, P.; Hagg, M.; Gotz, F.; Renner, K.; Otterbein, C.; Mages, J.; et al. Epidemiologically non-feasible singleton reactors at the final stage of BoHV-1 eradication: Serological evidence of BoHV-2 cross reactivity. Vet. Microbiol. 2012, 159, 282–290. [Google Scholar] [CrossRef]
- Petrini, S.; Righi, C.; Iscaro, C.; Viola, G.; Gobbi, P.; Scoccia, E.; Rossi, E.; Pellegrini, C.; De Mia, G.M. Evaluation of passive immunity induced by immunisation using two inactivated gE-deleted marker vaccines against infectious bovine rhinotracheitis (IBR) in calves. Vaccines 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Petrini, S.; Martucciello, A.; Grandoni, F.; De Matteis, G.; Cappelli, G.; Giammarioli, M.; Scoccia, E.; Grassi, C.; Righi, C.; Fusco, G.; et al. Evaluation of safety and efficacy of an inactivated marker vaccine against bovine alphaherpesvirus 1 (BoHV-1) in water buffalo (Bubalus bubalis). Vaccines 2021, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Marnila, P.; Korhonen, H. Immunoglobulins. In Encyclopedia of Dairy Science; Academic Press: London, UK, 2002; pp. 1950–1956. [Google Scholar]
- Van Oirschot, J.T. Diva vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef]
- Muratore, E.; Bertolotti, L.; Nogarol, C.; Caruso, C.; Lucchese, L.; Iotti, B.; Ariello, D.; Moresco, A.; Masoero, L.; Nardelli, S. Surveillance of infectious bovine Rhinotracheitis in marker-vaccinated dairy herds: Application of a recombinant gE-ELISA on bulk milk samples. Vet. Immunol. Immunopathol. 2017, 185, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wellenberg, G.J.; Verstraten, E.R.A.M.; Mars, M.H.; Van Oirschot, J.T. Detection of bovine herpesvirus 1 glycoprotein E antibodies in individual milk samples by enzyme-linked immunosorbent assays. J. Clin. Microbiol. 1998, 36, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król, J.; Litwińczuk, Z.; Brodziak, A.; Barłowska, J. Lactoferrin, lysozyme and immunoglobulin G content in milk of four breeds of cows managed under intensive production system. Pol. J. Vet. Sci. 2010, 13, 357–361. [Google Scholar] [PubMed]
- OIE. Principles and Methods of Validation of Diagnostic Assays for Infectious Diseases. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Chapter 1.1.6; Office Intl Des Epizooties: Paris, France, 2021; pp. 1–16. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/1.01.06_VALIDATION.pdf (accessed on 13 October 2021).
- OIE. Statistical Approaches to Validations. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Chapter 2.2.5; Office Intl Des Epizooties: Paris, France, 2021; pp. 210–221. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.02.05_STATISTICAL_VALIDATION.pdf (accessed on 13 October 2021).
- European Union. Commission Decision 2004/558/EC of 15 July 2004 Implementing Council Directive 64/432/EEC as Regards Additional Guarantees for Intra-Community Trade in Bovine Animals Relating to Infectious Bovine Rhinotracheitis and the Approval of the Eradication Programmes Presented by Certain Member States (Notified under Document Number C (2004) 2104); European Union: Brussels, Belgium, 2020; Volume 249, pp. 20–25. Available online: http://data.europa.eu/eli/dec/2004/558/oj (accessed on 24 November 2021).
- OIE. Infectious Bovine Rhinotracheitis/Infectious Pustular Vulvovaginitis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Chapter 3.4.11; Office Intl Des Epizooties: Paris, France, 2018; pp. 1139–1157. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.11_IBR_IPV.pdf (accessed on 31 January 2022).
