The Effects of Bacterial Lipopolysaccharide (LPS) on Turkey Poults: Assessment of Biochemical Parameters and Histopathological Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Animals
2.3. Chemicals
2.4. Experimental Design
2.5. Blood Sample Collection
2.6. Serum Biochemical Analysis
2.7. Serum Protein Electrophoresis
2.8. Histopathological Assessment
2.9. Statistical Analysis
3. Results
3.1. Effects of LPS on Serum Biomarkers
3.2. Effects of LPS on Serum Protein Fractionation
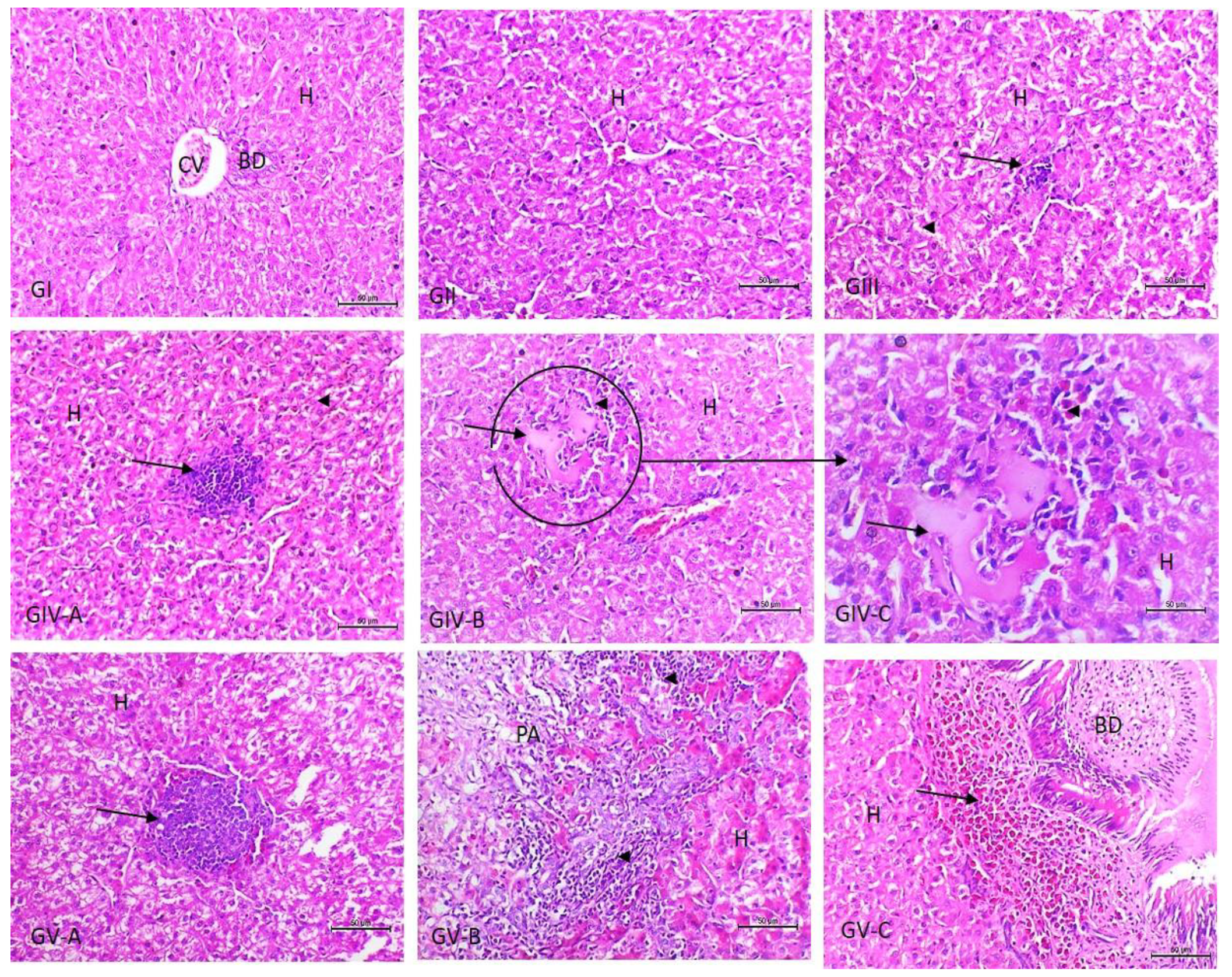
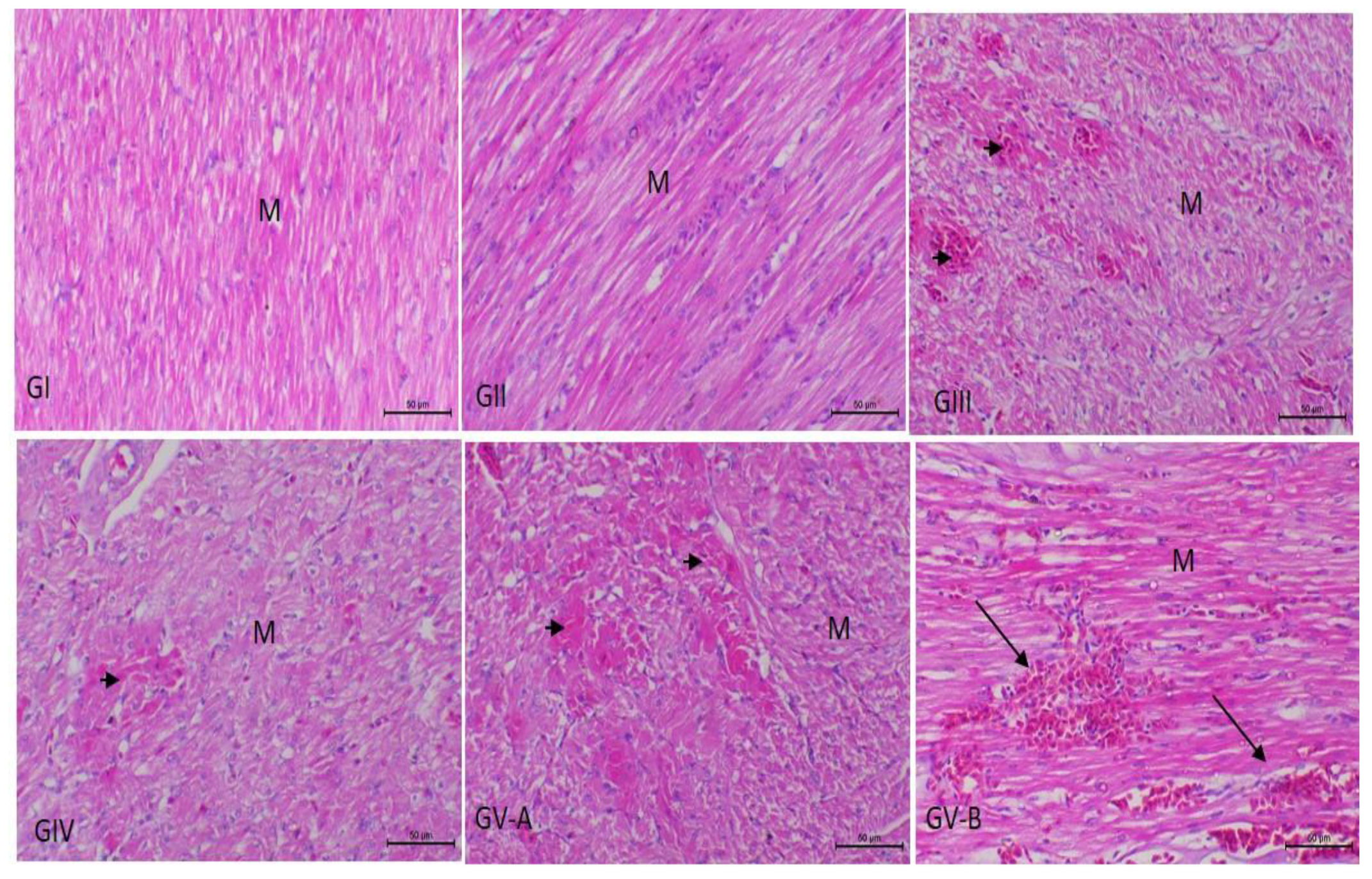
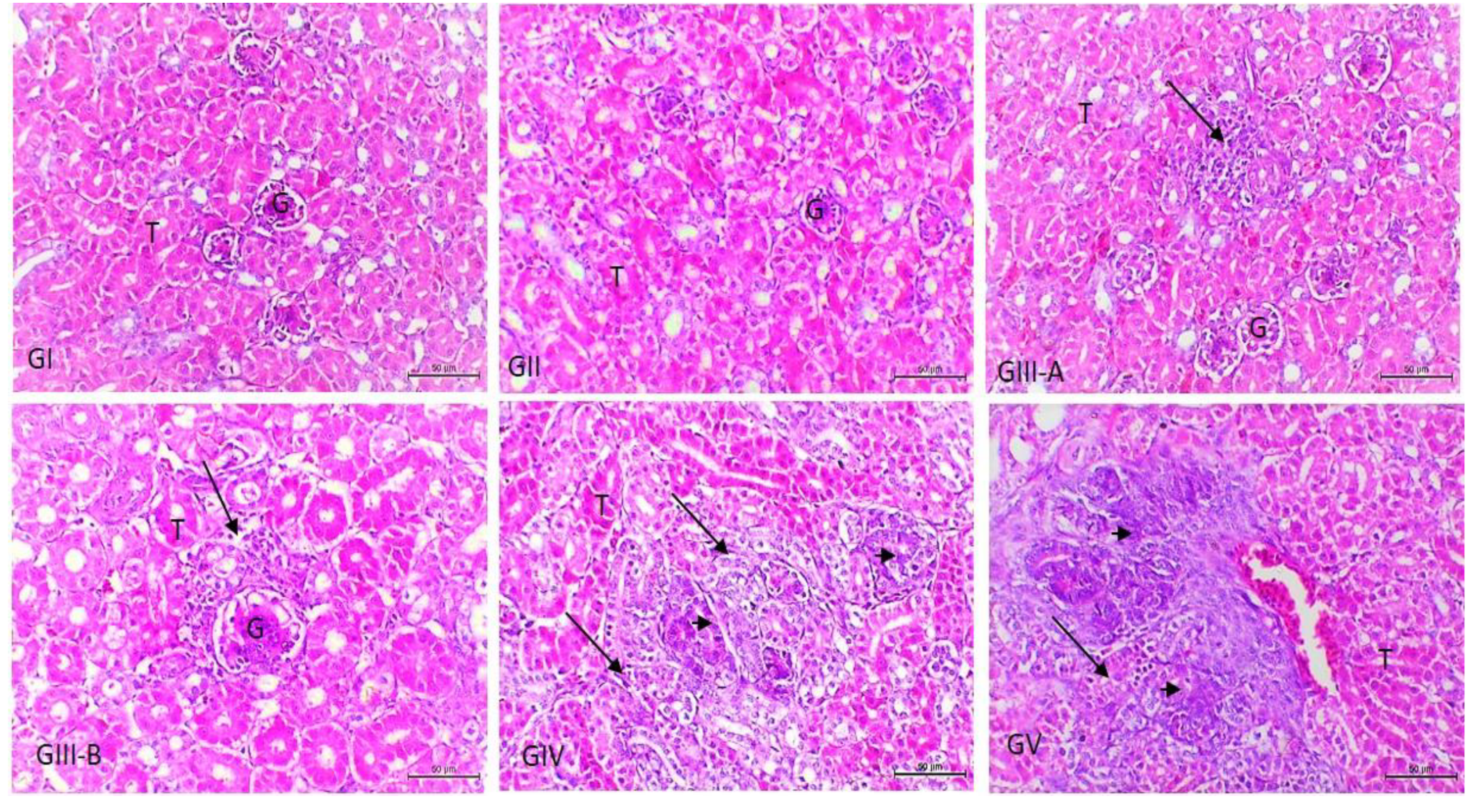
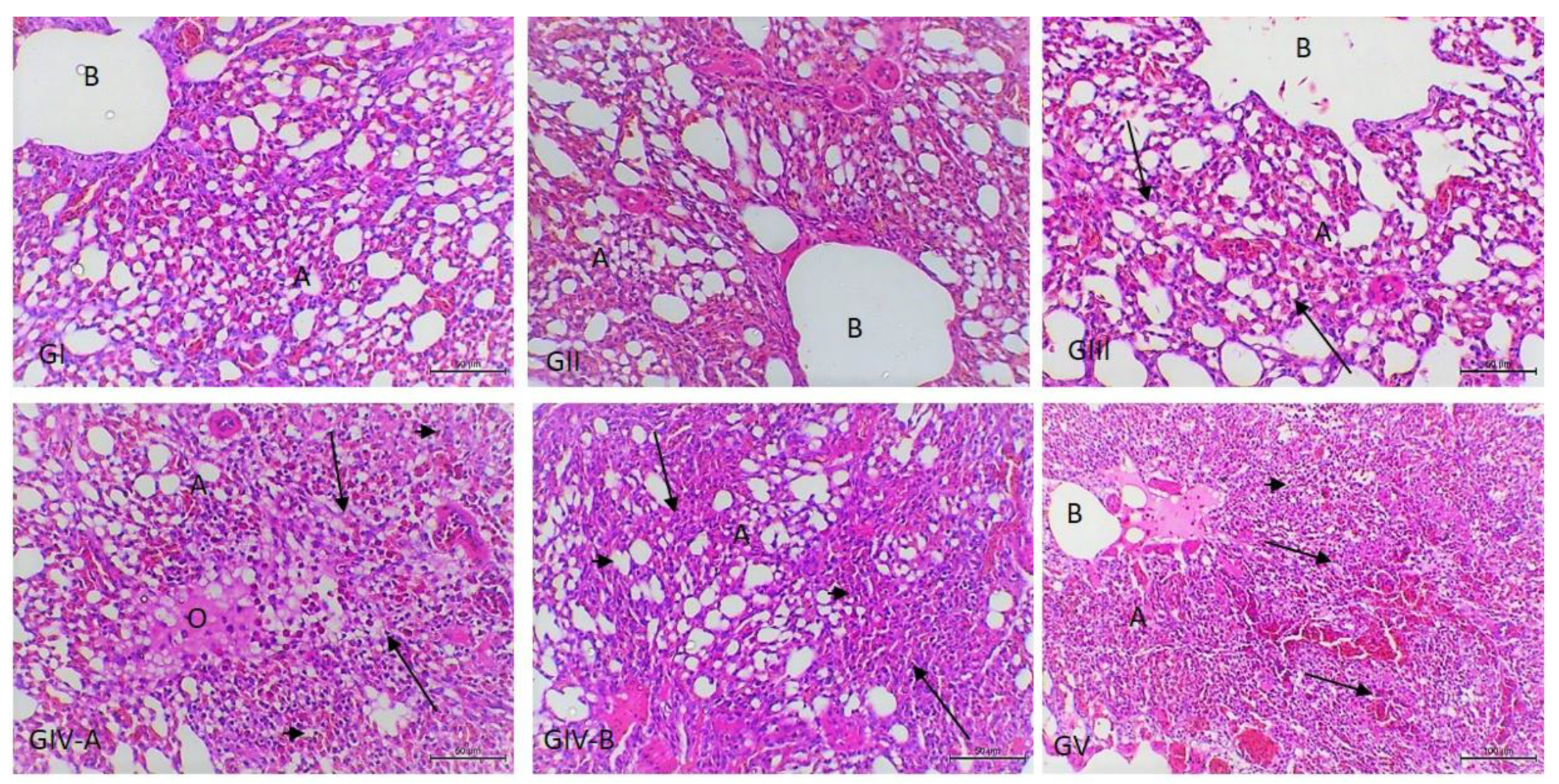
3.3. Histopathological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H. Current knowledge and prospective risk analysis related to ongoing turkey diseases. In Proceedings of the Istanbul: XIV International Congress of the World Poultry Association, Istanbul, Turkey, 22–26 August 2005; Lebib Yalkin Yaimlari ve Basim Isleri Anonim Sirketi: Istanbul, Turkey, 2005; pp. 138–149. [Google Scholar]
- Sackey, B.A.; Mensah, P.; Collison, E.; Sakyi-Dawson, E. Campylobacter, Salmonella, Shigella and Escherichia coli in live and dressed poultry from metropolitan Accra. Int. J. Food Microbiol. 2001, 71, 21–28. [Google Scholar] [CrossRef]
- Barnes, H.; Gross, W. Colibacillosis. Diseases of Poultry, 10th ed.; Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R., Saif, Y.M., Eds.; Iowa State University Press: Ames, IA, USA, 1997; pp. 131–141. [Google Scholar]
- De Oliveira, A.L.; Newman, D.M.; Sato, Y.; Noel, A.; Rauk, B.; Nolan, L.K.; Barbieri, N.L.; Logue, C.M. Characterization of avian pathogenic escherichia coli (APEC) associated with Turkey cellulitis in Iowa. Front. Vet. Sci. 2020, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Sadeyen, J.-R.; Kaiser, P.; Stevens, M.P.; Dziva, F. Analysis of immune responses induced by avian pathogenic Escherichia coli infection in turkeys and their association with resistance to homologous re-challenge. Vet. Res. 2014, 45, 19. [Google Scholar] [CrossRef] [Green Version]
- Pierson, F.W.; Larsen, C.; Domermuth, C. The production of colibacillosis in turkeys following sequential exposure to Newcastle disease virus or Bordetella avium, avirulent hemorrhagic enteritis virus, and Escherichia coli. Avian Dis. 1996, 40, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Vahsen, T.; Zapata, L.; Guabiraba, R.; Melloul, E.; Cordonnier, N.; Botterel, F.; Guillot, J.; Arné, P.; Risco-Castillo, V. Cellular and molecular insights on the regulation of innate immune responses to experimental aspergillosis in chicken and turkey poults. Med. Mycol. 2021, 59, 465–475. [Google Scholar] [CrossRef]
- Morales-Mena, A.; Martínez-González, S.; Teague, K.D.; Graham, L.E.; Señas-Cuesta, R.; Vuong, C.N.; Lester, H.; Hernandez-Patlan, D.; Solis-Cruz, B.; Fuente-Martinez, B. Assessment of fermented soybean meal on Salmonella Typhimurium infection in neonatal turkey poults. Animals 2020, 10, 1849. [Google Scholar] [CrossRef]
- Xu, L.; Eicher, S.D.; Applegate, T.J. Effects of increasing dietary concentrations of corn naturally contaminated with deoxynivalenol on broiler and turkey poult performance and response to lipopolysaccharide. Poult. Sci. 2011, 90, 2766–2774. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Zhai, J.-H.; Chai, Y.-F. Recent advances in the molecular mechanisms underlying pyroptosis in sepsis. Mediat. Inflamm. 2018, 2018, 5823823. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, Y.; Zhang, A.-L.; Xu, T.; Zhou, D.-D.; Li, X.-F.; Yang, J.-F.; Zhang, L.; Wang, X. ZEB2 attenuates LPS-induced inflammation by the NF-κB pathway in HK-2 cells. Inflammation 2018, 41, 722–731. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [Green Version]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [Green Version]
- Riedemann, N.C.; Guo, R.-F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524. [Google Scholar] [CrossRef]
- Kaukonen, K.-M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. New Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C. Endotoxin removal: History of a mission. Blood Purif. 2014, 37, 5–8. [Google Scholar] [CrossRef] [PubMed]