- Gwet, K.L. Computing inter-rater reliability and its variance in the presence of high agreement. Br. J. Math. Stat. Psychol. 2008, 61, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Gwet, K.L. Handbook of Inter-Rater Reliability, 4th ed.; Advanced Analytics, LLC: Trumbull, CT, USA, 2014; ISBN 978-0970806284. [Google Scholar]
- Altman, D.G. Practical Statistics for Medical Research, 1st ed.; Chapman & Hall: London, UK, 1991; ISBN 978-0412276309. [Google Scholar]
- Parreno, V.; Alejandra Romera, S.; Makek, L.; Rodriguez, D.; Malacari, D.; Maidana, S.; Compaired, D.; Combessies, G.; Vena, M.M.; Garaicoechea, L.; et al. Validation of an indirect ELISA to detect antibodies against BoHV-1 in bovine and guinea-pig serum samples using ISO/IEC 17025 standards. J. Virol. Methods 2010, 169, 143–153. [Google Scholar] [CrossRef]
- Jacobson, R.H. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. 1998, 17, 469–486. [Google Scholar] [CrossRef]
- Nylin, B.; Stroger, U.; Ronsholt, L. A retrospective evaluation of a Bovine Herpesvirus-1 (BHV-1) antibody ELISA on bulk-tank milk samples for classification of the BHV-1 status of Danish dairy herds. Prev. Vet. Med. 2000, 47, 91–105. [Google Scholar] [CrossRef]
- Reber, A.; Reist, M.; Schwermer, H. Cost-effectiveness of bulk-tank milk testing for surveys to demonstrate freedom from infectious bovine rhinotracheitis and bovine enzootic leucosis in Switzerland. Schweiz. Arch. Tierheilkd. 2012, 154, 189–197. [Google Scholar] [CrossRef]
| Process Steps | Protocols | |||
|---|---|---|---|---|
| gE I | gE II | gE III | ||
| Diagnostic Matrix | Whole Milk | Whole Milk | Skimmed Milk | |
| Purification and concentration of IgG | Incubation | 5 min on ice with rennet-based casein precipitation | 20 min at 37 °C with precipitating reagent A; curd broken by manual agitation and incubated on ice for 10 min | 10 min at 37 °C followed by 50 min with precipitating reagent A, always in a water bath |
| Centrifugation | 3600× g for 10 min at 4 °C | 3600× g for 10 min at 4 °C | 2500× g for 15 min at 23 ± 3 °C | |
| Separation from lipids and curd | 6 mL milk with affinity matrix for 10 min | 7 mL whey added with equal volume of precipitating reagent B; 60 min incubation on a platform shaker at 23 ± 3 °C | 7 mL whey milk added with equal volume of precipitating reagent B; 30 min incubation on a platform shaker at 23 ± 3 °C | |
| Centrifugation and wash | Centrifuge and wash twice | 3600× g for 10 min at 18 ± 3 °C and tube upside down (1–2 min) | 2500× g for 20 min at 23 ± 3 °C and tube upside down (1–2 min) | |
| Elution | In 200 μL glycine buffer | In 400 μL serum dilution buffer | In 800 μL serum dilution buffer | |