- De Boever, S.; Beyaert, R.; Vandemaele, F.; Baert, K.; Duchateau, L.; Goddeeris, B.; De Backer, P.; Croubels, S. The influence of age and repeated lipopolysaccharide administration on body temperature and the concentration of interleukin-6 and IgM antibodies against lipopolysaccharide in broiler chickens. Avian Pathol. 2008, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Wang, X.; Hao, M.; Li, H.; Cheng, G.; Liu, D.; Yang, Y.; Li, Y. Forsythiaside attenuates Escherichia coli lipopolysaccharide-induced liver acute inflammatory response in chicken. Eur. J. Inflamm. 2019, 17, 2058739219826793. [Google Scholar] [CrossRef] [Green Version]
- Reisinger, N.; Emsenhuber, C.; Doupovec, B.; Mayer, E.; Schatzmayr, G.; Nagl, V.; Grenier, B. Endotoxin translocation and gut inflammation are increased in broiler chickens receiving an oral lipopolysaccharide (LPS) bolus during heat stress. Toxins 2020, 12, 622. [Google Scholar] [CrossRef]
- Lew, W.Y.; Bayna, E.; Molle, E.D.; Dalton, N.D.; Lai, N.C.; Bhargava, V.; Mendiola, V.; Clopton, P.; Tang, T. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS ONE 2013, 8, e61057. [Google Scholar]
- Couch, Y.; Trofimov, A.; Markova, N.; Nikolenko, V.; Steinbusch, H.W.; Chekhonin, V.; Schroeter, C.; Lesch, K.-P.; Anthony, D.C.; Strekalova, T. Low-dose lipopolysaccharide (LPS) inhibits aggressive and augments depressive behaviours in a chronic mild stress model in mice. J. Neuroinflamm. 2016, 13, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 126–138. [Google Scholar]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoud, A.; Saad Allah, M.; Dahran, N.A.; Nasr, N.E.; El-Fkharany, I.; Ahmed, M.S.; Alsharif, K.F.; Elmahallawy, E.K.; Derbalah, A.S. Toxicological effects of Malathion at low dose on Wister male rats with respect to Biochemical and Histopathological alterations. Front. Environ. Sci. 2022, 10, 530. [Google Scholar] [CrossRef]
- Alexander, C.; Rietschel, E.T. Invited review: Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Ci, X.; An, N.; Fan, J.; Cui, J.; Deng, X. Effects of florfenicol on early cytokine responses and survival in murine endotoxemia. Int. Immunopharmacol. 2008, 8, 982–988. [Google Scholar] [CrossRef]
- Schumann, R.R. Old and new findings on lipopolysaccharide-binding protein: A soluble pattern-recognition molecule. Biochem. Soc. Trans. 2011, 39, 989–993. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Fang, H.; Wei, W.; Kan, C.; Xie, C.; Dahmen, U.; Dirsch, O. G-CSF pretreatment aggravates LPS-associated microcirculatory dysfunction and acute liver injury after partial hepatectomy in rats. Histochem. Cell Biol. 2014, 142, 667–676. [Google Scholar] [CrossRef]
- Fink, M.P. Animal models of sepsis. Virulence 2014, 5, 143–153. [Google Scholar] [CrossRef]
- Ferluga, J.; Allison, A. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet 1978, 312, 610–611. [Google Scholar] [CrossRef]
- Curtis, M.; Butler, E. Response of caeruloplasmin to Escherichia coli endotoxins and adrenal hormones in the domestic fowl. Res. Vet. Sci. 1980, 28, 217–222. [Google Scholar] [CrossRef]
- Kaur, G.; Tirkey, N.; Bharrhan, S.; Chanana, V.; Rishi, P.; Chopra, K. Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatoxicity in rodents. Clin. Exp. Immunol. 2006, 145, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative stress, inflammation, and apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, J.J. Serum proteins and the dysproteinemias. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 117–138. [Google Scholar]
- Eckersall, D. Proteins, Proteomics, and the Dysproteinemias. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press Inc.: San Diego, CA, USA, 1997; pp. 116–155. [Google Scholar]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.W. Clinical Chemistry of Birds. In Veterinary Hematology and Clinical Chemistry, 2nd ed.; Thrall, M.A., Weiser, G., Allison, R.W., Campbell, T.W., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 582–598. [Google Scholar]
- O’Connell, T.; Horita, T.J.; Kasravi, B. Understanding and interpreting the serum protein electrophoresis. Am. Fam. Physician 2005, 71, 105–112. [Google Scholar] [PubMed]
- Sakaguchi, O.; Sakaguchi, S. Alterations of lipid metabolism in mice injected with endotoxin. Microbiol. Immunol. 1979, 23, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-J.; Li, Y.; Lou, B.; Wu, M.-P. Beneficial effects of ApoA-I on LPS-induced acute lung injury and endotoxemia in mice. Life Sci. 2006, 79, 210–215. [Google Scholar] [CrossRef]