| Confirm the presence of IgG | Bradford Quantification assay Bovine Gamma Globulin (BIORAD Quick Start) standard curve | Once a compact pellet becomes visible, detach it and vortex to obtain a consistent solution | Once a compact pellet becomes visible, detach it and vortex to obtain a consistent solution | |
| ELISA | Volume sample (μL) | Dilution 1:2 in phosphate-buffered saline (PBS) containing 1.25% casein | 200 μL IgG concentrated sample | 200 μL IgG concentrated sample |
| Incubation | 60 min at 23 ± 3 °C | 2 h at 23 ± 3 °C | 16 ± 2 h at 2–8 °C | |
| Addition of peroxidase-labelled secondary antibody | Diluted at 10 ng/mL in PBS containing 1.25% casein | Diluted at 10 ng/mL in PBS containing 1.25% casein | Before use, dilute the 100 X conjugate in conjugate dilution buffer | |
| Group | Cow ID | Samples | |||||
|---|---|---|---|---|---|---|---|
| Serum | Individual Milk | ||||||
| gE-ELISA d | gB-ELISA e | VNT f | Whole-Virus ELISA g | gE-ELISA d | gB-ELISA e | ||
| 1 a | 31 | + | + | 1:512 | + | + | + |
| 37 | + | + | 1:512 | + | + | + | |
| 40 | + | + | 1:256 | + | + | + | |
| 44 | + | + | 1:256 | + | + | + | |
| 2 b | 28 | + | + | 1:1024 | + | + | + |
| 36 | + | + | 1:2048 | + | + | + | |
| 41 | + | + | 1:1024 | + | + | + | |
| 43 | + | + | 1:1024 | + | + | + | |
| 3 c | 29 | + | + | 1:2048 | + | + | + |
| 30 | + | + | 1:4096 | + | + | + | |
| 34 | + | + | 1:4096 | + | + | + | |
| 42 | + | + | 1:4096 | + | + | + | |
| Cow ID | OD Values a | S/P% e | Reactivity | Dilutions Performed | No. of Positive Reference Samples | ||
|---|---|---|---|---|---|---|---|
| Ag+ b | Ag– c | Net d | |||||
| 44 | 1.314 | 0.155 | 1.159 | 264.6 | Weak | 1:10 1:20 1:40 | 4 4 4 |
| 37 | 1.491 | 0.262 | 1.229 | 280.6 | |||
| 31 | 1.581 | 0.213 | 1.368 | 312.3 | |||
| 40 | 1.616 | 0.158 | 1.458 | 332.9 | |||
| 36 | 2.322 | 0.2 | 2.122 | 484.5 | Medium | 1:10 1:20 1:40 | 4 4 4 |
| 28 | 2.492 | 0.279 | 2.213 | 505.3 | |||
| 41 | 2.703 | 0.46 | 2.243 | 512.1 | |||
| 43 | 2.545 | 0.166 | 2.379 | 543.2 | |||
| 29 | 2.985 | 0.272 | 2.713 | 619.4 | High | 1:10 1:20 1:40 | 4 4 4 |
| 34 | 3.002 | 0.25 | 2.752 | 628.3 | |||
| 30 | 4.029 | 0.958 | 3.071 | 701.1 | |||
| 42 | 3.467 | 0.206 | 3.261 | 744.5 | |||
| Total of positive reference samples | 36 | ||||||
| Reactivity | Cow ID | Dilution | Replicates (S/P%) | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| Weak | id-44 | 1:10 | 167.3 | 191.1 | 207.1 | 195.0 |
| id-37 | 126.9 | 154.9 | 159.2 | 157.1 | ||
| id-31 | 183.6 | 214.0 | 213.3 | 213.9 | ||
| id-40 | 172.1 | 207.7 | 239.8 | 229.5 | ||
| id-44 | 1:20 | 93.8 | 109.8 | 98.0 | 108.7 | |
| id-37 | 83.8 | 96.6 | 98.7 | 97.2 | ||
| id-31 | 102.9 | 126.2 | 128.8 | 130.4 | ||
| id-40 | 94.0 | 111.5 | 121.7 | 113.4 | ||
| id-44 | 1:40 | 54.7 | 62.6 | 59.7 | 56.8 | |