- Curtis, M.; Scott, B.; Butler, E. The influence of Escherichia coli 078 endotoxin on carbohydrate metabolism in the domestic fowl. Res. Vet. Sci. 1981, 30, 57–61. [Google Scholar] [CrossRef]
- Raetzsch, C.F.; Brooks, N.L.; Alderman, J.M.; Moore, K.S.; Hosick, P.A.; Klebanov, S.; Akira, S.; Bear, J.E.; Baldwin, A.S.; Mackman, N. Lipopolysaccharide inhibition of glucose production through the Toll-like receptor-4, myeloid differentiation factor 88, and nuclear factor κb pathway. Hepatology 2009, 50, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Xianchu, L.; Lan, Z.; Ming, L.; Yanzhi, M. Protective effects of rutin on lipopolysaccharide-induced heart injury in mice. J. Toxicol. Sci. 2018, 43, 329–337. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Chang, X.; Gao, J.; Zhu, L.; Miao, M.; Yan, T. Salidroside mitigates sepsis-induced myocarditis in rats by regulating IGF-1/PI3K/Akt/GSK-3β signaling. Inflammation 2015, 38, 2178–2184. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Luo, F.; Zhou, R.; Ma, C.; Liu, Y.; Yan, T. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis 2015, 20, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Artner-Dworzak, E.; Lechleitner, P.; Smidt, J.; Wagner, I.; Dienstl, F.; Puschendorf, B. Cardiac troponin T in diagnosis of acute myocardial infarction. Clin. Chem. 1991, 37, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Almalki, M.; Elmenshawy, O.; Abdel-Moneim, A. In vivo evaluation of the protective effects of arjunolic acid against lipopolysaccharide-induced septic myocardial injury. PeerJ 2022, 10, e12986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wen, K.; Zhang, Z.; Ma, C.; Zheng, N. 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model. Open Life Sci. 2021, 16, 1313–1320. [Google Scholar] [CrossRef]
- Ahmed, N.; El-Agamy, D.S.; Mohammed, G.A.; Abo-Haded, H.; Elkablawy, M.; Ibrahim, S.R.M. Suppression of LPS-induced hepato-and cardiotoxic effects by pulicaria petiolaris via NF-κB dependent mechanism. Cardiovasc. Toxicol. 2020, 20, 121–129. [Google Scholar] [CrossRef]
- Thorley, A.J.; Ford, P.A.; Giembycz, M.A.; Goldstraw, P.; Young, A.; Tetley, T.D. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J. Immunol. 2007, 178, 463–473. [Google Scholar] [CrossRef]
- Fehrenbach, H.; Brasch, F.; Uhlig, S.; Weisser, M.; Stamme, C.; Wendel, A.; Richter, J. Early alterations in intracellular and alveolar surfactant of the rat lung in response to endotoxin. Am. J. Respir. Crit. Care Med. 1998, 157, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Burrell, R.; Lantz, R.C.; Hinton, D.E. Mediators of Pulmonary Injury Induced by Inhalation of Bacterial Endotoxin1-3. Am. Rev. Respir. Dis. 1988, 137, 100–105. [Google Scholar] [CrossRef]
- Luster, M.I.; Germolec, D.R.; Yoshida, T.; Kayama, F.; Thompson, M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology 1994, 19, 480–488. [Google Scholar] [CrossRef]
- Bautista, A.P.; Spitzer, J.J. Acute endotoxin tolerance downregulates superoxide anion release by the perfused liver and isolated hepatic nonparenchymal cells. Hepatology 1995, 21, 855–862. [Google Scholar] [PubMed]
- Nakao, A.; Taki, S.; Yasui, M.; Kimura, Y.; Nonami, T.; Harada, A.; Takagi, H. The fate of intravenously injected endotoxin in normal rats and in rats with liver failure. Hepatology 1994, 19, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, B.M.; Stephens, R.C.; Feavers, I.M.; Montgomery, H. Role of bacterial endotoxin in chronic heart failure: The gut of the matter. Shock 2007, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Brieland, J.K.; Kunkel, R.G.; Fantone, J.C. Pulmonary alveolar macrophage function during acute inflammatory lung injury. Am. Rev. Respir. Dis. 1987, 135, 1300–1306. [Google Scholar] [CrossRef]
- Nanji, A.A.; Griniuviene, B.; Yacoub, L.K.; Fogt, F.; Tahan, S.R. Intercellular adhesion molecule-1 expression in experimental alcoholic liver disease: Relationship to endotoxemia and TNFα messenger RNA. Exp. Mol. Pathol. 1995, 62, 42–51. [Google Scholar] [CrossRef]
- Dellepiane, S.; Marengo, M.; Cantaluppi, V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care 2016, 20, 61. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.Z.; Badr, K.F. Endotoxin and renal function: Perspectives to the understanding of septic acute renal failure and toxic shock. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 1999, 14, 814–818. [Google Scholar] [CrossRef]