| id-37 | 60.6 | 62.3 | 33.9 | 52.4 | ||
| id-31 | 56.5 | 68.9 | 72.4 | 69.3 | ||
| id-40 | 55.6 | 67.2 | 68.4 | 69.6 | ||
| Medium | id-36 | 1:10 | 268.0 | 306.4 | 332.1 | 341.7 |
| id-28 | 318.8 | 378.3 | 379.3 | 380.0 | ||
| id-41 | 288.8 | 335.3 | 359.9 | 365.6 | ||
| id-43 | 277.5 | 340.6 | 340.1 | 330.4 | ||
| id-36 | 1:20 | 179.3 | 213.4 | 207.7 | 220.5 | |
| id-28 | 217.7 | 260.6 | 223.2 | 245.5 | ||
| id-41 | 208.4 | 244.3 | 258.7 | 260.1 | ||
| id-43 | 208.4 | 244.3 | 229.6 | 238.7 | ||
| id-36 | 1:40 | 98.6 | 118.7 | 136.2 | 135.1 | |
| id-28 | 151.1 | 163.2 | 174.2 | 170.3 | ||
| id-41 | 148.0 | 181.7 | 174.5 | 170.3 | ||
| id-43 | 103.4 | 126.8 | 110.2 | 117.7 | ||
| High | id-29 | 1:10 | 306.2 | 367.7 | 321.7 | 342.2 |
| id-34 | 373.7 | 427.7 | 407.7 | 412.3 | ||
| id-30 | 572.3 | 696.8 | 808.2 | 779.2 | ||
| id-42 | 447.5 | 537.0 | 568.6 | 586.8 | ||
| id-29 | 1:20 | 213.1 | 257.9 | 233.9 | 233.5 | |
| id-34 | 239.9 | 297.2 | 266.8 | 286.1 | ||
| id-30 | 559.7 | 683.4 | 793.4 | 749.1 | ||
| id-42 | 316.4 | 416.6 | 397.4 | 407.3 | ||
| id-29 | 1:40 | 116.9 | 152.1 | 148.0 | 155.7 | |
| id-34 | 141.5 | 183.2 | 163.3 | 177.4 | ||
| id-30 | 552.8 | 659.1 | 744.6 | 728.5 | ||
| id-42 | 216.7 | 297.0 | 257.1 | 266.7 | ||
| Reactivity | Cow ID | Dilution | Replicates I–II | Replicates I–IV | ||||
|---|---|---|---|---|---|---|---|---|
| Mean * | SD ‡ | CV% | Mean * | SD ‡ | CV% | |||
| Weak | id-44 | 1:10 | 179.2 | 16.8 | 9.4 | 190.1 | 16.7 | 8.8 |
| id-37 | 140.9 | 19.8 | 14.1 | 149.5 | 15.2 | 10.2 | ||
| id-31 | 198.8 | 21.5 | 10.8 | 206.2 | 15 | 7.3 | ||
| id-40 | 189.9 | 25.1 | 13.2 | 212.3 | 29.9 | 14.1 | ||
| id-44 | 1:20 | 101.8 | 11.3 | 11.1 | 102.6 | 7.9 | 7.7 | |
| id-37 | 90.2 | 9 | 10 | 94.1 | 6.9 | 7.3 | ||
| id-31 | 114.5 | 16.4 | 14.3 | 122.1 | 12.9 | 10.6 | ||
| id-40 | 102.7 | 12.4 | 12.1 | 110.1 | 11.7 | 10.6 | ||
| id-44 | 1:40 | 58.6 | 5.5 | 9.4 | 58.5 | 3.4 | 5.8 | |
| id-37 | 61.5 | 1.2 | 2 | 52.3 | 13 | 24.9 | ||
| id-31 | 62.7 | 8.8 | 14.1 | 66.8 | 7.1 | 10.6 | ||
| id-40 | 61.4 | 8.2 | 13.4 | 65.2 | 6.5 | 9.9 | ||
| Medium | id-36 | 1:10 | 287.2 | 27.2 | 9.5 | 312.1 | 33 | 10.6 |
| id-28 | 348.5 | 42.1 | 12.1 | 364.1 | 30.2 | 8.3 | ||
| id-41 | 312.1 | 32.9 | 10.5 | 337.4 | 35 | 10.4 | ||
| id-43 | 309 | 44.7 | 14.5 | 322.1 | 30.2 | 9.4 | ||
| id-36 | 1:20 | 196.4 | 24.1 | 12.3 | 205.2 | 18 | 8.8 | |
| id-28 | 239.2 | 30.3 | 12.7 | 236.8 | 19.9 | 8.4 | ||
| id-41 | 226.3 | 25.3 | 11.2 | 242.9 | 24.1 | 9.9 | ||
| id-43 | 226.3 | 25.3 | 11.2 | 230.2 | 15.7 | 6.8 | ||
| id-36 | 1:40 | 108.7 | 14.2 | 13.1 | 122.2 | 17.6 | 14.4 | |
| id-28 | 157.2 | 8.5 | 5.4 | 164.7 | 10.1 | 6.2 | ||
| id-41 | 164.9 | 23.8 | 14.4 | 168.6 | 14.5 | 8.6 | ||
| id-43 | 115.1 | 16.5 | 14.4 | 114.5 | 10 | 8.8 | ||