- Konishi, N.; Ward, J. Increased levels of DNA synthesis in hyperplastic renal tubules of aging nephropathy in female F344/NCr rats. Vet. Pathol. 1989, 26, 6–10. [Google Scholar] [CrossRef]
- Tochitani, T.; Mori, M.; Matsuda, K.; Kouchi, M.; Fujii, Y.; Matsumoto, I. Histopathological characteristics of renal changes in humanrenin-angiotensinogen double transgenic rats. J. Toxicol. Pathol. 2016, 29, 125–129. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Control Groups | Treated Groups | |||
|---|---|---|---|---|---|
| Group I (NC) | Group II (NS) | Group III (LPS 0.01) | Group IV (LPS 0.1) | Group V (LPS 1) | |
| Triglycerides (mg/dL) | 92.00 ± 3.16 a | 89.20 ± 6.31 a | 86.00 ± 3.90 a | 78.50 ± 4.59 b | 76.50 ± 7.12 b |
| Total cholesterol (mg/dL) | 132 ± 6.32 a | 129 ± 4.52 a | 114 ± 4.10 b | 101 ± 10.32 c | 95 ± 14.24 c |
| High-density lipoprotein (mg/dL) | 85.00 ± 1.90 a | 87.67 ± 5.94 a | 79.12 ± 3.31 ab | 72.50 ± 9.87 bc | 69.00 ± 10.95 c |
| Low-density lipoprotein (mg/dL) | 29.00 ± 2.53 a | 27.50 ± 2.59 a | 19.50 ± 1.97 c | 14.00 ± 1.26 d | 11.50 ± 0.55 e |
| Glucose (mg/dL) | 293 ± 10.82 a | 288 ± 7.54 a | 269 ± 9.00 b | 271 ± 2.68 b | 264 ± 10.21 b |
| Urea (mg/dL) | 2 ± 0.63 a | 2 ± 0.00 a | 1 ± 0.00 b | 1 ± 0.00 b | 1 ± 0.00 b |
| Creatinine (mg/dL) | 0.02 ± 0.01 c | 0.02 ± 0.01 c | 0.02 ± 0.01 c | 0.07 ± 0.02 b | 0.1 ± 0.00 a |
| Uric acid (mg/dL) | 3.37 ± 0.34 a | 3.4 ± 0.22 a | 2.63 ± 0.31 b | 2.17 ± 0.10 c | 1.87 ± 0.18 d |
| Aspartate transaminase (U/L) | 269 ± 9.49 d | 276 ± 13.59 d | 310 ± 8.94 c | 335 ± 8.94 b | 353 ± 4.38 a |
| Alanine transaminase (U/L) | 4.00 ± 0.89 c | 4.00 ± 0.00 c | 5.00 ± 0.89 b | 8.00 ± 0.63 a | 7.50 ± 0.55 a |
| Alkaline phosphatase (U/L) | 1600 ± 79.06 c | 1630 ± 61.67 c | 2100 ± 89.44 b | 2350 ± 89.44 a | 2340 ± 70.11 a |
| Lactate dehydrogenase (U/L) | 985 ± 15.81 d | 1040 ± 68.90 cd | 1080 ± 27.39 bc | 1130 ± 38.82 ab | 1170 ± 100.78 a |
| Creatine kinase CK (U/L) | 947 ± 18.97 d | 958 ± 24.01 d | 1300 ± 89.44 c | 1530 ± 81.65 b | 1660 ± 21.91 a |
| Cardiac troponin T (ng/mL) | 67.32 ± 8.46 c | 60.26 ± 7.47 c | 61.46 ± 5.58 c | 99.12 ± 8.58 b | 198.00 ± 14.30 a |
| Parameters | Control Groups | Treated Groups | |||
|---|---|---|---|---|---|
| Group I (NC) | Group II (NS) | Group III (LPS 0.01) | Group IV (LPS 0.1) | Group V (LPS 1) | |
| Total protein (g/L) | 3.80 ± 0.09 ab | 3.83 ± 0.05 ab | 3.77 ± 0.14 b | 3.90 ± 0.09 a | 3.83 ± 0.06 ab |
| Albumin (g/L) | 1.43 ± 0.05 a | 1.37 ± 0.05 b | 1.13 ± 0.05 c | 1.17 ± 0.05 c | 1.17 ± 0.05 c |
| α1-globulin (g/L) | 0.20 ± 0.02 c | 0.25 ± 0.05 b | 0.19 ± 0.02 c | 0.17 ± 0.01 c | 0.39 ± 0.05 a |
| α2-globulin (g/L) | 0.94 ± 0.02 c | 0.93 ± 0.03 c | 1.01 ± 0.02 b | 1.19 ± 0.02 a | 0.79 ± 0.03 d |
| β-globulin (g/L) | 0.88 ± 0.01 bc | 0.90 ± 0.03 b | 0.88 ± 0.03 bc | 0.84 ± 0.05 c | 0.94 ± 0.02 a |
| γ-globulin (g/L) | 0.35 ± 0.01 b | 0.39 ± 0.04 b | 0.55 ± 0.07 a | 0.53 ± 0.11 a | 0.55 ± 0.02 a |
| Albumin/Globulin (ratio) | 0.61 ± 0.01 a | 0.55 ± 0.03 b | 0.43 ± 0.02 c | 0.41 ± 0.02 c | 0.44 ± 0.02 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Elazab, M.F.; Nasr, N.E.; Ahmed, M.S.; Alrashdi, B.M.; Dahran, N.; Alblihed, M.A.; Elmahallawy, E.K. The Effects of Bacterial Lipopolysaccharide (LPS) on Turkey Poults: Assessment of Biochemical Parameters and Histopathological Changes. Vet. Sci. 2022, 9, 240. https://doi.org/10.3390/vetsci9050240
Abou Elazab MF, Nasr NE, Ahmed MS, Alrashdi BM, Dahran N, Alblihed MA, Elmahallawy EK. The Effects of Bacterial Lipopolysaccharide (LPS) on Turkey Poults: Assessment of Biochemical Parameters and Histopathological Changes. Veterinary Sciences. 2022; 9(5):240. https://doi.org/10.3390/vetsci9050240
Chicago/Turabian StyleAbou Elazab, Mohamed F., Nasr E. Nasr, Mohamed S. Ahmed, Barakat M. Alrashdi, Naief Dahran, Mohamed A. Alblihed, and Ehab Kotb Elmahallawy. 2022. "The Effects of Bacterial Lipopolysaccharide (LPS) on Turkey Poults: Assessment of Biochemical Parameters and Histopathological Changes" Veterinary Sciences 9, no. 5: 240. https://doi.org/10.3390/vetsci9050240
APA StyleAbou Elazab, M. F., Nasr, N. E., Ahmed, M. S., Alrashdi, B. M., Dahran, N., Alblihed, M. A., & Elmahallawy, E. K. (2022). The Effects of Bacterial Lipopolysaccharide (LPS) on Turkey Poults: Assessment of Biochemical Parameters and Histopathological Changes. Veterinary Sciences, 9(5), 240. https://doi.org/10.3390/vetsci9050240