| High | id-29 | 1:10 | 336.9 | 43.5 | 12.9 | 334.4 | 26.6 | 8 |
| id-34 | 400.7 | 38.2 | 9.5 | 405.3 | 22.8 | 5.6 | ||
| id-30 | 634.5 | 88 | 13.9 | 714.1 | 105.7 | 14.8 | ||
| id-42 | 492.3 | 63.3 | 12.9 | 535 | 61.8 | 11.6 | ||
| id-29 | 1:20 | 235.5 | 31.7 | 13.5 | 234.6 | 18.3 | 7.8 | |
| id-34 | 268.6 | 40.5 | 15.1 | 272.5 | 25.1 | 9.2 | ||
| id-30 | 621.6 | 87.5 | 14.1 | 696.4 | 101.7 | 14.6 | ||
| id-42 | 366.5 | 70.9 | 19.3 | 384.4 | 46.1 | 12 | ||
| id-29 | 1:40 | 134.5 | 24.9 | 18.5 | 143.2 | 17.8 | 12.4 | |
| id-34 | 162.3 | 29.5 | 18.2 | 166.3 | 18.6 | 11.2 | ||
| id-30 | 606 | 75.2 | 12.4 | 671.3 | 87.2 | 13 | ||
| id-42 | 256.9 | 56.8 | 22.1 | 259.4 | 33.2 | 12.8 | ||
| Test Results | Reference Samples | Total | |
|---|---|---|---|
| True Positive | True Negative | ||
| Non-negative * | 36 | 2 | 38 |
| Negative | 0 | 78 | 78 |
| Total | 36 | 80 | 116 |
| Test Results | Reference Sample Panel | Total | ||
|---|---|---|---|---|
| True Positive | Doubtful | True Negative | ||
| Positive | 35 | 0 | 1 | 36 |
| Doubtful | 1 | 0 | 1 | 2 |
| Negative | 0 | 0 | 78 | 78 |
| Total | 36 | 0 | 80 | 116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righi, C.; Iscaro, C.; Ferroni, L.; Rosati, S.; Pellegrini, C.; Nogarol, C.; Rossi, E.; Dettori, A.; Feliziani, F.; Petrini, S. Validation of a Commercial Indirect ELISA Kit for the Detection of Bovine alphaherpesvirus1 (BoHV-1)-Specific Glycoprotein E Antibodies in Bulk Milk Samples of Dairy Cows. Vet. Sci. 2022, 9, 311. https://doi.org/10.3390/vetsci9070311
Righi C, Iscaro C, Ferroni L, Rosati S, Pellegrini C, Nogarol C, Rossi E, Dettori A, Feliziani F, Petrini S. Validation of a Commercial Indirect ELISA Kit for the Detection of Bovine alphaherpesvirus1 (BoHV-1)-Specific Glycoprotein E Antibodies in Bulk Milk Samples of Dairy Cows. Veterinary Sciences. 2022; 9(7):311. https://doi.org/10.3390/vetsci9070311
Chicago/Turabian StyleRighi, Cecilia, Carmen Iscaro, Laura Ferroni, Sergio Rosati, Claudia Pellegrini, Chiara Nogarol, Elisabetta Rossi, Annalisa Dettori, Francesco Feliziani, and Stefano Petrini. 2022. "Validation of a Commercial Indirect ELISA Kit for the Detection of Bovine alphaherpesvirus1 (BoHV-1)-Specific Glycoprotein E Antibodies in Bulk Milk Samples of Dairy Cows" Veterinary Sciences 9, no. 7: 311. https://doi.org/10.3390/vetsci9070311
APA StyleRighi, C., Iscaro, C., Ferroni, L., Rosati, S., Pellegrini, C., Nogarol, C., Rossi, E., Dettori, A., Feliziani, F., & Petrini, S. (2022). Validation of a Commercial Indirect ELISA Kit for the Detection of Bovine alphaherpesvirus1 (BoHV-1)-Specific Glycoprotein E Antibodies in Bulk Milk Samples of Dairy Cows. Veterinary Sciences, 9(7), 311. https://doi.org/10.3390/vetsci9070311






